Abstract
Background
For patients with inadequate control of cholesterol using moderate-dose statins in the secondary prevention of cardiovascular diseases (CVD), either doubling the dose of statins or adding ezetimibe should be considered. The cost-effectiveness of them is unknown in the Chinese context. The aim of this study is to compare the cost and effectiveness of the two regimens, and estimate the incremental cost-effectiveness ratio (ICER).
Methods
A Markov model of five health statuses were used to estimate long-term costs and quality-adjusted life-years (QALYs) of the two treatment regimens from the healthcare perspective. The effectiveness data used to calculate the transition probability was based on a previously published randomized trial. The utility data was gathered from literature and the costs were gathered from the electronic medical record system of West China Hospital in Chinese Yuan (CNY) in 2017 price. One-way sensitivity analysis and probabilistic sensitivity analysis were conducted.
Results
The ICER for ezetimibe plus moderate-dose rosuvastatin was 47,102.99 CNY per QALY for 20 years simulation, which did not reach the threshold of per capita gross domestic product (GDP) of 59,660 CNY per QALY in 2017 in China. Non-CVD-related mortality and CVD-related mortality contributed most to the ICER.
Conclusion
Adding ezetimibe to the moderate-dose statin in secondary prevention for CVD is cost-effective, compared with the high-dose statin in the Chinese context whose low-density lipoprotein cholesterol (LDL-c) was not inadequately controlled by moderate-dose statin alone.
Introduction
Cardiovascular diseases (CVD) are the leading causes of death, disability and disease burden worldwide including China.Citation1 Impaired lipid profile, which presents in over 40% of Chinese adults,Citation2 is one of the top risk factors and the key treatment target of CVD.Citation3
Moderate-dose statins are recommended as the first-line agents for both primary and secondary prevention of CVD in the latest guidelines.Citation4,Citation5 Rosuvastatin is one of the most widely used and guideline-recommended first-line lipid-lowering agents in China.Citation4,Citation6 However, approximately 75% of the patients receiving moderate-dose rosuvastatin or other statins in the secondary prevention of CVD could not achieve the treatment goal of low-density lipoprotein cholesterol (LDL-c)Citation7–Citation9 and require dose doubling of rosuvastatin or combing a second-line lipid-lowering agent.Citation5
Ezetimibe, which inhibits cholesterol absorption in the intestine, has been widely used and recommended by the current guidelines as the second-line lipid-lowering agent in the secondary prevention of CVD.Citation4,Citation5 Although several Chinese and international trials confirmed its efficacy in lowering LDL-c level and reducing the risk of future CVD,Citation10–Citation15 it is still unclear if ezetimibe is cost-effective in the Chinese context. A study conducted by Ran and colleagues is one of the featured trials comparing the efficacy of ezetimibe plus moderate-dose rosuvastatin versus high-dose rosuvastatin in the secondary prevention of CVD in China.Citation16 Based on the data of the costs of patients in West China Hospital, Sichuan University (a tertiary hospital in China), we investigated whether ezetimibe plus moderate-dose statin, when compared with its alternative, high-dose statins, is cost-effective in the Chinese patients in healthcare perspective with inadequately controlled blood lipid level by moderate-dose statin alone for the secondary prevention of CVD.
Materials and Methods
Comparison of Treatment Regimens
With the outcome data the costs calculated below, the cost and effectiveness of the two regimens, high-dose rosuvastatin (namely high-dose group, treated 20mg once daily) and ezetimibe combined with moderate-dose rosuvastatin (namely combination group, treated with ezetimibe 10mg once daily plus rosuvastatin 10mg once daily), were compared. The incremental cost-effectiveness ratio (ICER) was used to estimate the cost-effective across two groups.
Model Structure
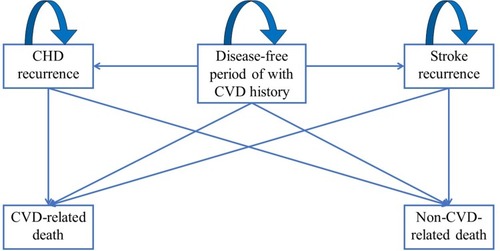
A Markov model established from the healthcare perspective under the Chinese context () was adopted to estimate the health outcomes, which was measured by the comprehension of all the outcome statuses in this model. Five health statuses included in this model were disease-free status after the first CVD episode (including coronary heart disease [CHD] and stroke), CHD recurrence and rehospitalization, stroke recurrence and rehospitalization, CVD-related death and non-CVD-related death. Patients in the disease-free status were recruited after their first episode of CVD, attack which might turn to CHD or stroke rehospitalization with CVD event.
Figure 1 Transition diagram.
The base-case population was set with an average age of 60 years old, a balanced male/female ratio and newly diagnosed with CVD, according to the Chinese trial population.Citation16 The duration of the clinical trial study used to calculate the transition probability was 3 months, which was adopted as the duration of the cycle over the 20-year time horizon when the base-case reached the life expectancy of the Chinese population. The annual discount rates of both the future costs and effectiveness were 3% according to the recommendation of China Guidelines for Pharmacoeconomic Evaluations and Manual (2013 Edition).Citation17 Cost-effectiveness was determined to assume a threshold of per capita gross domestic product (GDP) of ChinaCitation17 (59,660 Chinese Yuan [CNY] in 2017Citation18). This Markov model was constructed using decision analysis software (TreeAge Pro 2011, Williamstown, MA).
Model Inputs
Effectiveness Calculation
The effectiveness was measured in quality-adjusted life-years (QALYs) per patient, which were calculated using the duration and the utility.Citation17 In our study, the utility of health valued from 0 (death) to 1 (perfect health). The baseline utility of the participants was 0.79 or 0.50 due to their previous history of CHD or stroke, respectively, based on the previous literature ().Citation19,Citation20 The utility decrement was the proportion of subsequent hospitalization due to non-fatal CHD and non-fatal stroke ().Citation21 Quality of life was decremented with each admission of non-fatal CHD or non-fatal stroke 2.3.2 Event risks.
Table 1 Utility Values
Table 2 Disutility Values for Disease Recurrence
Previously reported event probabilities of CHD recurrence, stroke recurrence, cardiovascular death, natural death and adverse reaction (ADR) in Chinese population (Supplementary Tables 1 and 2)Citation13,Citation16,Citation22 were used in our study. The only serious adverse event in the trial was a new onset myopathy (CK ≥ 5 times of upper limit of normal) in the high-dose group, which led to the patient withdrawal.Citation16 No events of elevated transaminases were reported in the trial, and thus such events were not considered in the current model.
Cost Calculation
The costs of the two groups were calculated using the daily consumption of the drugs according to the prescriptive pattern in Chinese Yuan (CNY, Supplementary Table 3). The costs of the hospitalization of CHD, stroke and myopathy caused by statins were calculated using the sum of the hospitalized patient costs with a primary discharge diagnosis with CHD (ICD-10: I20-I25), stroke (ICD-10: I61-I65) or unspecific myopathy (ICD-10: G70.90X) from West China Hospital, Sichuan University in 2017, respectively (Supplementary Tables 4–6). The cost of the withdrawn patients due to the adverse events included direct medical costs and elevated cardiovascular risk due to the discontinuation of the drug.Citation19,Citation22 All the cost data were collected according to the context in 2017.
Comparison of Cost and Effectiveness
The hypothetical cohort of 1,000 patients in each group was recruited in the Markov model. Cumulative cost and effectiveness were calculated by Markov queue simulations. The ICER was calculated using the difference in costs divided by QALYs. The group with lower costs was considered to be cost-effective if the ICER surpasses the per capita GDP in that year, while the group with higher costs was to be cost-effective otherwise.Citation17
Sensitivity Analysis
One-way sensitivity analysis was performed to evaluate the robustness of the model using key parameters in the model including the costs, utility values and transition probabilities. The annual discount rate of costs and utilities in one-way sensitivity analysis ranged from 0% to 5%, and time horizon ranged from 1 year to 30 years. Other parameter ranges were assigned between 25% of base-case values when neither the confidence interval (CI) nor the standard deviation (SD) was available, according to guideline recommendation.Citation17 Beta and gamma distributions were assigned to event probabilities, utilities, and cost estimates, respectively in the probabilistic sensitivity analysis. The probabilistic sensitivity analysis was performed by Monte Carlo simulations set to simulate 1,000 times.
Results
Results of the Base-Case Analysis
The hypothetical cohort of 1,000 patients in each group was recruited in our study. The combination treatment regimen cost about 9037.40 CNY annually, while the high-dose treatment regimen cost about 7194.15 CNY (Supplementary Table 3). The costs of hospitalization due to CHD and stroke were 8864 CNY and 14,340.95 CNY, respectively. After a 20-year simulation, the cumulative cost and effectiveness were 68,322.18 CNY at the price of 2017 per person and 6.63 QALYs per person in the high-dose rosuvastatin group, and 84,780.69 CNY per person and 6.98 QALYs per person in the combination group (). The ICER of the combination group compared with the high-dose group was 47,102.99 CNY per QALY gained, which did not surpass the per capita GDP (59,660 CNY/QALY) in China in 2017,Citation18 indicating ezetimibe plus moderate-dose rosuvastatin was cost-effective compared with high-dose rosuvastatin.
Table 3 Base-Case Results of Cost-Effectiveness Analysis for Two Treatment Regimens
Sensitivity Analysis
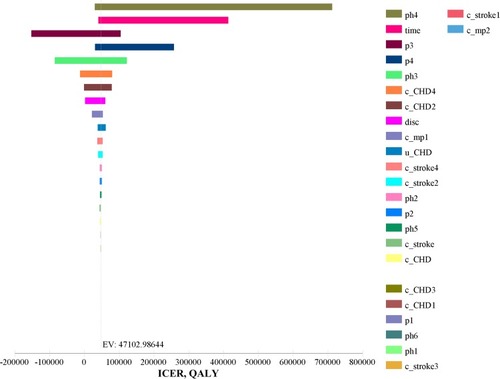
One-way sensitivity analyses of the cost, effectiveness, transition probabilities, discount rate and time horizon were shown in . The ICER were closely associated with non-CVD-related mortality in high-dose group (ph4), CVD-related mortality in combination group (p3), non-CVD-related mortality in combination group (p4), CVD-related mortality in high-dose group (ph3), outpatient prescription costs of CHD in combination group (c_CHD2) and outpatient prescription costs of CHD in high-dose group (c_CHD4). The combination group was showed to be cost-effective with longer follow-up duration. Within the range of the values, the results showed robustness and the trend kept consistent. Besides, larger ICER was observed with higher non-CVD-related mortality and higher CVD-related mortality in the combination group, lower non-CVD-related mortality and lower CVD-related mortality in the high-dose group, or shorter time horizon, which might lead to the high-dose group cost-effective.
Figure 2 Tornado diagram.
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years.
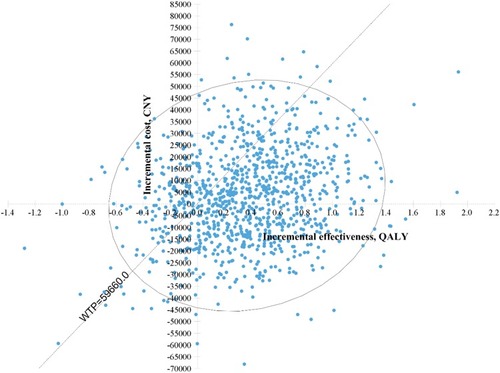
The Monte Carlo simulation scatters plot () showed 70% of the simulated population are willing to pay the threshold cost (59,660 CNY/QALY) for the combination treatment rather than the high-dose treatment. A few spots fell in the second quadrant, where ezetimibe was less effective and costly. Some spots fell in the fourth quadrant, where ezetimibe was more effective and less costly than high-dose statins. These dominant spots may be related to the CVD- and non-CVD-related mortality of the high-dose group and combination group according to one-way sensitivity analysis.
Figure 3 Scatter plot of Monte Carlo simulation.
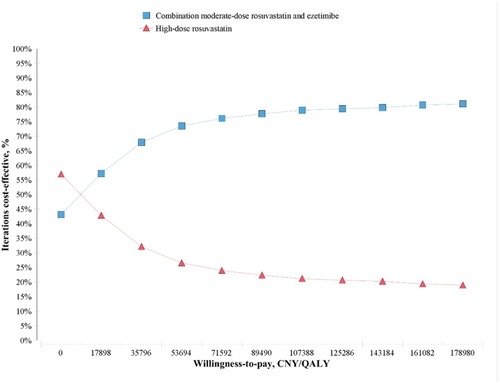
The cost-effectiveness acceptability curve () indicated that the combination treatment was more acceptable when the willingness to pay threshold was over 17,898 CNY/QALY.
Discussion
Our study developed a 20-year model to evaluate the costs and effectiveness of moderate-dose rosuvastatin and ezetimibe versus high-dose rosuvastatin in patients whose LDL-c level was not adequately controlled by moderate-dose statin alone for the secondary prevention of CVD in China 2017. With an ICER of 47,103 CNY/QALY, which does not surpass per capita GDP of 59,660 in China 2017, our data suggest that the combination of moderate-dose rosuvastatin and ezetimibe was cost-effective in preventing CVD events compared to high-dose rosuvastatin in China 2017 despite the cost increase of 20% in the combination group. This is the first cost-effectiveness analysis of ezetimibe as part of the second-line lipid-lowering therapy in the secondary prevention of CVD in the context of China.
Lipid-lowering treatment is the cornerstone of the secondary prevention of CVD.Citation23 Ezetimibe and proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors are two new non-statin lipid-lowering drugs, which were suggested to reduce the cardiovascular events.Citation14,Citation24 Compared to PCSK9 inhibitors, ezetimibe reduced only borderline risk of cardiovascular diseases (hazard ratio of ezetimibe, 0.936; 95% CI, 0.89 to 0.99 according to IMPROVE-IT trial vs relative risk of PCSK9 inhibitors, 0.84; 95% CI, 0.79 to 0.89 according to recent systematic review).Citation14,Citation24 However, according to recent cost-effectiveness analysis, PCSK9 inhibitors are too costly to be reasonably used in the US health system.Citation25 Although the prices of the two major PCSK9 inhibitors (alirocumab and evolocumab) were reduced consequently, they are still beyond the threshold of per capita GDP to be cost-effective in most countries. Being widely used in China and the western countries,Citation26–Citation28 ezetimibe is considered to be affordable (compared to PCSK9 inhibitors) and it does not cause major adverse events.Citation29 Our study further suggested that adding-on ezetimibe can benefit patients cost-effectively in the current Chinese context compared to doubling the dose of statin. The results were mainly driven by the safety issues of the two treatments.
High-dose statins were reported to be associated with increased risk of myopathy and elevated transaminases, especially simvastatin.Citation30–Citation32 Though such adverse events were relatively rare,Citation33,Citation34 more frequent creatine kinase (CK) elevation was observed in patients receiving high-dose rosuvastatin compared to the combination of moderate-dose rosuvastatin and ezetimibe.Citation16 The adverse events may lead to the discontinuation of the drug and sometimes hospitalization,Citation34 which could increase the cost of treatment.
Our study was in line with previous studies investigated the cost-effectiveness of ezetimibe combined with statin using different models in different clinical contexts.Citation35–Citation43 de Labry Lima et alCitation35 and Korman et alCitation36 suggested that the combination of ezetimibe and statins were cost-effective in the primary and secondary prevention of CVD compared with add-on PCSK9 inhibitors. Almalki et alCitation37 and Davies et alCitation38 suggested the superiority of the cost-effectiveness of the combined regimen compared with statin monotherapy. Laires et alCitation39 reported that it is cost-effective to treat patients with high cardiovascular risk with ezetimibe plus atorvastatin than switching to rosuvastatin. In particular patients with acute coronary syndrome (ACS)Citation37,Citation40 or chronic kidney disease (CKD),Citation41 the cost-effectiveness of the combined regimen of ezetimibe and moderate-dose statins may not be promising.
To be noted, our data also suggested that patients taking add-on ezetimibe to moderate-dose rosuvastatin spent more (5.05 CNY more per day during hospitalization and 7.87 CNY more per day after discharge) than patients taking high-dose rosuvastatin, increasing financial burden to the patients and the medical system in short-term. Price reduction of ezetimibe may thus help patients in a cost-effective manner. As the patent of ezetimibe has been expired in 2017, its generic counterparts are looking forward to with comparable effectiveness and a lower price in the developing countries like China.
There were some limitations in our study. Firstly, the effectiveness of the regimen was based on a short-term small sample trial,Citation16 which might not accurately represent the effectiveness of the real population. We thus introduced several sensitivity analyses to evaluate the robustness of the results. However, long-term study in China is warranted to better compare the cost and effectiveness of ezetimibe plus rosuvastatin versus high-dose rosuvastatin in the future. Secondly, the cost data was solely based on a tertiary hospital in China, which may not necessarily stay consistent throughout the country because of the unbalanced development across provinces and cities. However, we simulated the sensitivity of patients to cost change in the sensitivity analyses and the results suggested the relative cost-effectiveness of these two regimens should be similar even taking into consideration of a change of the costs.
Conclusions
In conclusion, adding ezetimibe to moderate-dose rosuvastatin is more costly but cost-effective compared to doubling the dose of rosuvastatin in the secondary prevention of CVD in the Chinese context when the LDL-c levels of the patients are still beyond target after being treated with moderate-dose rosuvastatin alone. A price reduction of ezetimibe may improve its cost-effectiveness. As several policies of drug price regulation are on their way in China, updated analyses are warranted along with the reformation of the medical system in China.
Author Contributions
HT, MH and SL conceived the study. NL and YZ collected the data. HY performed the model and statistical analysis. MH guaranteed the quality of the statistical procedure. HY and SL interpreted the results. HY, ZX and SL drafted the manuscript.
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
An abstract of this paper was presented as a poster presentation in ISPOR 2019 (PDG20).
Funding
This study was supported by the Cholesterol Fund by China Cardiovascular Foundation and China Heart House, the International Visiting Program for Excellent Young Scholars of Sichuan University, Sichuan Science and Technology Program (No. 2019YFH0150), the National Natural Science Foundation of China (No. 81400811 and 21534008) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYGD18022). The funders played no role in the planning, design, conduction, analysis and publication of the study.
References
- MoranA, ZhaoD, GuD, et al. The future impact of population growth and aging on coronary heart disease in China: projections from the coronary heart disease policy model-China. BMC Public Health. 2008;8(1):394. doi:10.1186/1471-2458-8-39419036167
- Health and Family Planning Commission of the People’s Republic of China. Graphic: report on nutrition and chronic disease status of Chinese residents; 2015 Available from: http://www.nhc.gov.cn/jkj/s5879/201506/4505528e65f3460fb88685081ff158a2.shtml. Accessed 8 1 2020.
- CritchleyJ, LiuJ, ZhaoD, WeiW, CapewellS. Explaining the increase in coronary heart disease mortality in Beijing between 1984 and 1999. Circulation. 2004;110(10):1236–1244. doi:10.1161/01.CIR.0000140668.91896.AE15337690
- Joint committee for guideline revision National Expert Committee on Cardiovascular Diseases, National Center for Cardiovascular Diseases Chinese Society of Cardiology, Chinese Medical Association Chinese Diabetes Society, Chinese Medical Association Chinese Society of Endocrinology, Chinese Medical Association Chinese Society of Laboratory Medicine, Chinese Medical Association. Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2016;2018(15):1–29.
- JellingerPS, HandelsmanY, RosenblitPD, et al. American Association of Clinical Endocrinologists And American College of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(4):479–497. doi:10.4158/EP171764.GL28156151
- Chinese Medical Association Cardiovascular Diseases Committee, Chinese Gerontology Society Cardiovascular and Cerebrovascular Diseases Committee. Clinical experts on selective cholesterol absorption inhibitors (2013 Edition). Chin J Intern Med. 2013;52:617–620.
- ZhaoS, WangY, MuY, et al. Prevalence of dyslipidaemia in patients treated with lipid-lowering agents in China: results of the DYSlipidemia International Study (DYSIS). Atherosclerosis. 2014;235(2):463–469. doi:10.1016/j.atherosclerosis.2014.05.91624950001
- WangF, YeP, HuD, et al. Lipid-lowering therapy and lipid goal attainment in patients with metabolic syndrome in China: subgroup analysis of the Dyslipidemia International Study-China (DYSIS-China). Atherosclerosis. 2014;64(16):C109–C110.
- HouQ, YuC, LiS, et al. Characteristics of lipid profiles and lipid control in patients with diabetes in a tertiary hospital in Southwest China: an observational study based on electronic medical records. Lipids Health Dis. 2019;18(1):13. doi:10.1186/s12944-018-0945-830636643
- Cholesterol Treatment Trialists’ CTT Collaborator. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statin. Lancet. 2005;366(9493):1267–1278. doi:10.1016/S0140-6736(05)67394-116214597
- BaysH, DujovneC. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother. 2003;4:779–790.12740000
- HPS THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi:10.1093/eurheartj/eht05523444397
- ArmitageJ, BowmanL, WallendszusK, et al.; for Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376(9753):1658–1669.21067805
- CannonCP, BlazingMA, GiuglianoRP. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi:10.1056/NEJMoa141048926039521
- ClarkeAT, JohnsonPCD, HallGC, et al. High dose atorvastatin associated with increased risk of significant hepatotoxicity in comparison to simvastatin in UK GPRD cohort. PLoS One. 2016;11(3):e0151587. doi:10.1371/journal.pone.015158726983033
- RanD, NieHJ, GaoYL, et al. A randomized, controlled comparison of different intensive lipid-lowering therapies in Chinese patients with non-ST-elevation acute coronary syndrome (NSTE-ACS): ezetimibe and rosuvastatin versus high-dose rosuvastatin. Int J Cardiol. 2017;235(Complete):49–55. doi:10.1016/j.ijcard.2017.02.09928291622
- Chinese Pharmacoeconomics. China guidelines for pharmacoeconomic evaluations and manual. J Pharm Econ (2013 Ed). 2013.
- Bureau of Statistics of China. China Statistical Yearbook 2018 Available from: http://www.stats.gov.cn/tjsj/ndsj/2018/indexeh.htm. Accessed 8 1 2020.
- LiewD, ParkHJ, KoSK. Results of a Markov model analysis to assess the cost-effectiveness of a single tablet of fixed-dose amlodipine and atorvastatin for the primary prevention of cardiovascular disease in Korea. Clin Ther. 2009;31(10):2189–2203. doi:10.1016/j.clinthera.2009.10.01519922890
- StevanovicJ, PehlivanoglouP, PostmaMJ. Meta-analysis of preference-based quality of life values in heart failure. PLoS One. 2016;11(3):e0152030. doi:10.1371/journal.pone.015203027011260
- McconnachieA, WalkerA, RobertsonM, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J. 2014;35(5):290–298. doi:10.1093/eurheartj/eht23223839541
- AraR, PandorA, TumurI, et al. Estimating the health benefits and costs associated with ezetimibe coadministered with statin therapy compared with higher dose statin monotherapy in patients with established cardiovascular disease: results of a Markov model for UK costs using data registries. Clin Ther. 2008;30(8):0–1523.
- CatapanoAL, GrahamI, De BackerG, et al. ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;2016(37):2999–3058. doi:10.1093/eurheartj/ehw272
- DuH, LiX, SuN, et al. Proprotein convertase subtilisin/kexin 9 inhibitors in reducing cardiovascular outcomes: a systematic review and meta-analysis. Heart. 2019;105(15):1149–1159. doi:10.1136/heartjnl-2019-314763
- KaziDS, PenkoJ, CoxsonPG, et al. Cost-effectiveness of alirocumab: a just-in-time analysis based on the ODYSSEY outcomes trial. Ann Intern Med 2019;170(4):221–229. doi:10.7326/M18-1776
- RossJS, FrazeeSG, GaravagliaSB, et al. Trends in use of ezetimibe after the ENHANCE trial, 2007 through 2010. JAMA Intern Med. 2014;174(9):1486. doi:10.1001/jamainternmed.2014.340425070672
- LuL, KrumholzHM, TuJV, et al. Impact of the ENHANCE Trial on the use of ezetimibe in the United States and Canada. Am Heart J. 2014;167(5):683–689. doi:10.1016/j.ahj.2014.01.01424766978
- LuL, KrumholzHM, TuJV, et al. Impact of drug policy on regional trends in ezetimibe use. Circ Cardiovasc Qual Outcomes. 2014;7(4):589. doi:10.1161/CIRCOUTCOMES.114.00102324895451
- BettersJL. Transporters as drug targets: discovery and development of NPC1L1 inhibitors. Clin Pharmacol Ther. 2010;87(6):117–121. doi:10.1038/clpt.2009.20919907422
- ThompsonPD, PanzaG, ZaleskiA, et al. Statin-associated side effects. J Am Coll Cardiol. 2016;67(20):2395–2410. doi:10.1016/j.jacc.2016.02.07127199064
- GrahamJH, SanchezRJ, SaseenJJ, et al. Clinical and economic consequences of statin intolerance in the United States: results from an integrated health system. J Clin Lipidol. 2017;11(1):70. doi:10.1016/j.jacl.2016.10.00328391913
- SaxonDR, EckelRH. Statin intolerance: a literature review and management strategies. Prog Cardiovasc Dis. 2016;59(2):153–164. doi:10.1016/j.pcad.2016.07.00927497504
- BaysH, CohenDE, ChalasaniN, et al. An assessment by the statin liver safety task force: 2014 update. J Clin Lipidol. 2014;8(3):S47–S57. doi:10.1016/j.jacl.2014.02.01124793441
- GrahamDJ. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292(21):2585. doi:10.1001/jama.292.21.258515572716
- de Labry LimaAO, BallesterVG, SánchezJF, et al. Cost-effectiveness and budget impact of treatment with evolocumab versus statins and ezetimibe for hypercholesterolemia in Spain. Rev Esp Cardiol (Engl Ed) 2018;71(12):1027–1035. doi:10.1016/j.rec.2018.05.003
- KormanM, WisløffT. Modelling the cost-effectiveness PCSK9 inhibitors vs. ezetimibe through LDL-C reductions in a Norwegian setting. Eur Heart J Cardiovasc Pharmacother. 2018;4:15–22. doi:10.1093/ehjcvp/pvx01028444187
- AlmalkiZS, GuoJJ, AlahmariA, et al. Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in patients with a history of acute coronary syndrome: analysis of results of the IMPROVE-IT trial. Heart Lung Circ. 2018;27:656–665. doi:10.1016/j.hlc.2017.05.13928716519
- DaviesGM, VyasA, BaxterCA. Economic evaluation of ezetimibe treatment in combination with statin therapy in the United States. J Med Econ. 2017;1–20.
- LairesPA, EjzykowiczF, HsuTY, et al. Cost–effectiveness of adding ezetimibe to atorvastatin vs switching to rosuvastatin therapy in Portugal. J Med Econ. 2015;18(8):1–8. doi:10.3111/13696998.2015.103179425271379
- RecklessJ, DaviesG, TunceliK, et al. Projected cost-effectiveness of ezetimibe/simvastatin compared with doubling the statin dose in the United Kingdom: findings from the INFORCE study. Value Health. 2010;13(6):726–734. doi:10.1111/j.1524-4733.2010.00742.x20561328
- MihaylovaB, SchlackowI, HerringtonW, et al. Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in CKD: results of the study of heart and renal protection (SHARP). Am J Kidney Dis. 2016;67(4):576–584. doi:10.1053/j.ajkd.2015.09.02026597925
- NootenFV, DaviesGM, JukemaJW, et al. Economic evaluation of ezetimibe combined with simvastatin for the treatment of primary hypercholesterolaemia. Neth Heart J. 2011;19(2):59–60. doi:10.1007/s12471-011-0078-421461034
- AraR, PandorA, TumurI, et al. Cost effectiveness of ezetimibe in patients with cardiovascular disease and statin intolerance or contraindications: a Markov model. Am J Cardiovasc Drugs. 2008;8(6):419–427. doi:10.2165/0129784-200808060-0000519159125