Abstract
Background
Gliomas are one of the most common types of primary brain tumors. It is usually evaluated by gadolinium(III)-based contrast agents by magnetic resonance imaging (MRI) in the clinic. Methotrexate (MTX), as a type of folate analog that inhibits the enzyme dihydrofolate reductase, is widely used as a chemotherapeutic agent to treat gliomas in the experiment.
Purpose
In this study, a novel theranostic agent MTX-DOTA-Gd (MTX-Gd) was synthesized, which integrates magnetic resonance imaging (MRI) with anticancer treatment.
Methods
MTX-Gd was synthesized by connecting MTX and Gd through 1,4,7,10-tetraazacy-clododecane-1,4,7,10-tetraacetic acid (DOTA). The characterization of MTX-Gd was detected by ultraviolet (UV) and infrared spectroscopy (IR). To confirm the antitumor effect of MTX-Gd, the cytotoxicity of MTX-Gd was examined by the MTT assay. The contrast enhancement of the MTX-Gd was measured through MRI in vitro. Then, nude mice bearing C6 tumor xenografts were used to study in vivo imaging capabilities.
Results
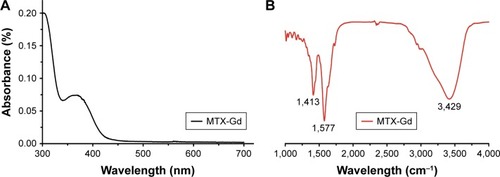
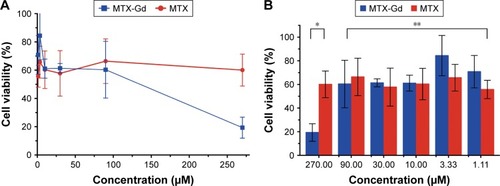
The ultraviolet-visible-near infrared radiation (UV-NIR) absorption curve indicated that MTX-Gd had a broad absorption in the region of 500-700 nm. The formation of MTX-Gd was confirmed from the characteristic bands of MTX-DOTA-Gd in the 1413 cm−1 (C-N), 1577 cm−1 (−NH2), and 3429 cm−1 (N-H), in the fourier-transform infrared (FTIR) spectra. MTX-Gd showed little difference in the cell viability compared with MTX, except for the highest concentration (270 μM). In vitro, the imaging of MTX-Gd was significantly brighter than Gd-DOTA at the same concentration, and the brightness and signal intensity of MRI were increased followed by the increased concentration of MTX-Gd. And it also showed that MTX was not visualized on MRI. The other images revealed that the concentration of 4 mM MTX-Gd had the same imaging effect with the concentration of 10 mM Gd-DOTA. Then, MTX-Gd was injected in nude mice bearing C6 tumor xenografts through the tail vein. Significant contrast enhancement was observed at the tumor site from 0.5 h to 3 h. The signal of tumor area was strongest at 3 h due to accumulation by size effect of macromolecules.
Conclusion
A novel stable and unique theranostic agent (MTX-Gd) was successfully synthe-sized, and it has good stability, strong anticancer ability and excellent magnetic capacity. The methotrexate component of MTX-Gd, as a chemotherapeutic agent, played an important role in targeted therapies of cancer. The DOTA-Gd component of MTX-Gd performed as the MRI contrast agent. The superior MRI imaging performance and synergetic chemical antineoplastic ability of MTX-Gd was revealed, and it has great potential in the diagnosis and treatment of glioma and potentially other cancers, with prospects of clinical application in the near future.
Keywords:
Introduction
Methotrexate (MTX) is a folate analog that inhibits the enzyme dihydrofolate reductase. It is widely used to treat psoriasis, rheumatoid arthritis, and other autoimmune diseases. In addition, malignant diseases such as acute lymphoblastic leukemia (ALL), lymphoma, medulloblastoma, and osteosarcoma are treated with high doses of MTX or intrathecal injection of MTX.Citation1,Citation37–Citation39 MTX at a dose of ≥1 g/m2 (high-dose MTX, HDMTX) is the most efficient treatment against primary central nervous system lymphoma (PCNSL) and is the most widely used drug in prospective clinical trials.Citation2,Citation27–Citation32 Numerous studies suggest that HDMTX (1 g/m2) has a moderate blood–brain barrier (BBB) permeability (approximately 5% of plasma levels).Citation3
Glioma is a common type of primary brain tumor that has high morbidity and mortality due to its location and locally invasive growth. Currently, gadolinium(III)-based contrast-enhanced magnetic resonance imaging (MRI) is a preferred choice for the clinical diagnosis of glioma and preoperative localization.Citation1,Citation33 Some studies have reported that MRI can make a good evaluation for the grading of gliomas.Citation4,Citation5,Citation34–Citation36 Measurement of relative cerebral blood volume (rCBV) can be used to improve the sensitivity of grading a tumor; the preoperative distinction between high-grade and low-grade gliomas showed that rCBV in intratumoral and peritumoral regions was significantly different. The combination of intratumoral and peritumoral rCBV provided overall better diagnostic accuracy and helped to reduce invasive interventions for nonsurgical candidates.Citation4–Citation9 Moreover, longitudinal relaxation rate, transverse relaxation rate (R1, R2), and rCBV decreased as the distance from the contrast enhancement portion of the tumor increased. There was a significant increase in the R1 gradient after contrast agent injection (P<0.0001). There was a heterogeneous pattern of relaxation values in the peritumoral edema adjacent to the contrast enhancement portion of the tumor. This pattern might reflect an invisible tumor infiltrating into the surrounding tissue. This information might be useful for the planning of surgery and radiotherapy.Citation8 In addition to rCBV, diffusion kurtosis imaging (DKI) and neurite orientation dispersion and density imaging (NODDI) scalar markers could be used effectively as glioma grade biomarkers and had a significant difference (P<0.05) for grading between low-grade and high-grade gliomas, especially for glioma II vs glioma III and glioma III vs glioma IV.Citation5 According to the abovementioned reports, MRI is a very good method for the clinical diagnosis of glioma.
In MRI, MRI contrast agents can enhance the relaxation rates of water molecules and can therefore be used to provide additional information in the image relative to the areas that have not been treated with the agent.Citation10 Currently, gadolinium(III)-based contrast agents have been used for >40% MRI scans in clinical practice and play an important role in angiography-enhanced MRI studies.Citation11–Citation13 There are several MRI contrast agents that have been successfully used for the clinical diagnosis of specific diseases, including Dotarem (Gd-DOTA), ProHance (Gd-HP-DO3A), and Gadovist (Gd-DO3A-burol).Citation14,Citation15 Gd-DOTA, a complex prepared with the chelating agent 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), exhibits the fastest rate of bound water.Citation16 Therefore, many studies currently use Gd-DOTA as a target for drug synthesis studies.Citation17–Citation23,Citation40–Citation42
Theranostics, which combines diagnostic and therapeutic effects, is a promising method of personalized medicine, especially for cancer.Citation24,Citation25 On the basis of the advantage of permeating the BBB with HDMTX, Gd-DOTA was conjugated to MTX. The fabricated drug, MTX-Gd, would have a broad impact on cancer diagnosis and treatment.
Materials and methods
Materials
All chemicals were obtained from commercial suppliers and were used as received. MTX was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Dimethyl sulfoxide (DMSO) was purchased from Shanghai Chemical Co. Ltd, Shanghai, PR China. All solutions were of analytical grade in this study. The C6 glioma cell line was purchased from the China Center for Type Culture Collection (Wuhan University, Wuhan, PR China) and cultured in DMEM (Beijing Dingguo Changsheng Biotechnology Co. Ltd, Beijing, PR China), supplemented with 10% FBS and 1% antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) at 37°C in a 5% CO2 atmosphere.
Synthesis and characterization of MTX-Gd
Tri-tert-butyl-2,2′,2″-(10-(2-ethoxy-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl) triacetate (Tris-DOTA) (1.447 g, 2.4 mmol) and MTX (2.3 g, 4.8 mmol) were dissolved in N,N-dimethylformamide (DMF), placed in a bottle for mixing, and stirred at room temperature for 48 hours. After that, the resulting mixture was evaporated to obtain a light yellow solid. Then, the solid mixture was dissolved in 22 mL HCl aqueous solution (37%), and the solution was stirred for 30 minutes at room temperature. HCl and water were removed under vacuum to obtain a light yellow solid. Light-yellow solid was added to 20 mL acetone, and the suspension was stirred for 1 hour at room temperature. The white precipitates were filtered and washed with 10 mL acetone three times. The solvent was removed under reduced pressure to gain the product MTX-DOTA as a light yellow powder.
MTX-Gd was prepared according to the reported procedure in the literature.Citation18 In short, the 10 mL DMF solution without metal MTX-DOTA (346 mg, 0.3 mmol) and gadolinium acetate hydrate (352 mg, 1 mmol) was stirred for 24 hours at 50°C. The solvent was removed and dissolved in water. The precipitates were filtered and washed with acetone three times and then purified by alumina column chromatography (toluene acetone–DMSO as eluent, DMSO/H2O =1/0–8/2 fraction).
In vitro cytotoxicity assay by the MTT assay
C6 cells were plated in 96-well plates at 5.0×104 cells/well and cultured in 100 μL DMEM with 10% FBS at 37°C in 5% CO2 atmosphere for 24 hours. Subsequently, the cells were incubated under concentrations of 0.04–270 μM of MTX or MTX-Gd for 48 hours. After incubation, the media were removed, and the cells were washed with PBS. To each well, 20 μL MTT solution (5 mg/mL in PBS) and 80 μL DMEM were added and incubated for 3–4 hours. The media were then completely removed, and 150 μL of DMSO was added to each well; as a result, the color changed from yellow to blue. The absorbance of the solution was measured using a microplate reader at 450 nm. The degree of cell viability in each sample was calculated by the following formula: cell viability (%) = Asample/Acontrol×100%, where Asample and Acontrol are the absorbance values for the treated cells and the untreated control cells, respectively. The Asample and Acontrol values were obtained after subtracting the absorbance of DMSO. Data are presented as the average ± SD (n=4).
T1-weighted and T1-mapping images of MTX-Gd in vitro and in vivo
All animals received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Wuhan University of China Animal Care and Use Committee. Female BALB/c mice were obtained from Beijing Huafukang Bioscience Co. Ltd, Beijing, PR China. with weights of 14–16 g and provided and maintained with free access to food and water.
The T1-weighted and T1-mapping images of MTX-Gd were measured at 37°C using the Siemens Prisma 3T MRI scanner. MTX-Gd was dispersed in double-distilled H2O (ddH2O) at different concentrations. For MRI measurements, each of the 96-well plates was filled with 150 μL solution to achieve T1-weighted and T1-mapping images. For in vivo MRI, tumor-bearing BALB/c nude mice were injected with 200 μL of MTX-Gd solution (2 mM) through the tail vein. Then, the mice were scanned using the Siemens Prisma 3T MRI scanner at 30 minutes, 1 hour, 2 hours, and 3 hours after the injection. The T1-weighted image parameters were described as follows: echo time, 12 ms; repetition time, 700 ms; field of view, 120×120 mm; matrix size, 400×400; slice thickness, 2.0 mm; and the number of acquisitions, 14. The T1 mapping parameters were described as follows: repetition time, 15 ms; echo time, 2.7 ms; field of view, 160×160 mm; image size, 768×768; slice thickness, 2.0 mm; and the number of acquisitions, 14. The T1-mapping value was measured three times at each MTX-Gd concentration, and the mean of the T1 values was recorded. Taking the longitudinal relaxation time (r1) as the abscissa and the inverse longitudinal relaxation time (1/T1) as the ordinate, a fitting curve was made to represent the MTX-Gd function. The control groups of MTX or Gd-DOTA also used the abovementioned method for data collection.
Results and discussion
Synthesis and characterization of MTX-Gd
MTX-Gd was prepared as shown in , and MTX-Gd was synthesized by connecting MTX and Gd through DOTA. To detect whether the MTX-Gd was synthesized successfully, it was detected by ultraviolet (UV) and infrared radiation (IR) spectroscopy. The ultraviolet-visible-near infrared radiation (UV-NIR) absorption curve indicated that MTX-Gd had a broad absorption in the NIR region (500–700 nm; ). The formation of MTX-Gd was confirmed from the characteristic bands of MTX-DOTA-Gd in the 1,413 cm−1 (C-N), 1,577 cm−1 (−NH2), and 3,429 cm−1 (N–H), in the Fourier transform IR (FTIR) spectra ().
Figure 1 The synthesis pathway of MTX-Gd.
In vitro cytotoxicity analysis by the MTT assay
To confirm the antitumor effect of MTX-Gd, the cytotoxicity of MTX-Gd was examined by the MTT assay. C6 cells were cultured in 96-well plates. At 24 hours after incubation, MTX-Gd or MTX was added to the cells, and the complex was incubated for 48 hours. Subsequently, the absorbance of the solution was measured using a microplate reader at 450 nm. shows that the MTX-Gd showed little difference in the cell viability compared with the control group, except for the highest concentration. The cytotoxicity experiments demonstrated that the synthesis of MTX-Gd had the same treatment effect as MTX at the same dose. However, the reason why MTX-Gd had more cytotoxicity than MTX at the highest concentration still needs to be explored. In addition, it is also revealed that the MTX-Gd conjugates do not lose the function of MTX after they are linked to DOTA-Gd. Furthermore, a previous study reported that Gd-DOTA is generally not toxic to normal cells at low concentrations. The data showed that it had cytotoxicity to normal cells when the concentration of Gd-DOTA was above 400 μM, otherwise it showed no toxicity.Citation26
Figure 3 After treating the cells with various concentrations of complexes for 48 hours, the cytotoxic effects of MTX-Gd and MTX were detected using the MTT assay.
Abbreviations: MTX, methotrexate; Gd, gadolinium(III).
T1-weighted and T1-mapping images of MTX-Gd in vitro and in vivo
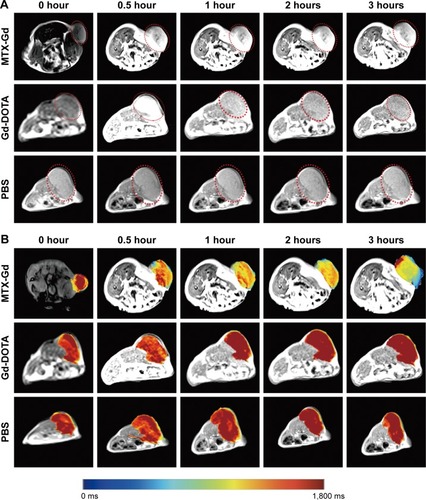
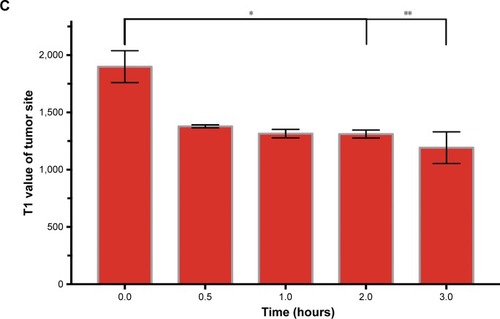
To investigate the contrast enhancement of the MTX-Gd, MRI was measured in vitro. As expected, due to the paramagnetic relaxivity enhancement (PRE) effect, MTX-Gd showed high proton relaxivity (r1) in ddH2O under the 3T MRI system (). As shown in , the imaging of MTX-Gd was significantly brighter than the control groups at the same concentration, and the brightness and signal intensity of MRI were increased followed by the increased concentration of MTX-Gd. In addition, it also showed that MTX was not visualized on MRI. In , the images revealed that the concentration of 4 mM MTX-Gd had the same imaging effect with the concentration of 10 mM Gd-DOTA. The images demonstrated that MTX-Gd at the low concentration could obtain a high signal intensity, while Gd-DOTA needed a high concentration. Moreover, as a key parameter of the contrast agent, r1 was obtained from the slope of the fit linear of the reciprocal of the T1 relaxation time. As shown in , when MTX-Gd and Gd-DOTA had the same signal intensity, the r1 of MTX-Gd was 7.7 mM−1 s−1, while the r1 of the commercial imaging agent Gd-DOTA was 2.8 mM−1 s−1. Then, nude mice bearing C6 tumor xenografts were used to study in vivo imaging capabilities. After the tail vein injection of MTX-Gd, significant contrast enhancement was observed at the tumor site from 0.5 to 3 hours (); the signal of tumor area was strongest at 3 hours due to accumulation by the size effect of macromolecules. This finding provided a longer viewing window than the clinic contrast agent such as Gd-DOTA, which will rapidly metabolize within 30 minutes. The results revealed that MTX-Gd had a better MRI contrast effect than Gd-DOTA, which further suggests that MTX-Gd has a good MRI contrast characteristic.
Figure 4 The signal intensity of MTX-Gd, MTX, and Gd-DOTA was measured by MRI in vitro.
Abbreviations: DOTA, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; MRI, magnetic resonance imaging; MTX, methotrexate; Gd, gadolinium(III).
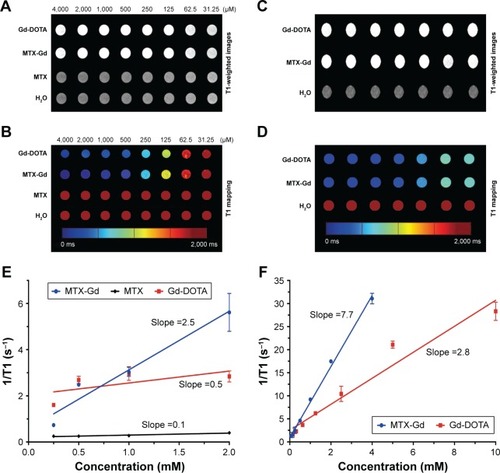
Figure 5 (A) T1-weighted images and (B) the corresponding mapping images of tumors after injection of MTX-Gd, Gd-DOTA, and PBS at different time points. (C) T1-mapping values of tumors were measured after different injection times of MTX-Gd (*P<0.05, **P<0.005).
Conclusion
A novel stable and unique theranostic agent (MTX-Gd) was successfully synthesized, which was specifically designed for cancer diagnosis and treatment. The data showed that MTX-Gd has good stability, strong anticancer ability, and excellent magnetic capacity. The MTX component of MTX-Gd, as a chemotherapeutic agent, played an important role in targeted therapies of cancer. The DOTA-Gd component of MTX-Gd performed as the MRI contrast agent. The superior MRI performance and synergetic chemical antineoplastic ability of MTX-Gd were revealed, and it has great potential in the diagnosis and treatment of gliomas and potentially other cancers, with prospects of clinical application in the near future.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (81701685, 81771819), the National Key Research and Development Project (2017YFC0108803), the Fundamental Research Funds for the Central Universities (2042018kf0245), and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (Project cxpy20160058, cxpy20160011).
Disclosure
he authors report no conflicts of interest in this work.
References
- StoneJBDeangelisLMCancer-treatment-induced neurotoxicity – focus on newer treatmentsNat Rev Clin Oncol20161329210526391778
- JoergerMHuitemaADIllerhausGFerreriAJRational administration schedule for high-dose methotrexate in patients with primary central nervous system lymphomaLeuk Lymphoma201253101867187522530664
- AngelovLDoolittleNDKraemerDFBlood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experienceJ Clin Oncol200927213503350919451444
- SolimanRKGamalSAEssaAAOthmanMHPreoperative Grading of Glioma Using Dynamic Susceptibility Contrast MRI: Relative Cerebral Blood Volume Analysis of Intra-tumoural and Peri-tumoural TissueClin Neurol Neurosurg2018167869229471287
- MaximovIITonoyanASProninINDifferentiation of glioma malignancy grade using diffusion MRIPhys Med201740243228712716
- NarangAKChaichanaKLWeingartJDProgressive Low-Grade Glioma: Assessment of Prognostic Importance of Histologic Reassessment and MRI FindingsWorld Neurosurg20179975175727108796
- FirouzniaSSabetrasekhPAhmadianAMultimodal MRI Techniques and Their Importance in Frameless Navigation Biopsy for Brain GliomaIran J Radiol2017144e65721
- BlystadIWarntjesJBMSmedbyÖLundbergPLarssonEMTisellAQuantitative MRI for analysis of peritumoral edema in malignant gliomasPLoS One2017125e017713528542553
- LeuKOttGALaiAPerfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II–III diffuse gliomasJ Neurooncol2017134117718828547590
- ChanKWongWSmall molecular gadolinium(III) complexes as MRI contrast agents for diagnostic imagingCoord Chem Rev200725117–2024282451
- WernerEJDattaAJocherCJRaymondKNHigh-relaxivity MRI contrast agents: where coordination chemistry meets medical imagingAngew Chem Int Ed Engl200847458568858018825758
- MajorJLMeadeTJBioresponsive, cell-penetrating, and multimeric MR contrast agentsAcc Chem Res200942789390319537782
- ChengWHaedickeIENofieleJComplementary strategies for developing Gd-free high-field T1 MRI contrast agents based on Mn(III) porphyrinsJ Med Chem201457251652024328058
- ChongHSSongHALimSA novel cholic acid-based contrast enhancement agent for targeted MRIBioorg Med Chem Lett20081872505250818337094
- LiYLiuGLouXChenZMaLIntra-individual comparison of different gadolinium-based contrast agents in the quantitative evaluation of C6 glioma with dynamic contrast-enhanced magnetic resonance imagingSci China Life Sci2017601111528078511
- Aydın TekdaşDGarifullinRŞentürkBDesign of a Gd-DOTA-phthalocyanine conjugate combining MRI contrast imaging and photosensitization properties as a potential molecular theranosticPhotochem Photobiol20149061376138625131633
- WuBLuSTZhangLJZhuoRXXuHBHuangSWCodelivery of doxorubicin and triptolide with reduction-sensitive lipid-polymer hybrid nanoparticles for in vitro and in vivo synergistic cancer treatmentInt J Nanomedicine2017121853186228331310
- WuBLiXQHuangTMRI-guided tumor chemo-photodynamic therapy with Gd/Pt bifunctionalized porphyrinBiomater Sci2017591746175028657073
- WuBWanBLuSTNear-infrared light-triggered theranostics for tumor-specific enhanced multimodal imaging and photothermal therapyInt J Nanomedicine2017124467447828670120
- TanMYeZLindnerDBrady-KalnaySMLuZRSynthesis and evaluation of a targeted nanoglobular dual-modal imaging agent for MR imaging and image-guided surgery of prostate cancerPharm Res20143161469147623471641
- ZhouZWuXKresakAGriswoldMLuZRPeptide targeted tripod macrocyclic Gd(III) chelates for cancer molecular MRIBiomaterials201334317683769323863450
- ZhangPZhangYLiBCell-assembled nanoclusters of MSC-targeting Gd-DOTA-peptide as a T2 contrast agent for MRI cell trackingJ Pept Sci2018244–5e307729582508
- WangHSunDLiaoHSynthesis and characterization of a bimodal nanoparticle based on the host-guest self-assembly for targeted cellular imagingTalanta201717181528551157
- LammersTAimeSHenninkWEStormGKiesslingFTheranostic nanomedicineAcc Chem Res201144101029103821545096
- KelkarSSReinekeTMTheranostics: combining imaging and therapyBioconjug Chem201122101879190321830812
- ZhangJLiYZhangQHaoXWangSEffects of gadolinium on proliferation, differentiation and calcification of primary mouse osteoblasts in vitroJ Rare Earths2012308831834
- AbreyLEMoskowitzCHMasonWPIntensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysisJ Clin Oncol200321224151415614615443
- BatchelorTCarsonKO’NeillATreatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07J Clin Oncol20032161044104912637469
- ChoiYJParkHLeeJSMethotrexate elimination and toxicity: MTHFR 677C.T polymorphism in patients with primary CNS lymphoma treated with high-dose methotrexateHematol Oncol201735450450927781293
- FritschKKasendaBSchorbEHigh-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study)Leukemia201731484685227843136
- Hoang-XuanKBessellEBrombergJDiagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-OncologyLancet Oncol2015167e322e33226149884
- OkitaYNaritaYMiyakitaYHealth-related quality of life in outpatients with primary central nervous system lymphoma after radiotherapy and high-dose methotrexate chemotherapyMol Clin Oncol20165317918527602217
- WellerMWickWAldapeKGliomaNat Rev Dis Primers201511501727188790
- EsmaeiliMStensjøenALBerntsenEMSolheimOReinertsenIThe Direction of Tumour Growth in Glioblastoma PatientsSci Rep201881119929352231
- SchuenkePPaechDKoehlerCFast and Quantitative T1ρ-weighted Dynamic Glucose Enhanced MRISci Rep201774209328169369
- XuesongDWeiXHengLEvaluation of neovascularization patterns in an orthotopic rat glioma model with dynamic contrast-enhanced MRIActa Radiol20175891138114627956462
- KhodadadeiFSafarianSGhanbariNMethotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery systemMater Sci Eng C Mater Biol Appl20177928028528629019
- VakilinezhadMAAlipourSMontaseriHFabrication and in vitro evaluation of magnetic PLGA nanoparticles as a potential Methotrexate delivery system for breast cancerJ Drug Deliv Sci Technol201844467474
- MaestáINiteckiRHorowitzNSEffectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: The New England Trophoblastic Disease Center experienceGynecol Oncol2018148116116729092742
- ParkJALeeYJKoIOIoKImproved tumor-targeting MRI contrast agents: Gd(DOTA) conjugates of a cycloalkane-based RGD peptideBiochem Biophys Res Commun20144553–424625025449282
- XiaoYXueRYouTGadolinium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid conjugate of arabinogalactan as a potential liver-targeting magnetic resonance imaging contrast agentCarbohydr Res201439591424995911
- LiYHanZRoelleSSynthesis and Assessment of Peptide Gd-DOTA Conjugates Targeting Extradomain B Fibronectin for Magnetic Resonance Molecular Imaging of Prostate CancerMol Pharm201714113906391528976766