Abstract
Background
Indirubin is the active component of Danggui Longhui Wan, a traditional Chinese medicine formulation. Due to its anti-inflammation and anti-tumor effects, indirubin has been widely used for the treatment of inflammation, cancer, and other chronic disease. Herein, we aimed to investigate the role and mechanism of indirubin in human ovarian cancer cell proliferation.
Materials and methods
The cell viability was determined by Cell Counting Kit-8 and colony formation assays by treatment with different dosages of indirubin over 72 hours. Apoptosis was examined by flow cytometry with fluorescein isothiocyanate Annexin V Apoptosis Detection Kit. Western blot assay was finally applied to analyze the expression of cancer-related STAT3 pathway and its downstream proteins.
Results
Indirubin was found to significantly inhibit cell viability and induce apoptosis in 2 human ovarian cancer cell lines. Mechanistic studies revealed that indirubin treatment led to reduced levels of phosphorylated-STAT3, thus repressing the downstream pro-survival proteins and elevating pro-apoptosis ones.
Conclusion
Our study provided the evidence for anti-survival activity of indirubin by inhibiting cell viability and inducing apoptosis in human ovarian cancer cells, which involved impaired STAT3 signaling pathway. Our findings further support indirubin as a potential drug candidate against human ovarian cancer.
Introduction
Ovarian cancer is the most common and second lethal type of gynecological cancer affecting women worldwide.Citation1,Citation2 Due to lack of evident symptoms, most of patients with ovarian cancer are diagnosed at the advanced stage, leading to a 5-year survival rate ranging from 25% to 30%.Citation3 Besides, high recurrence rate and chemo-resistance also contribute to poor prognosis and high mortality among these patients.Citation4,Citation5 Therefore, a refined investigation of the therapy strategies and identification of novel agents for ovarian cancer, especially high-grade ovarian cancer, are urgently required to improve the survival rate of this disease.
Till now, numerous natural products (mostly plant extracts) have been used to treat human diseases, including cancers.Citation6,Citation7 They constitute the basis for multiple prescriptions in traditional Chinese medicine and appear as a complementary approach to treat cancers.Citation8 Danggui Longhui Wan is a compound from traditional Chinese medicine consisting of 11 herbal medicines, and is used for treating chronic myeloid leukemia.Citation9 Among the 11 medicinal ingredients, Qing Dai (indigo naturalis) appears to be closely associated with the anti-leukemic activity, which is composed of high levels of indigo dye and a minor but active component indirubin. It has been discovered that indirubin and its derivatives are strong inhibitors of cyclin-dependent kinases (CDKs) by competing with ATP binding at the catalytic site, resulting in G2/M arrest and apoptosis in cancer cells.Citation10,Citation11 Indirubin derivative has been proved to inhibit tumor cell growth in many types of cancer cell lines and induced apoptosis.Citation12 Indirubin derivative E738 has been identified as a novel dual inhibitor of JAKs and SFKs, which leads to inhibition of STAT3 signaling.Citation13 And indirubin derivative 6BIO has been reported to exert the unique property of increasing stress tolerance and, in parallel, partially suppressing the nutrient-sensing pathway signaling.Citation14 Moreover, indirubin has been reported to decrease the susceptibility to influenza virus and promote the expression of interferon-β and interferon-inducible transmembrane 3 through promoting mitochondrial antiviral signaling pathway.Citation15 Over the last 2 decades, numerous studies relevant to anti-tumor activity of indirubin have been reported in the literature. However, whether it possesses oncotherapy potential in ovarian cancer remains unclear.
In this study, we focus our work on investigating the effect of simple indirubin on cell survival, including cell proliferation and apoptosis, in 2 different ovarian cancer cell lines and elucidate the significant cytotoxic mechanisms of this compound against ovarian cancer cells.
Materials and methods
This work was approved by the review board of The First Affiliated Hospital of Fujian Medical University.
Cell culture and reagent
Human cervical cancer cell lines A2780 and OVCAR3 were purchased from the cell bank of Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). The cells were cultured in Roswell Park Memorial Institute 1640 medium with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 100 U/mL of penicillin and 100 µg/mL of streptomycin (Thermo Fisher Scien tific) in a 5% CO2 incubator at 37°C. Indirubin was purchased from Sigma-Aldrich (San Francisco, CA, USA). Colivelin was purchased from Santa Cruz Biotechnology (sc-361153, Santa Cruz, CA, USA).
Cell Counting Kit-8 (CCK-8) assay
Cell viability assay was performed by CCK-8 methods. Briefly, cells were seeded in 96-well plate overnight at a density of 2×103 per well, and then treated with different concentrations of indirubin (2 and 5 µM) for 0, 24, 48, or 72 hours, respectively. After incubation, 10 µL Cell Counting Kit-8 (Dojindo, Tokyo, Japan) was added into each well for 1 hour. The OD of each sample was detected at 450 nm using a microplate reader. The experiments were performed in triplicate.
Colony formation assay
Cell viability assay was performed by colony formation assay and crystal violet staining methods. Briefly, the cells were seeded into a 6-well plate at 5×103 per well. After 12 hours, the cells were treated with indirubin at the concentration of 5 µM at 37°C. The culture medium was refreshed every 2 days. Ten days later, the cells were washed twice with PBS and fixed in 4% paraformaldehyde for 30 minutes. After washing twice with PBS buffer, the cells were stained with 1% crystal violet staining buffer for 30 minutes. The plates were then aspirated, washed, and allowed to air dry, followed by photograph by digital camera.
Flow cytometry analysis
Cell apoptosis assay was conducted by flow cytometry with fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) as per the manufacturer’s protocol. Briefly, the cells were treated with different concentrations of indirubin (0, 0.5, 1, 2, 5, 10, and 20 µM) for 72 hours, followed by harvesting and suspending in binding buffer at a concentration of 1.5×106 cells/mL. Then, 5 µL FITC Annexin V and 5 µL propidium iodide staining solution were added to 200 µL of the cell suspension. The mixture was incubated for 30 minutes at room temperature in dark and cells apoptosis was detected by BD FACSVerse™ (BD Biosciences). Apoptosis rate of cancer cells were analyzed by BD FACSuite software.
Western blot assay
A2780 and OVCAR3 cells were treated with different concentrations of indirubin (2 and 5 µM) or pretreated with 0.5 µM colivelin. Forty-eight hours later, the cells were washed by PBS and harvested by RIPA buffer (Beyotime, Shanghai, China) with 1 mM phenylmethylsulfonyl fluoride (Beyotime) on ice for 30 minutes. After centrifuging at 13,000 g at 4°C for 15 minutes, the supernatant fractions were collected and the protein concentration was quantified using a BCA Protein Assay Kit (Beyotime). The remaining supernatant was mixed with 2× loading buffer and boiled at 100°C for 15 minutes. The same amounts of protein were separated using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to nitrocellulose membrane (Millipore, Billerica, MA, USA). After transferring, the membrane was blocked with 5% fat-free milk in PBST (PBS with Tween 20). Primary antibodies were incubated at 4°C overnight, and secondary antibodies were incubated at room temperature for 1 hour. The membranes were washed in PBST and the proteins of interest were visualized using enhanced chemiluminescence Western blotting substrate (Pierce, Rockford, IL, USA). β-actin was used as an internal control. Anti-p-STAT (Tyr705) (1:1,000, #9145), anti-t-STAT3 (1:1,000, #9139), anti-Bcl-xL (1:1,000, #2764), anti-bax (1:1,000, #2774), anti-cleaved caspase 3 (1:1,000, #9664), and anti-β-actin (1:5,000, #3700) antibodies were from Cell Signaling Technology (San Jose, CA, USA). Anti-Cyclin D1 (1:1,000, sc-450) and anti-C-myc (1:1,000, sc-4084) antibodies were from Santa Cruz (Dallas, TX, USA).
Statistical analysis
All data were analyzed by GraphPad Prism 7.0 software. Comparison between groups was performed by one-way ANOVA followed by Student–Newman–Keuls test. The data were presented as mean ± SD. A P-value <0.05 was considered as statistically significant. All experiments were repeated thrice independently.
Results
Indirubin inhibits cell viability of human ovarian cancer cells
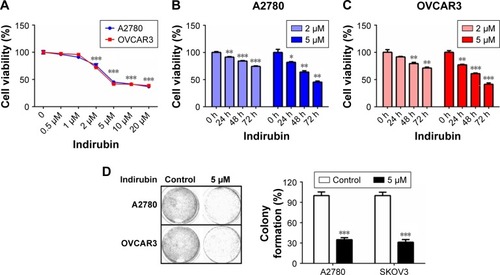
To characterize the cytotoxicity of indirubin on human ovarian cancer cells, we first treated 2 different ovarian cancer cell lines, A2780 and OVCAR3, with increasing dosages of indirubin (0, 0.5, 1, 2, 5, 10, and 20 µM) for 72 hours. Then cell viability was analyzed by CCK-8 assay. The results shown in revealed a similarly decreased cell viability following treatment with indirubin at >2 µM concentrations. And the half maximal inhibitory concentration value of indirubin for each cell line was ~4 µM. By treating the 2 cell lines with either 2 or 5 µM indirubin for 3 days continuously, we observed a similar time-dependent inhibition of cell viability, and that 5 µM indirubin made the faster suppression (). In addition, treatment with 5 µM indirubin significantly inhibited colony formation in both A2780 and OVCAR3 cell lines (). These results indicate that indirubin represses cell viability of ovarian cancer cells in vitro.
Figure 1 Indirubin inhibited cell viability in ovarian cancer cells.
Abbreviation: CCK-8, Cell Counting Kit-8.
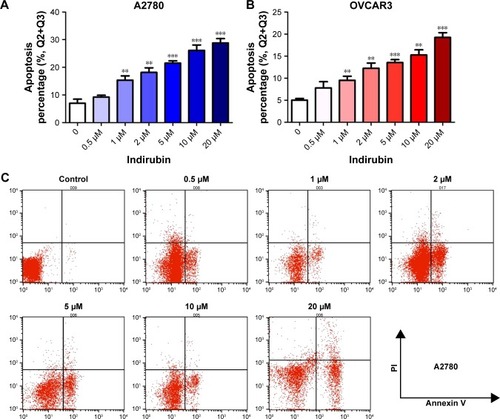
Indirubin induces apoptosis of human ovarian cancer cells
To examine whether indirubin represses cell viability via inducing cell apoptosis in the 2 ovarian cancer cell lines tested, we then evaluated the apoptosis rate of indirubin-treated cells through flow cytometry with FITC Annexin V Apoptosis Detection Kit. As shown in , after incubation with increasing concentrations of indirubin (0, 0.5, 1, 2, 5, 10, and 20 µM) for 72 hours, Annexin V-labeled cell apoptosis increased with the increased dosage of indirubin. These results suggested that indirubin treatment induces the apoptosis of ovarian cancer cells in vitro.
Figure 2 Indirubin induced apoptosis in ovarian cancer cells.
Cell viability inhibitory effects of indirubin was associated with the inhibition of STAT3 signaling
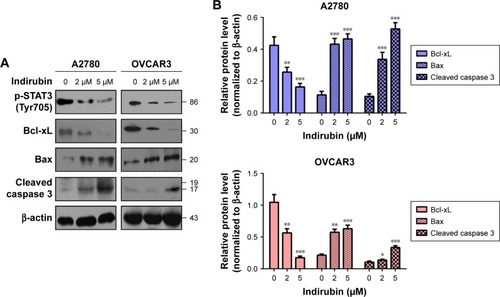
To further explore the molecular mechanism of anti-viability action of indirubin, we investigated the effect of indirubin on the cancer-related STAT3 signaling pathway. We found that indirubin caused downregulation of phospho-STAT3 (Tyr705) dose-dependently in both A2780 and OVCAR3 cells (). As is known, STAT3, activated by phosphorylation, plays a key role as a critical transcription activator in cell proliferation. We then analyzed the expression of STAT3 downstream genes. Results showed that indirubin significantly inhibited the expression of Cyclin D1 and C-myc (). On the other hand, STAT3 is also involved in the inhibition of apoptosis. Consistently, we found that the pro-apoptosis Bax and cleaved caspase 3 were activated, whereas the anti-apoptotic Bcl-xL was repressed by indirubin in the 2 cell lines dose-dependently (). These findings suggested that indirubin exerts anti-viability effects by inhibiting the activation of STAT3 signaling.
Figure 3 Indirubin-mediated expression of survival-related protein altered.
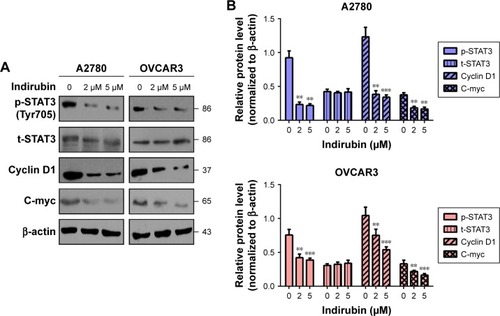
Figure 4 Indirubin-mediated expression of apoptosis-related protein altered.
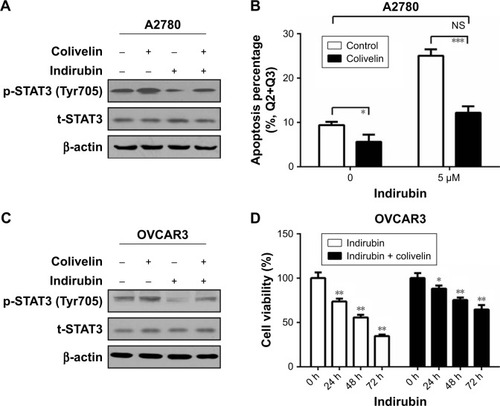
Colivelin partly reversed the indirubin-induced suppression of cell viability and phosphorylation of STAT3
To investigate whether indirubin played anti-viability roles through the phosphorylation of STAT3, colivelin, a STAT3 activator, was used in our system. A2780 cells were pretreated with 0.5 µM colivelin or dimethyl sulfoxide (DMSO) for 1 hour, followed by treated with or without 5 µM indirubin for 72 hours. The Western blot assay results showed that colivelin significantly induced the phosphorylation of STAT3, while indirubin significantly inhibited it. Moreover, we found that pretreatment of colivelin could partly reverse the indirubin-induced suppression of STAT3 phosphorylation (). Next, flow cytometry assay showed that colivelin also reversed the cell apoptosis phenotype induced by indirubin (). In order to make our conclusion more solid, we repeated the treatment in OVCAR3 cells (). CCK-8 assay showed that cell viability inhibition induced by indirubin was significantly impaired by pretreatment of colivelin (). Overall, these results suggested that indirubin exerts anti-viability effects partly through the activation of STAT3 signaling pathway.
Figure 5 Colivelin could partly reverse the indirubin-induced suppression of cell proliferation and phosphorylation of Stat3.
Abbreviation: NS, not significant.
Discussion
As an active component of the traditional Chinese herbal medicine, Danggui Longhui Wan, indirubin is known for treatment of chronic myelogenous leukemia.Citation9 Besides, recent studies demonstrate that indirubin as well as its derivatives act as potent inhibitors against CDKs and display potent growth inhibitory effects in human cancer cells.Citation16,Citation17 Specifically, indirubin inhibits the proliferation of a wide range of cancer cells, for example, lung cancer cell lines A549 and LXFL529L, breast cancer cell line MCF-7, prostate cancer cell line PC-12, and leukemic cell line K-562.Citation10,Citation11,Citation16,Citation18 Among most of these cell lines, indirubin inhibited cells proliferation in a dose-dependent manner. Induction of cell apoptosis could also be caused by arresting of cell cycle in the G1/S or G2/M by indirubin.Citation10,Citation17 It remains unclear if other mechanisms exist for explaining the apoptosis induction by indirubin.
Liu et al reported the anti-survival effect of MLS-2384 (a new 6-bromoindirubin derivative) on variant cancer cells, including ovarian cancers.Citation19 Yu et al demonstrated an inhibitory effect of (2Z, 3E)-6-bromoindirubin-3′-oxime (a bromoindirubin derivative, known as “BIO”) on cell proliferation, cell cycle, migration, and invasion in ovarian cancer cells.Citation20 In the present study, we conducted experiments on 2 different ovarian cancer cell lines. Our results showed that simple indirubin significantly inhibited cell viability and induced apoptosis in ovarian cancer cells. Detailed mechanism studies revealed that indirubin repressed the expression of phosphorylated-STAT3, thus repressing the downstream pro-survival proteins and elevating pro-apoptosis ones. Mechanistic studies by using STAT3 activator showed that indirubin exerts anti-viability effects partly through activation of STAT3 signaling pathway.
As is known, signal transducer and activator of transcription (STAT) proteins play an important role in cancer cell survival.Citation21,Citation22 Among the protein family members, STAT3 is often constitutively activated in many human cancer cells, and has been implicated as a promising target for cancer therapy.Citation21 Its persistent signaling significantly contributes to oncogenesis by promoting cell proliferation and preventing apoptosis.Citation22 STAT3 activates tumorigenesis through directly upregulating of genes encoding apoptosis inhibitors and cell cycle regulators like Bcl-xL, Cyclin D1, and C-myc.Citation21,Citation22 The link between indirubin and STAT3 activation has also been discovered by several other groups previously.Citation23–Citation25
Conclusion
Our study provided solid evidence for anti-survival activity of indirubin by inhibiting cell viability and inducing apoptosis in human ovarian cancer cells, which involved impaired STAT3 signaling. Therefore, indirubin is a potent inhibitor of the STAT3 signaling and can serve as a potential drug candidate against human ovarian cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- KnutsonKLKaryampudiLLamichhanePPrestonCTargeted immune therapy of ovarian cancerCancer Metastasis Rev2015341537425544369
- TorreLABrayFSiegelRLGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- AuKKJosahkianJAFrancisJASquireJAKotiMCurrent state of biomarkers in ovarian cancer prognosisFuture Oncol201511233187319526551891
- HeQZLuoXZWangKIsolation and characterization of cancer stem cells from high-grade serous ovarian carcinomasCell Physiol Biochem201433117318424504111
- VaughanSCowardJIBastRCRethinking ovarian cancer: recommendations for improving outcomesNat Rev Cancer2011111071972521941283
- GrahamJGQuinnMLFabricantDSFarnsworthNRPlants used against cancer – an extension of the work of Jonathan HartwellJ Ethno pharmacol2000733347377
- NewmanDJCraggGMNatural products as sources of new drugs over the 30 years from 1981 to 2010J Nat Prod201275331133522316239
- YangGLiXLiXTraditional chinese medicine in cancer care: a review of case series published in the chinese literatureEvid Based Complement Alternat Med2012201275104622778776
- XiaoZHaoYLiuBQianLIndirubin and meisoindigo in the treatment of chronic myelogenous leukemia in ChinaLeuk Lymphoma20024391763176812685829
- HoesselRLeclercSEndicottJAIndirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinasesNat Cell Biol199911606710559866
- EisenbrandGHippeFJakobsSMuehlbeyerSMolecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicineJ Cancer Res Clin Oncol20041301162763515340840
- ChengXMerzKHVatterSIdentification of a Water-Soluble Indirubin Derivative as Potent Inhibitor of Insulin-like Growth Factor 1 Receptor through Structural Modification of the Parent Natural MoleculeJ Med Chem201760124949496228557430
- NamSWenWSchroederADual inhibition of Janus and Src family kinases by novel indirubin derivative blocks constitutively-activated Stat3 signaling associated with apoptosis of human pancreatic cancer cellsMol Oncol20137336937823206899
- TsakiriENGaboriaud-KolarNIliakiKKThe Indirubin Derivative 6-Bromoindirubin-3′-Oxime Activates Proteostatic Modules, Reprograms Cellular Bioenergetic Pathways, and Exerts Antiaging EffectsAntioxid Redox Signal201727141027104728253732
- JieCLuoZChenHIndirubin, a bisindole alkaloid from Isatis indigotica, reduces H1N1 susceptibility in stressed mice by regulating MAVS signalingOncotarget201786210561510562929285277
- MarkoDSchätzleSFriedelAInhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cellsBr J Cancer200184228328911161389
- LeclercSGarnierMHoesselRIndirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors?J Biol Chem200276251260
- DamiensEBaratteBMarieDEisenbrandGMeijerLAnti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrestOncogene200120293786379711439342
- LiuLGaboriaudNVougogianopoulouKMLS-2384, a new 6-bromoindirubin derivative with dual JAK/Src kinase inhibitory activity, suppresses growth of diverse cancer cellsCancer Biol Ther201415217818424100507
- YuASZhaoLEffects of the GSK-3β inhibitor (2Z,3E)-6-bromoindirubin-3′-oxime upon ovarian cancer cellsTumour Biol20163744857486426526575
- YuHJoveRThe STATs of cancer – new molecular targets come of ageNat Rev Cancer2004429710514964307
- BuettnerRMoraLBJoveRActivated STAT signaling in human tumors provides novel molecular targets for therapeutic interventionClin Cancer Res20028494595411948098
- ZhangXSongYWuYIndirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cellInt J Cancer2011129102502251121207415
- NamSBuettnerRTurksonJIndirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cellsProc Natl Acad Sci U S A2005102175998600315837920
- SchwaibergerAVHeissEHCabaravdicMIndirubin-3′-monoxime blocks vascular smooth muscle cell proliferation by inhibition of signal transducer and activator of transcription 3 signaling and reduces neointima formation in vivoArterioscler Thromb Vasc Biol201030122475248120847306
