Abstract
Purpose
Baicalein, a widely used Chinese herbal medicine, has shown anticancer effects on many types of human cancer cell lines. However, little is known about the underlying mechanism in human breast cancer cells. In this study, we examined the apoptotic and autophagic pathways activated following baicalein treatment in human breast cancer cells in vitro and in vivo.
Materials and methods
In in vitro study, we used MTT and clone formation assay to confirm the inhibitory role of baicalein on proliferation of MCF-7 and MDA-MB-231 breast cancer cells. Apoptosis was detected employing Hoechst 33258 staining, JC-1 staining, and flow cytometry. Autophagy was monitored by acridine orange staining and transmission electron microscopy observation. Quantitative real-time PCR and Western blot analysis were employed to study the effects of baicalein on PI3K/AKT signaling components of MCF-7 and MDA-MB-231 breast cancer cells. In in vivo study, the effect of baicalein was tested with a breast cancer cells transplantation tumor model.
Results
Our study showed that baicalein has the potential to suppress cell proliferation, induce apoptosis and autophagy of breast cancer cells in vitro and in vivo. Furthermore, baicalein significantly downregulated the expression of p-AKT, p-mTOR, NF-κB, and p-IκB while enhancing the expression of IκB in MCF-7 and MDA-MB-231 cells. It also decreased the p-AKT/AKT and p-mTOR/mTOR ratios.
Conclusion
Our study demonstrated that baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting the PI3K/AKT signaling pathway in vivo and vitro. Our study revealed that baicalein may be a potential therapeutic agent for breast cancer.
Introduction
Breast cancer, one of the most commonly occurring female malignant tumors, with the increased incidence and much younger onset age recently, is a serious threat to women’s health.Citation1,Citation2 Based on GLOBOCAN estimates, approximately 1.7 million new cancer cases and 521,900 deaths occurred in 2012 worldwide.Citation3 Although there have been noteworthy advances in screening, surgery, and chemoradiotherapy techniques, the prognosis of patients remains little known.Citation4,Citation5 Hence, it is urgent to provide a new therapeutic strategy in cancer therapy.
Baicalein, a bioactive component extracted from the root of Scutellaria baicalensis Georgi, has been shown to have anti-tumor, anti-inflammatory, anti-cardiovascular disease, and antimicrobial activities,Citation6 and so on.Citation7,Citation8 Numerous studies have revealed the anti-tumor properties of baicalein in many types of human cancer cell lines both in vitroCitation9,Citation10 and in vivo.Citation11,Citation12 The molecular mechanisms involved in the anti-tumor effects of baicalein are conjectured to be due to the modulation of multiple pathways including the PI3K/AKT signaling pathway and inhibiting cell proliferation and inducing cell apoptosis, activating the caspase cascade and the intrinsic (mitochondrial) apoptotic pathway,Citation13,Citation14 DNA fragmentation in malignant cells.Citation15,Citation16 Cao et alCitation17 reported that apigenin has also demonstrated anticancer activities, involving apoptosis- and autophagy-induction in breast cancer cells. Significantly, the study illuminated that autophagy plays a vital cytoprotective role in apigenin-induced apoptosis.Citation17
The PI3K/AKT signaling pathway plays a pivotal role in mammalian cell proliferation, differentiation, apoptosis, autophagy, and survival,Citation18 and is a key regulator of authophagy.Citation19 Activation/inhibition of the PI3K/AKT signaling pathway has been clarified to regulate survival of human cancer cells in vitro,Citation20 as well as carcinogenicity, invasion and metastasis of human cancer cells in vivo.Citation21 Baicalein-induced apoptosis and autophagy have been illustrated to be mediated through inhibition of the PI3K/AKT signaling pathway in human renal carcinoma cells,Citation16 glioma,Citation18 human epidermoid carcinoma cells,Citation22 and bladder cancer cells.Citation23 Nevertheless, few studies have clarified the latent molecular mechanism of anticancer activity of baicalein on human breast cancer cells. Hence, the purpose of the present study was to ascertain potential mechanisms through which baicalein induces apoptosis and autophagy in MCF-7 and MDA-MB-231 breast cancer cells. And for all we know, the current study will provide new direct evidence that baicalein induces apoptosis and autophagy in breast cancer cells by inhibiting the PI3K/AKT signaling pathway.
Ethical approval
Female BALB/c nude mice (3–6 weeks old, body weight 18–20 g) were obtained from the Experimental Animal Center of Xi’an Jiaotong University (Xi’an, China). Animal experiments in this study were conducted according to the institutional guidelines for the care and use of animals and approved by the ethics committee of Xi’an Jiaotong University. Ethical approval for use of human cell lines was not needed as per Xi’an Jiaotong University ethical committee guidelines. The breast cancer cell lines used in the present study are all commonly used and subcultured cell lines obtained from public cell banks. They were not derived from our patients or healthy donors.
Materials and methods
Cell lines and chemicals
The MCF-7 and MDA-MB-231 breast cancer cell lines were obtained from Shanghai Cell Biological Institute of the Chinese Academy of Science (Shanghai, China). DMEM, Giemsa stain, and FBS were obtained from Gibco (Thermo Fisher Scientific, Waltham, MA, USA). DMSO, Hoechst 33258, acridine orange (AO) stain Kit, Mitochondrial membrane potential assay kit with JC-1, the Annexin V-FITC Apoptosis Detection kit, MTT, LY294002, and baicalein (molecular weight [MW] 270.24) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All antibodies (GAPDH, AKT, p-AKT, mTOR, p-mTOR, NF-κB, IκB, p-IκB, Bcl-2, Bax, BECN1, and LC3) were purchased from Abcam (Cambridge, UK). Baicalein was dissolved in DMSO.
Cell culture
MCF-7 and MDA-MB-231 breast cancer cells were cultured in DMEM supplemented with 10% FBS, 50 IU/mL penicillin, and 50 µg/mL streptomycin and maintained in humidified 5% CO2 air at 37°C.
Animals
Female BALB/c nude mice (3–6 weeks old, body weight 18–20 g) were obtained from the Experimental Animal Center of Xi’an Jiaotong University. Animal experiments in this study were conducted according to the recommended guidelines for the care and use of laboratory animals issued by the Chinese Council on Animal Research, and approved by the ethics committee of Xi’an Jiaotong University.
Morphological cell changes
MCF-7 and MDA-MB-231 cells were seeded in 12-well plates at a density of 3×105 cells/well and grown for 24 hours. Cells were treated with various concentrations (0, 10, 20, and 40 µM) of baicalein and grown at 37°C, in 5% CO2 and 95% air for 24 hours. For examining morphological changes, cells treated with baicalein were observed and photographed under a phase-contrast microscope.
MTT assay
The cell viability was assessed by MTT assay. Briefly, MCF-7 and MDA-MB-231 (4×103 cells/well) cells were seeded into 96-well plates and incubated in 5% CO2 air at 37°C. After 12 hours of incubation, the cells were treated with different concentrations of baicalein (0, 10, 20, and 40 µM) for 24, 48, and 72 hours. Subsequently, MTT (20 µL of 5 mg/mL) was added to each well and incubated at 37°C for 4 hours. The formazan crystals that formed were dissolved in 100 mL of DMSO after removal of the supernatant, and the cells were then incubated for another 10 minutes. The OD was recorded at 490 nm on a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). The inhibition ratio (IR) was calculated as follows: IR = (1-mean OD value of experimental group/mean OD value of control group)×100%. Approximately 50% inhibition concentration (IC50) of baicalein at different time points was calculated using the Logit method. All experiments were repeated at least three times.
Colony formation assay
MCF-7 and MDA-MB-231 cells (1,000 cells/well) were seeded into 6-well plates. After adhesion cells were treated with baicalein (0, 10, 20, and 40 µM) for 48 hours and then cultured in DMEM at 37°C for 14 days. During this period, the cells were washed with PBS every 3 days, fixed with paraformaldehyde (4%, 15 minutes), and stained with crystal violet (0.1%, 15 minutes) and the numbers of colonies with >50 cells were counted with an Olympus digital camera (Olympus Corporation, Tokyo, Japan). All experiments were repeated at least three times.
Hoechst 33258 staining for apoptosis
MCF-7 and MDA-MB-231 cells (2×105 cells/well) were seeded in 12-well plates for 12 hours and treated with various concentrations of baicalein (0, 10, 20, and 40 µM) for 48 hours, washed with PBS, and then fixed in paraformaldehyde (4%) for 15 minutes at room temperature. Sequentially, cells were stained with 100 µL Hoechst 33258 in PBS for 15 minutes at room temperature. The stained cells were visualized from randomly selected fields under a fluorescence microscope (Leica Microsystems, Wetzlar, Germany). The nuclear condensation and fragmentation of cells were identified as the apoptotic cells.
Measurement of mitochondrial membrane potential (ΔΨm)
MCF-7 and MDA-MB-231 cells were seeded in 12-well plates at a density of 2×105 cells/well for 12 hours and treated with different concentrations of baicalein (0, 10, 20, and 40 µM) for 48 hours to detect the changes of ΔΨm. Then, cells were harvested and washed with PBS and resuspended in JC-1 at 37°C for 30 minutes in the dark. The stained cells were analyzed by a fluorescence microscope.
AO staining
MCF-7 and MDA-MB-231 cells (1×105 cells/well) were suspended and seeded in a 12-well plate and incubated overnight. After adherence, cells were treated with baicalein (0, 10, 20, and 40 µM) for 48 hours. After 48 hours, cells were stained with AO (1 µg/mL) for 15 minutes in the dark, washed with PBS, and visualized under a fluorescence microscope (Olympus Corporation). All experiments were repeated for at least three times.
Transmission electron microscopy (TEM) observation
TEM was carried out to observe the ultrastructure of MCF-7 and MDA-MB-231 cells after incubation with 40 µg/mL baicalein for 48 hours. The cells were collected and fixed in ice-cold glutaraldehyde in 0.1 mol/L phosphate buffer (PH 7.4) overnight, and then fixed in 1% osmium tetroxide and dehydrated. Cells were impregnated with Epon. The ultrathin sections were contrasted with uranyl acetate and lead citrate for electron microscopy. Electron micrographs were observed through a transmission electron microscope (H7650; Hitachi Ltd., Tokyo, Japan).
Flow cytometry analysis
Flow cytometry assay was performed to measure cell apoptosis rates. Briefly, after treatment with various concentrations (0, 10, 20, and 40 µM) of baicalein for 48 hours, MCF-7 and MDA-MB-231 cells were collected and washed twice in PBS at room temperature. For apoptosis assays, cells (1×106) were counted and resuspended in 100 µL annexin-V binding buffer solution. Subsequently, 5 µL annexin V-FITC and 5 µL propidium iodide (PI) were added, and incubated at room temperature for 15 minutes in the dark. The percentage of apoptotic cells was analyzed by flow cytometry using CellQuest software (BC Epics XL, Miami, FL, USA).
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was conducted to quantify the expression of mRNA. Briefly, MCF-7 and MDA-MB-231 cells were treated with various concentrations (0, 10, 20, and 40 µM) of baicalein for 48 hours. Total cell RNA was extracted by Trizol according to the manufacturer’s instructions. RNA was then reverse transcribed into cDNA with RevertAid First-Strand cDNA Synthesis Kit (TaKaRa, Tokyo, Japan). Sequentially, qRT-PCR reaction system was prepared following the manufacturer’s instructions given in the SYBR®Premix Ex TaqII RT-PCR Kit (TaKaRa) using 100 ng cDNA. The primers applied in this current study are listed in . Finally, each sample was analyzed in triplicate by using a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). Relative quantification of genes was analyzed in accordance with the 2−ΔΔCt method (ΔΔCt = ΔCt [treated]−ΔCt [control]). GAPDH was used as endogenous control.
Table 1 Primers used for qRT-PCR analysis
Western blotting
The total proteins were collected from MCF-7 and MDA-MB-231 cells cultured with various concentrations (0, 10, 20, and 40 µM) of baicalein for 24 hours, 48 hours, and 72 hours. Protein concentrations were analyzed by the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Then, the proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk for 2 hours at room temperature followed by incubation overnight at 4°C with a primary antibody (GAPDH, AKT, p-AKT, mTOR, p-mTOR, NF-κB, IκB, and p-IκB) at a dilution of 1:1,000. Subsequently, the membranes were washed three times with TBST and incubated with the appropriate HRP-conjugated secondary antibody (1:5,000) for 2 hours at room temperature. Protein bands were visualized using the chemiluminescence gel imaging system (G: BOX; Syngene, Cambridge, UK) and quantitated using the Image-Pro Plus 6.0 software. GAPDH was used as an internal control.
Tumor xenograft study
Female BALB/c nude mice (3–6 weeks old) were purchased from Experimental Animal Center of Xi’an Jiaotong University. Xenografts were established by subcutaneous injection of in vitro-cultured MCF-7 (1×107/200 µL) and MDA-MB-231 cells (2×106 cells/200 µL) into the second left breast pad of nude mice. When the tumor size grew up to approximately 100 mm3, the mice were randomly divided into different groups (five mice in each group) and baicalein (100 mg/kg) or vehicle (1% carboxymethyl cellulose sodium) was administered by intra-gastric gavage once daily for 21 days. Additionally, tumor volume growth and body weight were measured every 3 days using vernier calipers. The tumor volume was measured as: v = a×b2/2 (a is the length; b is the width). Experiments were terminated at the 21st day and the animals were anesthetized and sacrificed. Tumors were removed for further analysis.
Immunohistochemistry
Histological analysis was performed on tissue samples isolated from mouse xenografts. Briefly, 5 µm sections were cut from all paraffin blocks and stained for p-AKT, Bax, and LC3. Immunohistochemical staining was conducted using Histostain®-SP Kits according to the manufacturer’s instructions. Sections were incubated with p-AKT (1:100), BAX (1:100), and LC3 (1:100) primary antibody at 4°C overnight. Images of sections were visualized using a Zeiss microscope (Carl Zeiss Meditec AG, Jena, Germany) and analyzed by Image-Pro Plus 6.0.
Statistical analysis
Experimental results are presented as mean ± SD from at least three separate experiments. Comparisons involving various baicalein concentrations or different incubation times were conducted using one-way ANOVA or Student’s t-test. Statistical significance was set at P<0.05. Statistical analyses were performed using SPSS software (version 11.0).
Results
Baicalein inhibited the proliferation of breast cancer cells
Morphological cell changes were examined using phase-contrast microscopy. Microscopic observations revealed that MCF-7 and MDA-MB-231 cells exposed to increasing concentrations (0, 10, 20, and 40 µM) of baicalein underwent significant morphological changes, including cell shrinkage and blebbing ().
Figure 1 Morphological changes and cell viability of MCF-7 and MDA-MB-231 cells following treatment with baicalein.
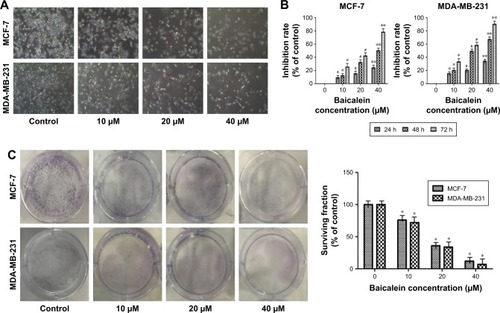
To measure the effect of baicalein on the proliferation of MCF-7 and MDA-MB-231 cells that were exposed to 0, 10, 20, and 40 µM of baicalein for 24, 48, and 72 hours, MTT assay was implemented. The results showed that baicalein significantly inhibited the proliferation of MCF-7 and MDA-MB-231 cells in a dose- and time-dependent manner (P<0.05, P<0.01, ). The IC50 values (µM) of baicalein in breast cancer cells are presented in . The IC50 values of baicalein at 24, 48, and 72 hours were 51.06, 22.16, and 13.98 µM and 60.12, 27.98, and 19.01 µM in MCF-7 and MDA-MB-231 cells, respectively.
Table 2 The IC50 values (µM) of baicalein in breast cancer cells
Baicalein also suppressed the colony formation of MCF-7 and MDA-MB-231 cells as shown by the plate colony formation assay. As shown in , the numbers of colonies that formed for preparations treated with baicalein at 10, 20, and 40 µM were 118±2.6, 63±6.2, 25±3.7 and 85±1.6, 51±6.7, 19±3.6 in MCF-7 and MDA-MB-231 cells respectively (P<0.05). These results suggest that baicalein has anti-proliferative effects on breast cancer cells.
Baicalein induced cell apoptosis in breast cancer cells
To evaluate the effect of baicalein on breast cancer cell apoptosis, Hoechst staining 33258, which is sensitive to DNA and is used to assess changes in cellular nuclear morphology, was conducted in our study. In our study, MCF-7 and MDA-MB-231 cells were cultured with different concentrations (0, 10, 20, and 40 µM) of baicalein for 48 hours. Results indicated that baicalein showed a potent effect on nuclear condensation when compared with the control cells demonstrated that baicalein was effective in inducing cellular apoptosis ().
Figure 2 Detection of apoptotic morphological changes in MCF-7 and MDA-MB-231 cells treated with baicalein at 0, 10, 20, and 40 µM concentrations for 48 hours.
Abbreviations: PI, propidium iodide; qRT-PCR, quantitative real-time PCR.
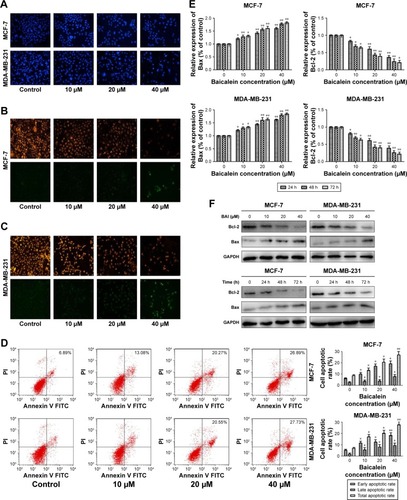
To further assess whether baicalein affects apoptosis, mitochondrial membrane potential (ΔΨm) changes, which are key events that take place during drug-induced apoptosis,Citation25,Citation26 were examined using the fluorescent probe JC-1. MCF-7 and MDA-MB-231 cells were pre-treated with various concentrations (0, 10, 20, and 40 µM) of baicalein for 48 hours to detect the changes of ΔΨm. In normal cells, JC-1 accumulates and forms aggregations characterized by red fluorescence in the mitochondria and also in the cytoplasm as a monomer characterized by green spectral fluorescence. During apoptosis, the ΔΨm changes, consequently, JC-1 aggregates transform into monomers leading to loss of red fluorescence. Hence, changes of the ΔΨm are manifested via reduced ratio of red to green fluorescence.Citation26 The red and green fluorescence were measured by a fluorescence microscope. With increasing dose of baicalein, the ΔΨm was decreased in MCF-7 and MDA-MB-231 cells. The results indicated that baicalein on the levels of ΔΨm were dose-dependent in breast cancer cells ().
Subsequently, we tested whether baicalein modulates cell apoptosis in MCF-7 and MDA-MB-231 cells by annexin V-FITC/PI staining and flow cytometry. The results revealed that baicalein increased the apoptosis rate of MCF-7 and MDA-MB-231 cells, with an apoptotic rate estimated at 26.89%±0.96% and 27.73%±0.23% in 40 µM baicalein group, 20.55%±0.62% and 20.27%±0.36% in 20 µM baicalein group, 13.08%±0.78% and 16.94%±0.86% in 10 µM baicalein group, and 8.62%±0.34% and 6.89%±0.65% in the control group respectively, and clearly indicated that baicalein induced breast cancer cells’ apoptosis in a dose-dependent manner (P<0.05, P<0.01, ).
Finally, to further confirm the apoptosis induced by baicalein, the expression of apoptosis-related genes and proteins was detected by qRT-PCR and Western blotting. As presented in (P<0.05, P<0.01), the levels of Bax were increased, by contrast, levels of Bcl-2 were decreased in MCF-7 and MDA-MB-231 cells after treatment with baicalein compared to the control group. Taken together, our experimental data demonstrated that baicalein could induce apoptosis in breast cancer cells in a dose- and time-dependent manner.
Baicalein induces autophagy in MCF-7 and MDA-MB-231 cells
AO staining revealed that baicalein induced the formation of autophagic vacuoles in MCF-7 and MDA-MB-231 cells in a dose-dependent manner. As presented in , compared with the control cells, baicalein treatment resulted in pronounced formation of autophagic vacuoles in MCF-7 and MDA-MB-231 cells.
Figure 3 Determination of the induction of autophagy in MCF-7 and MDA-MB-231 breast cancer cells.
Abbreviation: qRT-PCR, quantitative real-time PCR.
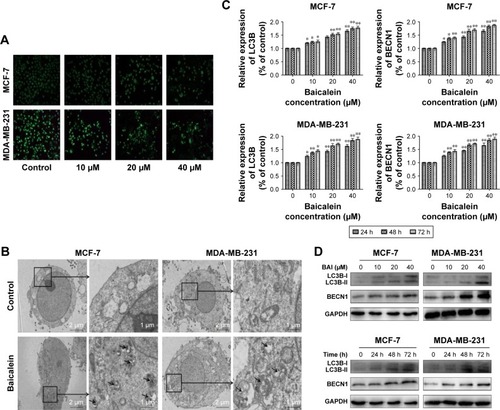
Morphological and ultrastructural features of MCF-7 and MDA-MB-231 cells were visualized by TEM. Cells were treated with 40 µM baicalein for 48 hours. Compared with the control cells, we monitored a large number of autophagy bodies and autophagolysosome in MCF-7 and MDA-MB-231 cells treated with baicalein ().
To further confirm the autophagy induced by baicalein, the expression of autophagy-related genes and proteins was detected by qRT-PCR and Western blotting. As presented in (P<0.05, P<0.01), the levels of LC3B and BECN1 were increased significantly in MCF-7 and MDA-MB-231 cells treated with baicalein in a dose- and time-dependent manner. These results provided real evidence of baicalein-induced autophagy of breast cancer cells.
Baicalein regulates the apoptosis and autophagy of breast cancer cells via the PI3K/AKT/AKT signaling pathway
In order to elucidate the underlying molecular mechanism of baicalein-induced apoptosis and autophagy, the levels of proteins associated with the PI3K/AKT signaling pathway were examined using Western blotting. The results suggested that compared with the control group, p-AKT, p-mTOR, NF-κB, and p-IκB protein expression was significantly downregulated in MCF-7 and MDA-MB-231 cells following treatment with different concentrations (0, 10, 20, and 40 µM) of baicalein for 24 hours, 48 hours, and 72 hours, whereas the expression of IκB was up-regulated at the protein level in a dose- and time-dependent manner (). Besides, the p-AKT/AKT and p-mTOR/mTOR ratios were also reduced in a dose- and time-dependent manner (, P<0.05, P<0.01). Collectively, these results illustrated that baicalein regulates the apoptosis and autophagy of breast cancer cells via the PI3K/AKT pathway.
Figure 4 Expression of proteins associated with the PI3K/Akt signaling following various treatment times of MCF-7 and MDA-MB-231 cells with baicalein (0, 10, 20, and 40 µM).
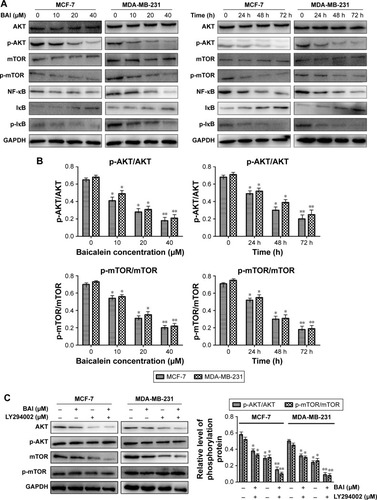
To further confirm the effects of baicalein on PI3K/AKT signaling pathway, LY294002, a specific PI3K inhibitor, was then used. Treatment of breast cancer cells with LY294002 resulted in a reduction in p-AKT. The levels of p-mTOR were also decreased compared with the controls (, P<0.05, P<0.01). These findings support the hypothesis that the induction of apoptosis and autophagy in cells by baicalein is mediated by the suppression of the PI3K/AKT signaling pathway.
In vivo effects of baicalein on breast cancer xenograft model
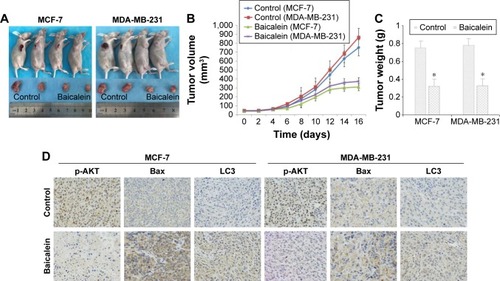
In vitro, we had clarified that baicalein can inhibit proliferation and induce apoptosis and autophagy in MCF-7 and MDA-MB-231 cells. Next, we established whether baicalein had similar effects in vivo. Consequently, we assessed the anticancer potential of baicalein using breast xenografts models (BALB/c-nude) as the testing model. After therapeutic treatment with baicalein, tumor tissues from breast xenograft models were collected and analyzed. The results demonstrated that the growth, volume, and weight of tumors were significantly suppressed in the baicalein-treated group compared with the control group (, P<0.05). Additionally, we assessed the anti-tumor efficacy of baicalein in vivo by immunohistochemistry staining methods. As presented in , baicalein remarkably reduced the expression of p-AKT, while increasing the expression of Bax and LC3 at the protein level. These results illustrated that baicalein can significantly induce apoptosis and autophagy through negative modulation of the PI3K/AKT pathway in vivo, in accordance with our in vitro research findings.
Figure 5 Baicalein inhibited MCF-7 and MDA-MB-231 tumor xenograft growth in vivo.
Discussion
Baicalein has been isolated from the roots of S. baicalensis, and it has been confirmed to be effective against various cancer cells both in vitroCitation9,Citation10 and in vivo.Citation11,Citation24 However, the properties of baicalein regarding anti-proliferation and induction of apoptosis and autophagy and the specific mechanism(s) in breast cancer cells have not been elucidated. The anti-tumor function of baicalein has not been investigated in clinical trials, further study of the mechanisms that underpin baicalein anti-tumor activity may provide possible clinical applications for the treatment of breast cancer. Our study illuminated that baicalein induces apoptosis and autophagy by inhibition of the PI3K/AKT signaling pathway.
Cell apoptosis and autophagy, as the critical formation of type I and II programmed cell death, have been closely associated with tumorigenicity and tumor development and could be regarded as a potential useful strategy in anti-tumor therapy.Citation25 Previous in vivo and in vitro studies have indicated that baicalein induces apoptosis in non-small-cell lung cancer,Citation26 human cervical cancer HeLa cells,Citation27 esophageal squamous cell carcinoma cells,Citation28 and Burkitt lymphoma cells.Citation29 However, there is a lack of information regarding the anticancer activity of baicalein in breast cancer cells, and its effects on the signaling pathways related to apoptosis and autophagy remain unclear. In this present study, first, we established that baicalein inhibited cell proliferation in a time-and dose-dependent manner in MCF-7 and MDA-MB-231 cells by MTT and clone formation assays. Then, we observed that baicalein induced apoptosis in two breast cancer cell lines via Hoechst staining, mitochondrial membrane potential (ΔΨm), and annexin V-FITC assay, respectively. In addition, the BCL-2 family is a crucial mediator and plays a vital role in cell apoptosis,Citation30 including anti-apoptotic protein (BCL-2) and pro-apoptotic protein (Bax).Citation31 These findings showed that baicalein induced apoptosis by increasing the Bax/BCL-2 ratio, as measured by qRT-PCR and Western blotting. Next, we assessed the potential of baicalein-induced autophagy. BECN1 and LC3, a central protein and the initiator of autophagy, respectively, are regarded as autophagy-related proteins, and participate in the autophagy signaling pathway, including autophagosome formationCitation32,Citation33 and autophagosome maturation.Citation34,Citation35 In mammals, LC3B, one of three isoforms (LC3A, B, and C), has extensive tissue specificities and is widely applied in the research of autophagy.Citation21 They are considered as vital molecular events, involving the conversion of LC3B-I to LC3B-II and increase of BECN1, observed in the process of autophagy. We analyzed autophagic cells through acidic vesicular organelle staining assay, and detected autophagosomes under TEM. The expression of BECN1 and LC3 was significantly increased as shown by qRT-PCR and Western blotting.
The PI3K/AKT signaling pathway plays a crucial role in not only regulating normal cell proliferation, differentiation, and apoptosis,Citation36 but also in modulating the development and progression of human cancers, once this signaling has been activated.Citation37,Citation38 AKT, NF-κB, and mTORCitation39,Citation40 are the downstream components of the PI3K/AKT signaling pathway,Citation41 and when continuously activated, they are thought to function significantly in maintenance of malignancies.Citation42 Previous studiesCitation21 revealed that the NF-κB and mTOR pathways play a vital role in cell growth, as well as progression, apoptosis, and metastasis in human cancer cells.Citation43,Citation44 To further illuminate the specific mechanisms involved in the effects of baicalein-apoptosis and autophagy on breast cancer cells in vitro, we applied Western blot to evaluate the expression level of various proteins (AKT, p-AKT, mTOR, p-mTOR, NF-κB, IκB, and p-IκB) in the PI3K/AKT pathway in MCF-7 and MDA-MB-231 cells. The results showed that baicalein remarkably reduced the expression of p-AKT, p-mTOR, NF-κB, and p-IκB, while increasing the expression of IκB at the protein level in baicalein-treated MCF-7 and MDA-MB-231 cells. Besides, the p-AKT/AKT and p-mTOR/mTOR ratios were also reduced in a dose- and time-dependent manner. Moreover, we observed that LY294002, a specific PI3K inhibitor, decreased the levels of p-AKT and p-mTOR. These findings further supported the hypothesis that the induction of apoptosis and autophagy in cells by baicalein is mediated by the suppression of the PI3K/AKT pathway. In addition, to acquire more reliable evidence to support and verify our in vitro experimental findings, we used the xenograft nude mouse model to clarify the underlying molecular mechanisms of baicalein-apoptosis and autophagy in breast cancer cells in vivo. Results acquired from the in vitro study were in accordance with those of in vivo.
Conclusion
Taken together, our results demonstrated that baicalein had the potential to suppress cell proliferation, induce apoptosis and autophagy in MCF-7 and MDA-MB-231 breast cancer cells through inhibiting the PI3K/AKT pathway both in vitro and in vivo. These results suggest that baicalein may have therapeutic potential for breast cancer treatment and deserves further study. The anti-tumor function of baicalein has not been investigated in clinical trials, further study of the mechanisms that underpin baicalein’s anti-tumor activity may provide possible clinical applications in the treatment of breast cancer.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Nos. 81274136, 81471670), Program for New Century Excellent Talents in Universities of China (No. NCET-110439). Shuqun Zhang provided funding for the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- BeikiOHallPEkbomAMoradiTBreast cancer incidence and case fatality among 4.7 million women in relation to social and ethnic background: a population-based cohort studyBreast Cancer Res2012141R522225950
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- ChristopheVDuprezCCongardAThe subjective experience of young women with non-metastatic breast cancer: the Young Women with Breast Cancer InventoryHealth Qual Life Outcomes2015137326036192
- MiocinovicRMcCabeNPKeckRWJankunJHamptonJASelmanSHIn vivo and in vitro effect of baicalein on human prostate cancer cellsInt J Oncol200526124124615586246
- SchmidtMKvan den BroekAJTollenaarRABreast Cancer Survival of BRCA1/BRCA2 Mutation Carriers in a Hospital-Based Cohort of Young WomenJ Natl Cancer Inst20171098
- KimuraYKuboMTaniTArichiSOhminamiHOkudaHStudies on Scutellariae radix. III. Effects on lipid metabolism in serum, liver and fat cells of ratsChem Pharm Bull1981298230823127318038
- LinCCShiehDEThe anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogoninAm J Chin Med199624131368739179
- LuYJoergerRWuCStudy of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis GeorgiJ Agric Food Chem20115920109341094221866919
- MaZOtsuyamaKLiuSBaicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cellsBlood200510583312331815626742
- MartínezMEUnkartJTTaoLPrognostic significance of marital status in breast cancer survival: A population-based studyPLoS One2017125e017551528475579
- ChenCHHuangLLHuangCCLinCCLeeYLuFJBaicalein, a novel apoptotic agent for hepatoma cell lines: a potential medicine for hepatomaNutr Cancer2002382287295
- LeeHZLeungHWLaiMYWuCHBaicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cellsAnticancer Res2005252A95996415868934
- HuangYHuJZhengJDown-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalinJ Exp Clin Cancer Res20123114822607709
- ZhangHBLuPGuoQYZhangZHMengXYBaicalein induces apoptosis in esophageal squamous cell carcinoma cells through modulation of the PI3K/Akt pathwayOncol Lett20135272272823420294
- HuangKFZhangGDHuangYQDiaoYWogonin induces apoptosis and down-regulates survivin in human breast cancer MCF-7 cells by modulating PI3K-AKT pathwayInt Immunopharmacol201212233434122182776
- SeoBRMinKJChoIJKimSCKwonTKCurcumin significantly enhances dual PI3K/Akt and mTOR inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma Caki cells through down-regulation of p53-dependent Bcl-2 expression and inhibition of Mcl-1 protein stabilityPLoS One201494e9558824743574
- CaoXLiuBCaoWAutophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cellsChin J Cancer Res201325221222223592903
- AnnovazziLMellaiMCalderaVValenteGTessitoreLSchifferDmTOR, S6 and AKT expression in relation to proliferation and apoptosis/autophagy in gliomaAnticancer Res20092983087309419661320
- SunHWangZYakisichJSNatural products targeting autophagy via the PI3K/Akt/mTOR pathway as anticancer agentsAnticancer Agents Med Chem20131371048105623293890
- LiHGaoQGuoLLuSHThe PTEN/PI3K/Akt pathway regulates stem-like cells in primary esophageal carcinoma cellsCancer Biol Ther2011111195095821467840
- LiBCheungPYWangXId-1 activation of PI3K/Akt/NFkappaB signaling pathway and its significance in promoting survival of esophageal cancer cellsCarcinogenesis200728112313232017638919
- AgarwalSAchariCPraveenDRoyKRReddyGVReddannaPInhibition of 12-LOX and COX-2 reduces the proliferation of human epidermoid carcinoma cells (A431) by modulating the ERK and PI3K-Akt signalling pathwaysExp Dermatol2009181193994619558494
- ChaoJISuWCLiuHFBaicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKTMol Cancer Ther20076113039304818025287
- LeeHZLeungHWLaiMYWuCHBaicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cellsAnticancer Res2005252A95996415868934
- MaCZhuLWangJAnti-inflammatory effects of water extract of Taraxacum mongolicum Hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathwayJ Ethnopharmacol201516834935525861954
- CathcartMCUseckaiteZDrakefordCAnti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivoBMC Cancer20161670727586635
- PengYGuoCYangYBaicalein induces apoptosis of human cervical cancer HeLa cells in vitroMol Med Rep20151132129213425373554
- ZhangHBLuPGuoQYZhangZHMengXYBaicalein induces apoptosis in esophageal squamous cell carcinoma cells through modulation of the PI3K/Akt pathwayOncol Lett20135272272823420294
- HuangYHuJZhengJDown-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalinJ Exp Clin Cancer Res2012314822607709
- AdamsJMCorySThe Bcl-2 protein family: arbiters of cell survivalScience19982815381132213269735050
- KimRUnknotting the roles of Bcl-2 and Bcl-xL in cell deathBiochem Biophys Res Commun2005333233634315922292
- KangRZehHJLotzeMTTangDThe Beclin 1 network regulates autophagy and apoptosisCell Death Differ201118457158021311563
- YorimitsuTKlionskyDJAutophagy: molecular machinery for self-eatingCell Death Differ200512Suppl 21542155216247502
- BarthSGlickDMacleodKFAutophagy: assays and artifactsJ Pathol2010221211712420225337
- HeCLevineBThe Beclin 1 interactomeCurr Opin Cell Biol201022214014920097051
- Kauffmann-ZehARodriguez-VicianaPUlrichESuppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKBNature199738566165445489020362
- SongLXiongHLiJSphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-κB pathway in human non-small cell lung cancerClin Cancer Res20111771839184921325072
- TsurutaFMasuyamaNGotohYThe phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondriaJ Biol Chem200227716140401404711842081
- KawauchiKOgasawaraTYasuyamaMOtsukaKYamadaOThe PI3K/Akt pathway as a target in the treatment of hematologic malignanciesAnticancer Agents Med Chem20099555055919519296
- VuCFrumanDATarget of rapamycin signaling in leukemia and lymphomaClin Cancer Res201016225374538020826559
- ChenYWangBCXiaoYPI3K: a potential therapeutic target for cancerJ Cell Physiol201222772818282121938729
- DazertEHallMNmTOR signaling in diseaseCurr Opin Cell Biol201123674475521963299
- SunSYRosenbergLMWangXActivation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibitionCancer Res200565167052705816103051
- XuGZhangWBertramPZhengXFMcleodHPharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR pathway in common human tumorsInt J Oncol200424489390015010827
