Abstract
Colorectal cancer is one of the most common cancer diseases with the increase of cases prevalence >5% every year. Multidrug resistance mechanisms and non-localized therapy become primary problems of chemotherapy drugs for curing colorectal cancer disease. Therefore, the enteric-coated nanoparticle system has been studied and proved to be able to resolve those problems with good performance for colorectal cancer. The highlight of our review aims to summarize and discuss the enteric-coated nanoparticle drug delivery system specific for colorectal cancer disease. The main and supporting literatures were collected from published research articles of journals indexed in Scopus and PubMed databases. In the oral route of administration, Eudragit pH-sensitive copolymer as a coating agent prevents the degradation of the nanoparticle system from the gastric fluid and releases drug to intestinal-colon track. Therefore, it provides a colon-specific targeting ability. Impressively, enteric-coated nanoparticles having a sustained release profile significantly increase the cytotoxic effect of chemotherapeutic drugs and achieve cell-specific target delivery. The enteric-coated nanoparticle drug delivery system represents an excellent modification to improve the effectiveness and performance of anticancer drugs for colorectal cancer disease in terms of the oral route of administration.
Introduction
Colorectal cancer is one of the most common cancers in the world with about 1.9 million new colorectal cancer (CRC) cases reported in 2018 and 900,000 deaths, making it the third most commonly diagnosed cancer worldwide.Citation1 In 2020, around 147,950 individuals will be predicted with CRC, 53,200 might die from these diseases.Citation2 Even though the prognosis of CRC patients has improved over the last few decades in many developed countries, mostly due to improved prevention and treatment,Citation3–Citation6 the incidence and mortality in low-and middle-income countries are rising rapidly, in part due to adaptation of western-lifestyle.Citation7
Conventional colon cancer treatment depending on the tumor stage consists mainly of surgery, chemotherapy, and radiation.Citation3,Citation8–Citation11 However, these methods bear several risks. Besides, the usual risks of surgery such as major blood loss and infections, colectomy might lead to serious tissue damage causing leakage of the anastomosis.Citation12–Citation19 It is also worth mentioning that the risk of fecal and urinary incontinence after radiation therapy is high.Citation5,Citation20–Citation25 Another problem associated with radiotherapy and chemotherapy is tissue toxicity as both cancerous and healthy cells are affected.Citation26–Citation28
In addition, the application of chemotherapeutics is greatly limited, ascribable to multi-drug resistance caused by efflux mechanisms, enhancement of drug inactivation, or mutations of the drug target.Citation28–Citation31 Besides that, common chemotherapeutics like 5-Fluorouracil have a poor site-specificity leading to the fact that a growing dose size of anti-cancer drugs is required, which increases toxic side effects.Citation32 For example, the overall response rate for 5-Fluorouracil regarding colorectal cancer alone is only 10% to 15%.Citation29 Furthermore, researchers are challenged to overcome the limitations of conventional cancer therapy with new approaches.
Targeted anti-cancer agents such as Bevacizumab and Cetuximab have been developed. Both are monoclonal antibodies specific for molecular targets, which block transduction pathways or cancer proteins. These targeted monoclonal agents are able to reach the tumor site specifically.Citation12,Citation33,Citation34 Currently, first-line treatments combine targeted therapy and fluoropyrimidine-based chemotherapy.Citation35,Citation36
In order to increase anti-tumor efficacy, current therapeutic methods involve the combination of different chemotherapeutic drugs in a series of cycles.Citation3,Citation12,Citation31 However, the emergence of drug-resistant tumor cells reduces the efficacy of chemotherapeutics and endure a crucial problem in colon cancer chemotherapy.Citation37
One promising approach to improve the efficacy and to reduce the systematic side effects of anti-cancer agents is nanoparticle drug delivery systems. They are biodegradable, nano to submicronic colloidal systems with a diameters range between 3 and 200 nm, able to effectively carry the anti-cancer agents to the tumor site,Citation30,Citation34,Citation38 attaining a high local drug concentration by site-specific targeting, enhanced permeability, and greater retention.Citation27,Citation39–Citation44 Thus, the use of nanocarriers can reduce the unwanted systemic side effects and drug resistance.Citation27,Citation45
However, nanoparticles for colon-targeted oral drug delivery systems have to overcome pH-sensitivity and transit time in the stomach. For oral administration, the formulation must be protected in order to prevent degradation, premature drug release and absorption before reaching the colon.Citation46,Citation47 These problems can be overcome by enteric coating of the nanoparticle delivery system. The enteric coating acts as a barrier protecting the loaded drug against the stomach acidic environment and controlling its release to reach sites in the lower gastrointestinal tract.Citation32,Citation48–Citation52
In recent years more and more nanoparticles for various purposes have been developed. Previous review articles discussed the application of polymers in delivering cancer drugs to colorectal cancer individually.Citation53–Citation55 This review article summarizes and discusses the enteric-coated nanoparticles as oral drug delivery systems for treating colorectal cancer. In this review, the comparison of different applied formulations and polymers for enteric-coated nanoparticles in colorectal cancer drug delivery system were discussed.
Methodology
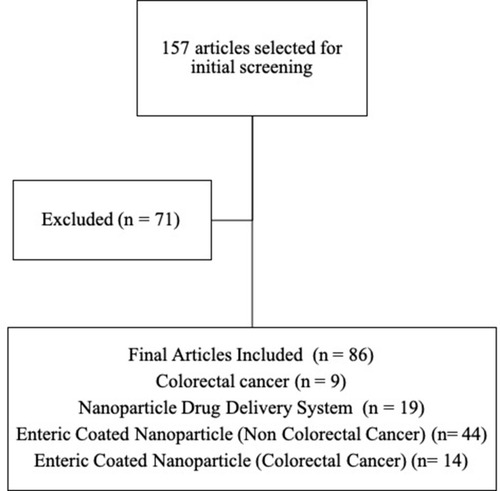
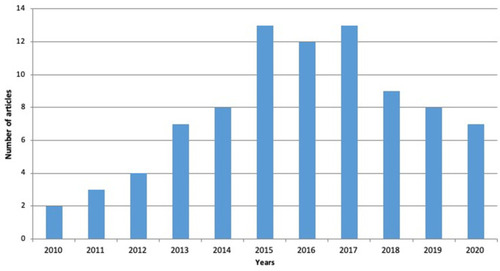
This review is focused on published articles indexed in Scopus and PubMed database using the keyword “enteric-coated nanoparticle colorectal cancer,” “enteric-coated nanoparticle cancer,” and “enteric-coated nanoparticles.” Opinions, assessments, and unrelated subjects such as pharmacological characteristics and bioactivities have been utilized for exclusion criteria. The flowchart of the methodology can be seen in . The distribution of articles based on the year of publication can be seen in .
In this review, we examined the studies on nano-sized cancer drugs with a macroporous sponge and multilayer dispersion system from macro to nano-sized. This system serves as a protection, targeted delivery to cancer cells, modification of release, and enhancing cell uptake in cancer drugs. Therefore, we also discussed the enteric-coated nanoparticle drug system for non-colorectal cancer and its development in targeted delivery to colorectal cancer.
Colorectal Cancer
Colorectal cancer is a type of cancer that grows in the large intestine (colon), or at the very end of the large intestine that is connected to the rectum. The term colorectal cancer refers to cancer that develops slowly. Its development starts from tumor or tissue growth in the inner lining of large intestine or rectum.Citation56 In general, colorectal cancer originates from the inner wall of colorectal epithelial layer as a polyp, which then invades the lymph node and muscles surrounding it. In the next stage, colorectal cancer will spread to other organs, especially the liver. Recurrence and widespread cancer (metastatic) distribution are the two main factors that are affecting the survival of patients with colorectal cancer. The chance of survival of colorectal cancer patients can reach up to around 90% for 5 years if colorectal cancer did not metastasize. But the survival rate is reduced to around 12% in patients with metastatic colorectal cancer.Citation1,Citation57,Citation58 Several factors are influencing the incidence of colorectal cancer including age, gender and genetic factors. Concomitant chronic diseases such as ulcerative colitis, diabetes, and obesity can increase the risk of developing colorectal cancer.Citation58–Citation61 At this time, patients with colorectal cancer are being treated by surgical removal of the tumor, chemotherapy as well as radiation therapy.Citation62,Citation63
Nanoparticle Drug Delivery System
Nanoparticles are defined as size structures ranging from 1 to 100 nm in at least one dimension. However, the “nano” prefix refers to nanostructured particles, is usually used for particles up to several hundred nanometers in secondary size contained nano-sized primary particles.Citation64,Citation65 Nanocarriers have certain physicochemical and biological characteristics, which makes it easier to enter cells in comparison to larger molecules, so they can successfully deliver active substances intracellularly,Citation66–Citation68 and have the ability to carry cancer drugs having small or large molecular weights including genes or proteins. Thus, nanocarriers can be used for targeted anticancer delivery approaches for better accumulation in cancer cells.Citation34,Citation69–Citation74 In addition, nanocarriers can increase the solubility of hydrophobic drugs, protect drugs from degradation, reduce renal clearance, increase the half-life, and can be used for controlled systemic release.Citation34,Citation75
Nanotechnology has been widely developed as a new strategy for drug delivery and cancer treatment. When compared to conventional drug delivery systems, nanotechnology-based drug delivery systems have superior potential in several aspects, for example, targeting specific organs, increased circulation times and controlled systemic release.Citation75 The application of nanotechnology has the potential to overcome drug resistance especially through reversing Multi-Drug Resistance (MDR). Thus, allowing sufficient drugs to accumulate in the cytoplasm resulting in remarkable improvement in chemotherapy efficiency.Citation76–Citation81
Enteric Nanoparticle Development
In 1930, the first ingredient used in the enteric coating system was shellac. The enteric coating technique was first introduced by Unna in 1984 in the form of keratin-coated pills. Micro- to nano-encapsulated forms were then developed due to various needs in improving the quality and stability of drugs such as controlling drug release, reducing gastrointestinal irritation, and preventing drug interactions. This delivery system was first developed by Bodmeier et al (1989) using chitosan and alginate as coatings.Citation82,Citation83 Furthermore, the development of nano-sized enteric coating systems has been developed to date with various coating modifications and the addition of cell targeting or magnetic labeling agents (see ).
Enteric-Coated Nanoparticle Formulations for Non-Colorectal Cancer
Each part of the digestive tract has a different pH. The stomach has an acidic pH environment (1–3) while the small intestine has a slightly acidic to neutral environment pH (5.9 −7.8). The colon has a pH ranging from 5 to 8.Citation84–Citation86 Based on physiological conditions of the gastrointestinal tract, targeted drug delivery systems that are suitable for certain parts of the gastrointestinal release the active compounds triggered by pH. pH-dependent coating technology is usually applied to protect active substances from degradation by gastric acid and as targeted delivery in certain parts of the digestive tract.Citation87,Citation88 Enteric-coated drug delivery systems are designed to be able to survive in the acidic environment and disintegrate at a higher pH environment, preventing the degradation of active compounds by gastric fluid components.Citation88,Citation89 Some examples of enteric-coated drug formulas for non-colorectal cancer are summarized in .
Table 1 Enteric-Coated Nanoparticle Formulations for Non-Colorectal Cancer
The application of enteric-coated in several studies has successfully delivered drugs through the gastric channel (low pH) by maintaining the stability of active substances, even those in the form of peptides and proteins such as vaccines,Citation99,Citation104,Citation125 and insulinCitation94,Citation95,Citation98 to be absorbed via lymphatic or bile pathways. These formulas can be used as innovators in the development of targeted delivery systems to colon cancer cells.
Natural or synthetic polymers are used in the enteric-coated system. However, in the colorectal drug delivery system, the polymers used need to be modified in order to deliver drugs to the targeted cells and increase its bioavailability. Therefore, mediators such as receptors, peptides, and other compounds are needed to be linked to the surface of the carrier. The mediators facilitate the attachment of the drug to the cell and increase its uptake.
Enteric-Coated Nanoparticle Formulations for Colorectal Cancer
Enteric-coated nanoparticles are one of the drug delivery technologies that can improve the bioavailability of drugs by oral administration,Citation50,Citation133–Citation135 increase intracellular penetration and retention time, control the release of encapsulated drugs and targeted delivery in specific parts of the gastrointestinal tract such as in the treatment of colorectal cancer.Citation136 Cellular uptake and efficacy of nanoparticle drug delivery systems for colorectal cancer therapy are influenced by several factors, namely, size, shape, and surface chemistry.Citation137–Citation139 The size of the drug delivery carrier plays an important role in colorectal cancer therapy. Nanoparticles sizes around 100–200 nm have better cancer-targeting properties than larger particles. This can increase selective accumulation in the colon tissue due to the epithelial-enhanced permeability and retention effect. This can increase selective accumulation in the colon tissue.Citation140 In order to be absorbed into the cells, the particle size of the drug should not exceed 104 nm. With a particle size of 102 nm, nanoparticle drugs are generally absorbed via the clathrin pathway.Citation141 The illustration of anticancer drug delivery and its release in the colon can be seen in .
Figure 4 The illustration of nanosized drug entrapped in polymeric matrix (A) and its mechanism in release and delivery at colon (B).
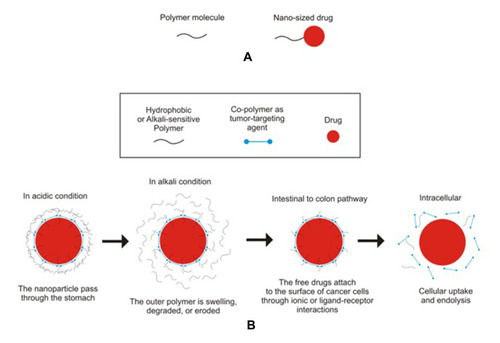
Materials used for enteric coating are usually water-resistant or pH-sensitive (). Enteric-coated systems use polymer coatings that do not dissolve in a gastric fluid consequently prevent or slowing down the release of drug compounds in stomach.Citation89 Eudragit is a pH-dependent enteric-coated polymer, dissolves in a pH>5.5 medium,Citation142 and has the highest entrapment ability compared to other polymers.Citation143,Citation144 It protects the active ingredient from gastric fluid, improves drug effectiveness, and enables targeting specific areas at intestine. Eudragit polymers are versatile polyacrylate polymers with various degrees of solubility, which make it suitable for sustained release formulation. Eudragit S100 has solubility characteristics above pH 7, making it suitable for use in colonic release targeting. Therefore, it is often used as an enteric coating in the drug delivery system for colorectal cancer.
Table 2 Enteric-Coated Nanoparticle Formulations for Colorectal Cancer
Chitosan Nanoparticle
In 2017, Sun et al tried to develop a nanoparticle system for 5-Fluorouracil as drug payload by using ionic gelation. In this study, they used chitosan as the main carrier. The observed mean particle size was around 283.9 nm, with 44.28% of entrapment efficiency and 20.12% loading capacity. The highest entrapment efficiency and drug-loading could be achieved by using a 1:1 mass ratio of 5-FU and Chitosan. The PDI was around 0.252, indicating that the distribution is relatively homogeneous. In addition, the nanoparticle system showed good stability with a zeta-potential of about 45.3 mV. Impressively, the in vitro and in vivo drug release study implies that compared to the normally used 5-FU solution, a notably sustained and extended drug release by 5-FU Chitosan nanoparticles can be observed. Regarding the bioavailability in rats, the AUC value of 5-FU Chitosan nanoparticles showed more than 2-fold increase to the solution. Furthermore, the nanoparticles show the same in vitro cytotoxic efficacy on gastric cancer SGC-7901 as conventional 5-FU injections.Citation145
Another study from Tummala et al in 2015 demonstrated that nanoparticle formulations with enteric coating become an excellent modification of the previously mentioned nanoparticles. In this study, the solvent emulsification evaporation technique was used to prepare the same type of nanoparticles, which were additionally coated with an enteric coating (Eudragit S100) to protect the nanoparticles from degradation in gastric fluid and to achieve drug release when the carrier reaches the intestine. Comparing the 1:1 mass ratio formulations of S. Tummala and L. Sun, the particle size with about 192 nm which is much smaller, even though having an extra enteric coating. Moreover, the average particle size of the formulation prepared using a 1:3 mass ratio was about 138 nm, which is even smaller. In addition, the study revealed a significantly higher entrapment efficiency (69.18%) and drug loading (28.14%), using a drug: polymer ratio of 1:3. In contrast to the study of Sun et al the drug loading did not increase with the increase of 5-FU. Other than that, the other particle characteristics regarding stability and distribution showed similar results.
In in vitro study, non-enteric-coated 5-FU chitosan nanoparticles released up to 70% of 5-FU before reaching the colonic fluid. Due to the Eudragit S100 coating, which contains acidic functional groups, dissolving only in alkaline colonic medium, the enteric-coated nanoparticles remain stable in gastric fluids and reach the tumor sites in the colon. Drug release of enteric-coated nanoparticles started only after 4h in simulated intestinal fluid and showed a sustained release profile over 24h of a time period, whereas non-enteric-coated nanoparticles released about 50% of 5-FU after 2 to 3 hours and up to 70% before reaching colonic fluid.Citation32
Continuing the previous study, Tummala et al focused on improving 5-FU anti-cancer activity of 5-FU loaded enteric-coated chitosan nanoparticles, they have evaluated their apoptotic activity in vitro using HCT 116 colorectal cancer cells. A decrease in the IC50 value of 5.5 folds compared to pure drugs was shown, whereas plain nanoparticles showed no toxicity indicating the safety of nanoparticles. Furthermore, the research demonstrated the ability in localizing the drug at the colon and releasing the majority of the payload once it arrives at the ascending colon after 4 h. Moreover, the improved apoptotic activity of enteric-coated nanoparticles was determined compared with free nanoparticles and pure 5-FUCitation133
In conclusion, an enteric-coated nanoparticle system with a significant sustained and localized release and enhanced anticancer activity for colorectal cancer treatment was successfully developed.
As for various cancer diseases including colorectal cancer hyaluronic acid (HA) receptors in tumor cells are overexpressed, making it a suitable target for anticancer drugs, which seems to be a promising approach to improve orally targeted drug delivery systems for colorectal cancer.
In a study from Jain et al in 2010 hyaluronic-acid coupled Oxaliplatin (L-OHP) loaded nanoparticles with Chitosan as the main carrier were prepared using ionotropic gelation as an attempt to develop an optimal targeted delivery system for colorectal cancer. Afterward, the nanoparticles were processed into pellets, which were then coated with Eudragit S100.
Characterization studies showed that HA-coupled chitosan nanoparticles had similar characteristics as the previously mentioned nanoparticles. However, compared to uncoupled nanoparticles, the entrapment efficiency of HA-coupled nanoparticles was significantly less.
Other than that, zeta potentials of uncoupled chitosan nanoparticles (CTNPOP) and HA-coupled chitosan nanoparticles (HACTNPOP) formulations were observed to be around 40.3 and 10.0 mV, which mean that the stability differs significantly. It was also concluded that the optimal total weight gain regarding the film thickness is 10%. In this range, the coat is stable enough to pass gastric fluids in the upper gastrointestinal tract without degradation but is still able to ensure an optimum drug release in the colon. In terms of the in vitro drug release, non-coupled nanoparticles had a higher drug release profile than the HA-coupled ones. HA coupling might act as an additional barrier for drug diffusion, which has to be overcome. Furthermore, the mural study could confirm, that coated nanoparticles mainly started to release the drug in the ascending colon after 4 to 5 hours, which implies, that colon-specific delivery of L-OHP was achieved after oral administration of enteric-coated nanoparticles and, that the enteric coating process was successful.
As expected, the biodistribution studies show, that HA-coupled nanoparticle increased the drug concentration at the tumor site more effectively than the uncoupled nanoparticles or the free drug, which means that the researchers were able to achieve a more specific targeted approach to the colorectal cancer site by using hyaluronic acid as targeting ligand. Impressively, tumor regression studies on C57 Balb/c mice could successfully confirm HA-coupled nanoparticles being able to stop tumor proliferation more effectively than free L-OHP and CTNPOEPs. It could be observed that the growth of tumor cells could be delayed for about 8 days, which is twice as long as non-coupled nanoparticles. These results imply that nanoparticles coupled with targeting ligand like hyaluronic acid have a significant effect.Citation39 Five years later in 2015, Jain et al used 5-FU instead of Oxaliplatin and attempted to optimize the chitosan nanoparticles. The preparation method was comparable to the study from 2010 and very similar results regarding the characterization could be observed.Citation155
Pectin-Eudragit Nanoparticle
Subudhi et al attempted to prepare Eudragit S100-coated nanoparticles, which are loaded with 5-FU for colon targeting in cancer therapy. Instead of using Chitosan like in the previously mentioned articles, citrus pectin was used. Conveniently, citrus pectin also acts as a target mediator at the same time since it additionally functions as a ligand for galectin-3 receptors. Like hyaluronic acid receptors, Galectin-3 receptors are also overexpressed in colorectal cancer, making it a promising and specific target for anticancer medication. With a mean particle size of about 218.12 nm the enteric-coated nanoparticles are slightly bigger than coated nanoparticles consisting of Chitosan.
Surprisingly the zeta potential of the coated citrus pectin nanoparticles could be found around the value of −27.5 mV. Compared to the same non-coated nanoparticles with −18.4 mV, an enormous improvement could be found, but the optimal stability could not be achieved as the zeta potential should be >|30| mV to provide good stability of colloidal dispersions. In contrast to chitosan nanoparticles of previously mentioned articles, citrus pectin nanoparticles showed less entrapment efficacy of only around 35.15%. That being said, regarding particle size and entrapment efficacy, citrus pectin nanoparticles seem to be less optimal for colon targeted delivery systems than chitosan nanoparticles.
Thus, the effective drug delivery depends on the size, stability of the suspension and provide well-dispersed nanoparticles. The morphology of the nanoparticles also plays an important role on the hydrodynamic of the drug delivery and consequently affect the kinetic reactivity of the colloidal systems.
The in vitro drug release study in simulated gastrointestinal fluid mediums at different pH values showed that the enteric-coated nanoparticles did not release a significant amount of 5-FU within the first 4 hours in simulated intestinal fluid, which is similar to the result of S. Tummala et al, whereas the release rate from nanoparticles starts to increase with the increasing pH values of the release medium. Therefore, as expected, the amount of drug release of non-coated and coated nanoparticles differs significantly. Eudragit S100 coating was able to reduce the drug release by about 19% after 24 hours. In medium containing 2% rat cecal content, it could be stated that the drug release of both coated and non-coated nanoparticles was drastically increased by the numerous anaerobic bacteria, which could digest pectin, leading to 5-FU being released.
All in all, it can be concluded, that the prepared coated citrus nanoparticles have a controlled and sustained release profile. Based on sulforhodamine B assay, it was successfully proven that the cytotoxicity could be increased by using citrus pectin nanoparticles as dosage form (LC50 = 36.4 μg/mL) compared to the free drug solution, but when coated with Eudragit S100 the nanoparticles (LC50 = 94.2 μg/mL) showed less cytotoxicity than the free 5-FU (LC50 = 56.7 μg/mL), which is probably due to the Eudragit S100 coating, that remains mostly stable in acidic pH of the medium used in the cell cytotoxicity study.
As expected, it was verified that the nanoparticles covered with Eudragit S100 released the least amount of drug in the upper GIT compared to the non-coated nanoparticles and free drug solution. In addition, there was a significant increase in colon 5-FU concentration in the case of Eudragit-coated citrus pectin nanoparticles due to facilitated microflora degradation in the colonic region.
The plasma drug concentration was analyzed to evaluate drug absorption. The maximum drug plasma level of non-coated nanoparticles was reached after 8 h, mainly released in the small intestine, also showing a prolonged and delayed-release profile due to low permeability. On the other hand, enteric-coated nanoparticles reached their maximum plasma level after 12h, slowly releasing the drug in the colon within 12 hours, achieving a higher drug concentration and prolonged effect in the colon site compared to the non-coated nanoparticle and the free solution. In addition, Eudragit-coated nanoparticles showed a relatively low drug plasma level at all time points, and as a result, the risk of severe systematical side effects is reduced and the exposure time to the tumor site in the colon is prolonged.Citation149
PMMA-Eudragit Nanoparticle
In 2015 another approach on developing nanoparticles as a colon targeted delivery system was attempted. Ma et al first prepared Cy5-labelled PMMA-Eudragit RS PO nanoparticles (Cy5 NPs). Subsequently, they were incorporated in IR750-dyed-chitosan-Hypromellose microcapsules using ionotropic gelation. The microcapsules were then coated with Eudragit S100 eventually. Other than the previously mentioned studies, the nanoparticles were not loaded with an anti-cancer agent. Thus, the nanoparticles were not coupled with a specific target mediator for tumor cells in the colon site. In the study, they tried to compare site-specificity of free nanoparticles and nanoparticles in a microcapsule-system and evaluated their potential for colon-targeting.
After 2 hours of in vitro incubation in simulated gastric fluid and 6 hours in intestinal fluid, only less than 4% of nanoparticles from Eudragit S100-coated IR750 MCs were released. Another 9% were released in the following 6 hours in simulated colonic fluid, which not only demonstrates an overall relatively slow release rate but also showing that Cy5 nanoparticles could be at least successfully released from Chitosan-HPMC microcapsules in colonic fluids. Moreover, a significant number of nanoparticles have been already released in the small intestine before reaching the colon. Therefore, the aim of colon-specificity could not be achieved.
To have a significant therapeutic effect, the NP must not only be released but also penetrate into the tumor tissue. In order to investigate the cellular uptake of Cy5 nanoparticles, human colon adenocarcinoma (HT29) and embryonic fibroblast (NIH/3T3) cells were used. Altogether it could be noted that the general NP from Eudragit S100-coated IR750 MCs uptake increased with the increase of NP concentration and time of exposure. Due to the higher proliferation rate of cancerous cells, the NP uptake in HT29 was higher than the uptake in NIH/3T3 cells.
Surprisingly, in contrast to the in vitro study, all of the drug was released in vivo in the lower small intestine. Altogether it could be concluded that there is an efficient, but non-specific uptake of nanoparticles in both normal and cancerous cells. After oral administration in mice, the biodistribution of free Cy5 NPs and Eudragit S100-coated Cy5 NP-in-IR750 microcapsules was examined by using fluorophore-based animal imaging. It could be stated that free Cy5 NPs and Eudragit S100-coated Cy5 NP-in-IR750 MCs, showed stomach retention over 24 hours, owing to adhesion to the gastric mucosal surface.
Over 24 hours after oral administration, the non-encapsulated nanoparticles had a restricted allocation in the colon. On the other hand, the enteric-coated MCs increased the delivery of nanoparticles to the colon due to prolonged nanoparticle residence in the gastrointestinal tract of the mouse and reduced nanoparticle excretion in feces, although the MCs had poor colon specificity.Citation150
PLGA-Eudragit Nanoparticle
In 2011, Nassar was able to prepare nanocapsules loaded with Docetaxel using PLGA [poly(lactic-co-glycolic acid)] as the main carrier, which was then embedded in microparticles coated with Eudragit L. The average diameter and zeta potential values of the NCs formed were around 300 nm and −60.1 mV to −37.7mV, which is the prevalent size for PLGA nanoparticles, and also demonstrates that the solution is stable. Interestingly, the nanoparticle-microparticle system showed a significant better oral absorption and higher bioavailability compared to both, the docetaxel solution and the free docetaxel NC formulation.
Cell viability assay, MTT, on Walker 256 cells could demonstrate, those blank microcapsules alone have a certain toxic effect due to the cytotoxicity of Eudragit L. A higher intrinsic cytotoxic activity can be seen when testing free docetaxel nanoparticles.
Interestingly, there were even more pronounced cytotoxic impacts of nanocapsulated docetaxel microparticles than blank microparticles and Docetaxel in solution. At a Docetaxel concentration around 5 µg/mL, it could be observed that cytotoxicity increased significantly compared with the free solution and blank nanocapsules. These results could be found for an incubation time of 72 hours in a cell growth medium. When incubating only for 3 hours, we can surprisingly see that the docetaxel solution has a significantly higher cytotoxic effect than the microparticle system. Comparable results could be observed in the studies of M. Subudhi, where coated nanoparticles had less cytotoxicity effect than free nanoparticles and free drug solution. It can be assumed that the enteric coating could be the reason for this result. Only 3 hours of incubation may not be enough time for the polymer to degrade, which might show a delayed-release profile because Eudragit L is only soluble at pH level above 6.Citation156 Furthermore, the microcapsules showed promising stability for one hour in a solution with a pH of 1.2, whereas a complete degradation of the enteric coating could be observed at pH value of 7.5.
The study was also able to show, that an enormous improvement in docetaxel oral absorption was achieved by this nanoparticle system. A higher bioavailability and lower clearance could be observed compared to both the docetaxel solution and the docetaxel NCs injected intravenously.Citation157
Sodium Alginate-Eudragit Nanoparticle
Research conducted by Ma (2013) shows a double coating of indomethacin-complexed Eudragit RS nanoparticles that are incorporated into alginate microcapsules successfully delivering indomethacin to the colon with 60% drug loading. The nanoparticles’ size ranges from 116 nm with a drug loading of 5%. The entrapment efficiency increases when the nanoparticle-microcapsule system was drained (0.7% alginate). The nanoparticles also release the drug immediately in the gastric and intestinal tract by 90%. But, the double coating using alginate as the outer layer can maintain the drug entrapment up to the colon and reduce systemic toxicity from indomethacin.Citation152 Meanwhile, research conducted by Rajpoot and Jain (2020) resulted in targeted cancer drug delivery to colon cancer cells using radio labelling and the addition of folic acid to solid lipid nanoparticles as ligand cell targeting. The solid lipid nanoparticles (SLN) that have been labeled and given the ligand were then encapsulated into the S-100 eudragit polymer and alginate. The results showed that the maximum drug accumulation was achieved to the cancerous tissue in the colon in the alb/c mouse model.Citation153
Albumin Nanoparticle
The delivery system of indomethacin to the colon has also been studied by Cerchiara et al by using a multi-coating system. The increased solubility of Indomethacin was significant with increasing cyclodextrin concentrations. However, the swelling index of the indomethacin-cyclodextrin complex was smaller than that of indomethacin-albumin. The swelling index of Indomethacin-cyclodextrin-albumin was greater in an alkaline atmosphere with the release rate following zero order. However, this system still releases some drugs in an acidic atmosphere. With a multi-coating system using Eudragit as the outer layer, the release of drugs in an acidic atmosphere can be reduced.Citation154
Size and Mechanism Comparison
Some of the encapsulation forms described in the previous sections vary in size and shape of the system used. This difference will lead to different deliveries and endocytosis mechanisms. The comparison of size and coating system can be seen in .
Figure 5 The comparison of the size and shape of the coating system in the enteric-coated nanoparticle formulations for colorectal cancer. (A) chitosan-PMMA-Eudragit-coated drug, (B) eudragit-coated drug-cyclodextrin-bovine serum albumin complexes, (C) eudragit-chitosan-coated drug, (D) eudragit-pectin-coated drug, (E) hyaluronic acid-chitosan-coated drug, (F) PLGA-coated drug, (G) pectin-coated drug, (H) chitosan-coated drug.
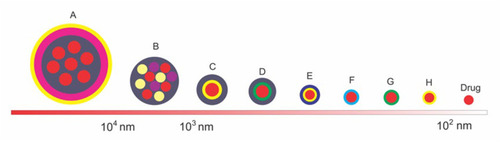
describes that the enteric-coated nanoparticle drug formulations for colorectal cancer system can be in the form of a single drug coating or in the form of a matrix. The single form of coating generally aims to prevent drug aggregation and targeted deliveryCitation39,Citation127,Citation153 while the matrix form aims to increase solubility and controlled release.Citation150,Citation154 For anionic charged drugs that reach target cells in the 102 nm size will be uptaken via the clathrin pathway. Whereas those with cationic and neutral charges with a larger size (not to exceed 104 nm) will be uptaken via other channels besides clathrin.Citation141
As enteric-coated polymer, eudragit releases nearly 90% drug in a zero-order pattern at 10 h time at small intestinal pH. Meanwhile, ethylcellulose and cellulose acetate phthalate released 90% of the drug at 20 hours. When compared with these polymers, shellac has a more sensitive response to intestinal pH where drug release reaches 100% at 10 hours.Citation158 However, when compared to natural polymers, eudragit is more reproducible and is not absorbed, so they do not give systemic side effects.Citation159
The Perspective of the Author
In the last decades, researchers have been developing and using nanoparticle systems with controlled drug release profiles. It was possible to elaborate nanoparticles for sustained and delayed drug release, which prolongs release and extends the drug effect, leading to a significant increase of bioavailability.
Chitosan polymer is often used in nanoparticle technology, as studies prove that it shows low toxicity. Besides, it is biodegradable and biocompatible.Citation160
In order to improve the delivery efficiency of oral nanoparticle systems even more, the enteric coating seems to be a promising approach, but every dosage form taken orally has to face the problem of degradation, resulting in reduced oral availability. This can be prevented by enteric coating. So far enteric-coated nanoparticles for various purposes have been developed. For instance, oral delivery of insulin,Citation94,Citation111,Citation161,Citation162 antihypertensive agents,Citation97 anti-glioma therapeutics,Citation163 and cancer therapeutics for colorectal cancer.
As for nanoparticle system in colorectal cancer treatment, Eudragit has been used commonly as an enteric coating. These copolymers are perfectly suitable for colon-specific targeting as their dissolution is pH-depended, which is necessary since there are several milieus of different pH levels throughout the gastrointestinal tract,Citation156 making it a perfect option as an enteric coating of oral nanoparticle systems. In the previously mentioned articles, Eudragit S100 was commonly used taking advantage of the fact that it dissolves above a pH level of 7, which makes it perfectly suitable for colon-specific release.
As a summary, the reviewed articles discussed the enteric coating of the nanoparticles significantly improves the delayed and prolonged drug release in the colon. Also, the sustained drug release profile can be enhanced. Thus, a more localized release at the tumor site could be achieved leading to a significantly enhanced antitumor activity.
Moreover, nanoparticles modified with ligand seem to be a promising attempt to improve specific active colorectal tumor targeting, although they did not show optimal characteristics compared to non-modified nanoparticles. Therefore, more research has to be conducted to optimize these modified nanoparticles, but other than that, combining enteric coating with modified nanoparticles could enhance the colon-specific targeting and release, which is essential for antitumor agents in colorectal cancer therapy.
As for the anticancer activity, in vitro studies should consider the characteristics of enteric coating. Cell toxicity assays should be tested in a medium similar to the colonic fluids instead of acidic cell medium. Only then, studies can be informative and comparable.
Further, more in vivo studies should be taken into consideration that optimal colon-targeted drug release depends on many various factors, which can hardly be simulated fully in vitro.
Conclusion
In recent years the field of nanotechnology in cancer treatment has been expanding rapidly. Nanoparticles used as oral drug delivery systems seem to be one of the most promising approaches in colorectal cancer therapy these days. The development of different formulations and technologies has been carried out to improve colon-specific drug delivery by controlled drug release. The overall research, which has been conducted so far, showed that enteric-coated nanoparticles were able to enhance the controlled drug release. An improved delayed, prolonged, and sustained drug release profile in the colonic area could be confirmed, leading to an enhanced antitumor activity on tumor cells. Enteric-coated nanoparticles showed a great potential to increase the effect of anticancer agents like 5-FU significantly, which could be a big next step in colorectal cancer therapy.
Furthermore, for the future development of drug delivery systems in colorectal cancer therapy not only target pH-stimulated release but also combined with specific targeting of colorectal cancer cells. The interaction between the targeted ligands decorated on the nanoparticle surface and certain receptors that are overexpressed at the diseased site or certain cells is expected to increase the adhesion and internalization of the nanoparticles. This effect will lead to selective drug accumulation at the target site which can increase therapeutic efficacy and reduce side effects.
Disclosure
The authors report no conflicts of interest for this work.
Acknowledgment
Many thanks to Lisna Meylina for the valuable advice and suggestions in the writing of the manuscript.
References
- BrayF, FerlayJ, SoerjomataramI, SiegelRL, TorreLA, JemalA. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.2149230207593
- SiegelRL, MillerKD, Goding SauerA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi:10.3322/caac.2160132133645
- BrouwerNPM, BosACRK, LemmensVEPP, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143(11):2758–2766. doi:10.1002/ijc.3178530095162
- HaggarFA, BousheyRP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. doi:10.1055/s-0029-124245821037809
- HäfnerMF, DebusJ. Radiotherapy for colorectal cancer: current standards and future perspectives. Visc Med. 2016;32(3):172–177. doi:10.1159/00044648627493944
- AielloP, SharghiM, MansourkhaniSM, et al. Medicinal plants in the prevention and treatment of colon cancer. Oxid Med Cell Longev. 2019;2019:1–51. doi:10.1155/2019/2075614
- ArnoldM, SierraMS, LaversanneM, SoerjomataramI, JemalA, BrayF. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi:10.1136/gutjnl-2015-31091226818619
- MishraJ, DrummondJ, QuaziSH, et al. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit Rev Oncol Hematol. 2013;86(3):232–250. doi:10.1016/j.critrevonc.2012.09.01423098684
- BertelsenCA, NeuenschwanderAU, JansenJE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16(2):161–168. doi:10.1016/S1470-2045(14)71168-425555421
- AngeneteE. The importance of surgery in colorectal cancer treatment. Lancet Oncol. 2019;20(1):6–7. doi:10.1016/S1470-2045(18)30679-X30545751
- GreenBL, MarshallHC, CollinsonF, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100(1):75–82. doi:10.1002/bjs.894523132548
- TerriereL, HolvoetJ, SchrijversD. Colorectal cancer. ESMO Handb Cancer Prev. 2008;1:127–135. doi:10.1038/nrdp.2015.65
- TevisSE, KennedyGD. Postoperative complications: looking forward to a safer future. Clin Colon Rectal Surg. 2016;29(3):246–252. doi:10.1055/s-0036-158450127582650
- ClimentM, MartinST. Complications of laparoscopic rectal cancer surgery. Mini-Invasive Surg. 2018;2018. doi:10.20517/2574-1225.2018.62.
- Chiu-C-C, LinW-L, ShiH-Y, et al. Comparison of oncologic outcomes in laparoscopic versus open surgery for non-metastatic colorectal cancer: personal experience in a single institution. J Clin Med. 2019;8(6):875. doi:10.3390/jcm8060875
- BedirliA, SalmanB, YukselO. Laparoscopic versus open surgery for colorectal cancer: a retrospective analysis of 163 patients in a single institution. Minim Invasive Surg. 2014;2014:1–6. doi:10.1155/2014/530314
- SongX-J, LiuZ-L, ZengR, YeW, LiuC-W. A meta-analysis of laparoscopic surgery versus conventional open surgery in the treatment of colorectal cancer. Medicine (Baltimore). 2019;98(17):e15347. doi:10.1097/MD.000000000001534731027112
- YangX-F, PanK. Diagnosis and management of acute complications in patients with colon cancer: bleeding, obstruction, and perforation. Chin J Cancer Res. 2014;26(3):331–340. doi:10.3978/j.issn.1000-9604.2014.06.1125035661
- KirchhoffP, ClavienP-A, HahnloserD. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4(1):5. doi:10.1186/1754-9493-4-520338045
- BeraldoFB, YusufSAI, PalmaRT, KharmandayanS, GoncalvesJE, WaisbergJ. Urinary dysfunction after surgical treatment for rectal cancer. Arq Gastroenterol. 2015;52(3):180–185. doi:10.1590/S0004-2803201500030000526486283
- CicchettiA, AvuzziB, PaloriniF, et al. Predicting late fecal incontinence risk after radiation therapy for prostate cancer: new insights from external independent validation. Int J Radiat Oncol. 2018;102(1):127–136. doi:10.1016/j.ijrobp.2018.05.013
- BirgissonH, PåhlmanL, GunnarssonU, GlimeliusB. Late adverse effects of radiation therapy for rectal cancer – a systematic overview. Acta Oncol (Madr). 2007;46(4):504–516. doi:10.1080/02841860701348670
- LangeMM, van de VeldeCJH. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol. 2011;8(1):51–57. doi:10.1038/nrurol.2010.20621135876
- LangeMM, den DulkM, BossemaER, et al. Risk factors for faecal incontinence after rectal cancer treatment. Br J Surg. 2007;94(10):1278–1284. doi:10.1002/bjs.581917579345
- LangeMM, van de VeldeCJH. Faecal and urinary incontinence after multimodality treatment of rectal cancer. PLoS Med. 2008;5(10):e202. doi:10.1371/journal.pmed.005020218842066
- KimJH, JenrowKA, BrownSL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J. 2014;32(3):103–115. doi:10.3857/roj.2014.32.3.10325324981
- MalamY, LoizidouM, SeifalianAM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi:10.1016/j.tips.2009.08.00419837467
- BriggerI, DubernetC, CouvreurP. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2012;64(SUPPL):24–36. doi:10.1016/j.addr.2012.09.006
- ZhangN, YinY, XuSJ, ChenWS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13(8):1551–1569. doi:10.3390/molecules1308155118794772
- ChoK, WangX, NieS, ChenZ, ShinDM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi:10.1158/1078-0432.CCR-07-144118316549
- LuqmaniYA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(SUPPL. 1):35–48. doi:10.1159/000086183
- TummalaS, Satish KumarMN, PrakashA. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm J. 2015;23(3):308–314. doi:10.1016/j.jsps.2014.11.01026106279
- ŠtabucB. Systemic therapy for colorectal cancer. Arch Oncol. 2003;11(4):255–263. doi:10.2298/AOO0304255S
- Pérez-HerreroE, Fernández-MedardeA. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi:10.1016/j.ejpb.2015.03.01825813885
- EdwardsMS, ChaddaSD, ZhaoZ, BarberBL, SykesDP. A systematic review of treatment guidelines for metastatic colorectal cancer. Color Dis. 2012;14(2):31–47. doi:10.1111/j.1463-1318.2011.02765.x
- TolJ, KoopmanM, CatsA, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009.
- LeeG, JoungJY, ChoJH, SonCG, LeeN. Overcoming P-glycoprotein-mediated multidrug resistance in colorectal cancer: potential reversal agents among herbal medicines. Evid Based Compl Altern Med. 2018;2018:1–9. doi:10.1155/2018/3412074
- BahramiB, Hojjat-FarsangiM, MohammadiH, et al. Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett. 2017;190:(April):64–83. doi:10.1016/j.imlet.2017.07.015
- JainA, JainSK, GaneshN, BarveJ, BegAM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine Nanotechnology, Biol Med. 2010;6(1):179–190. doi:10.1016/J.NANO.2009.03.002
- RuoslahtiE, BhatiaSN, SailorMJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188(6):759–768. doi:10.1083/jcb.20091010420231381
- DadwalA, BaldiA, Kumar NarangR. Nanoparticles as carriers for drug delivery in cancer. Artif Cells Nanomed Biotechnol. 2018;46(sup2):295–305. doi:10.1080/21691401.2018.145703930043651
- SykesEA, ChenJ, ZhengG, ChanWCWW. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano. 2014;8(6):5696–5706. doi:10.1021/nn500299p24821383
- YuX, TraseI, RenM, DuvalK, GuoX, ChenZ. Design of nanoparticle-based carriers for targeted drug delivery. J Nanomater. 2016;2016:1–15. doi:10.1155/2016/1087250
- GondaA, ZhaoN, ShahJV, et al. Engineering tumor-targeting nanoparticles as vehicles for precision nanomedicine. Med One. 2019;4. doi:10.20900/mo.20190021
- BazakR, HouriM, El AchyS, et al. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2016;141(5):769–784. doi:10.1007/s00432-014-1767-3.Cancer
- AmidonS, BrownJE, DaveVS. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS Pharm Sci Tech. 2015;16(4):731–741. doi:10.1208/s12249-015-0350-9
- PridgenEM, AlexisF, FarokhzadOC. Polymeric nanoparticle technologies for oral drug delivery. Clin Gastroenterol Hepatol. 2014;12(10):1605–1610. doi:10.1016/j.cgh.2014.06.01824981782
- HussanSD. A review on recent advances of enteric coating. IOSR J Pharm. 2012;2(6):05–11. doi:10.9790/3013-2610511
- LeopoldCS. Coated dosage forms for colon-specific drug delivery. Pharm Sci Technol Today. 1999;2(5):197–204. doi:10.1016/S1461-5347(99)00151-010322382
- HaoS, WangB, WangY, XuY. Enteric-coated sustained-release nanoparticles by coaxial electrospray: preparation, characterization, and in vitro evaluation. J Nanoparticle Res. 2014;16(2):2204. doi:10.1007/s11051-013-2204-2
- LiC, ZhouK, ChenD, et al. Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int J Nanomedicine. 2019;14:1619–1631. doi:10.2147/IJN.S18347930880969
- BhadraS, PrajapatiA, BhadraD. Development of pH sensitive polymeric nanoparticles of erythromycin stearate. J Pharm Bioallied Sci. 2016;8(2):135. doi:10.4103/0975-7406.17169127134466
- Shahdadi SardoH, SaremnejadF, BagheriS, AkhgariA, Afrasiabi GarekaniH, SadeghiF. A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int J Pharm. 2019;558:367–379. doi:10.1016/j.ijpharm.2019.01.02230664993
- KhotimchenkoM. Pectin polymers for colon-targeted antitumor drug delivery. Int J Biol Macromol. 2020;158:1110–1124. doi:10.1016/j.ijbiomac.2020.05.002
- LangX, WangT, SunM, ChenX, LiuY. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: a review. Int J Biol Macromol. 2020;154:433–445. doi:10.1016/j.ijbiomac.2020.03.14832194103
- MarleyRA, NanH. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;96(2):105–114. doi:10.2169/naika.96.200
- SiegelR, DeSantisC, JemalA. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi:10.3322/caac.2122024639052
- NaeemM, AwanUA, SubhanF, et al. Advances in colon-targeted nano-drug delivery systems: challenges and solutions. Arch Pharm Res. 2020;43(1):153–169. doi:10.1007/s12272-020-01219-031989477
- DongY, ZhouJ, ZhuY, et al. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies. Biosci Rep. 2017;37(6):1–12. doi:10.1042/BSR20170945
- MaY, YangY, WangF, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8:1. doi:10.1371/journal.pone.0053916
- TsilidisKK, KasimisJC, LopezDS, NtzaniEE, IoannidisJPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350(January):1–11. doi:10.1136/bmj.g7607
- YouX, KangY, HollettG, et al. Polymeric nanoparticles for colon cancer therapy: overview and perspectives. J Mater Chem B. 2016;4(48):7779–7792. doi:10.1039/c6tb01925k32263770
- JeonG, KoYT. Enhanced photodynamic therapy via photosensitizer-loaded nanoparticles for cancer treatment. J Pharm Investig. 2019;49(1). doi:10.1007/s40005-017-0363-3
- WilczewskaAZ, NiemirowiczK, MarkiewiczKH, CarH. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–1037. doi:10.1016/S1734-1140(12)70901-523238461
- TiwariJN, TiwariRN, KimKS. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog Mater Sci. 2012;57(4):724–803. doi:10.1016/j.pmatsci.2011.08.003
- LiZ, TanS, LiS, ShenQ, WangK. Cancer drug delivery in the nano era: an overview and perspectives (Review). Oncol Rep. 2017;38(2):611–624. doi:10.3892/or.2017.571828627697
- BehzadiS, SerpooshanV, TaoW, et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46(14):4218–4244. doi:10.1039/C6CS00636A28585944
- ForoozandehP, AzizAA. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. 2018;13(1):339. doi:10.1186/s11671-018-2728-630361809
- GouM. Promising application of nanotechnology in anticancer drug delivery. Drug Des Open Access. 2013;02:02. doi:10.4172/2169-0138.1000e117
- KumarM, SharmaHK. Targeted nanotechnology for anticancer drug delivery: current issue and challenge. J Drug Deliv Ther. 2018;8(5):23–27. doi:10.22270/jddt.v8i5.1882
- RodzinskiA, GuduruR, LiangP, et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci Rep. 2016;6(1):20867. doi:10.1038/srep2086726875783
- SutradharKB, AminML. Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnol. 2014;2014:1–12. doi:10.1155/2014/939378
- XinY, YinM, ZhaoL, MengF, LuoL. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol Med. 2017;14(3):228. doi:10.20892/j.issn.2095-3941.2017.005228884040
- SafhiMM, SivakumarSM, JabeenA, et al. Nanoparticle system for anticancer drug delivery: targeting to overcome multidrug resistance In: Grumezesc Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics. Elsevier; 2017:159–169. doi:10.1016/B978-0-323-52725-5.00008-3
- LiuJ, HuangY, KumarA, et al. PH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32(4):693–710. doi:10.1016/j.biotechadv.2013.11.00924309541
- KhatoonN, AlamH, KhanA, RazaK, SardarM. Ampicillin silver nanoformulations against multidrug resistant bacteria. Sci Rep. 2019;9(1):6848. doi:10.1038/s41598-019-43309-031048721
- YangJ, ZhangH, ChenB. Application of nanoparticles to reverse multi-drug resistance in cancer. Nanotechnol Rev. 2016;5:5. doi:10.1515/ntrev-2016-0023
- AlMatarM, MakkyEA, VarI, KoksalF. The role of nanoparticles in the inhibition of multidrug-resistant bacteria and biofilms. Curr Drug Deliv. 2018;15(4):470–484. doi:10.2174/156720181566617120716350429219055
- Chand DakalT, PalM. Nanotechnology for combating multi-drug resistance: a next generation antimicrobial therapy. J Drug Metab Toxicol. 2017;08:02. doi:10.4172/2157-7609-C1-009
- KwonS-I, KyungK-H, ParkJ-Y, et al. Uniform anti-reflective films fabricated by layer-by-layer ultrasonic spray method. Colloids Surf A Physicochem Eng ASP. 2019;580((August):123785):123785. doi:10.1016/j.colsurfa.2019.123785
- KotelevetsL, ChastreE, DesmaëleD, CouvreurP. Nanotechnologies for the treatment of colon cancer: from old drugs to new hope. Int J Pharm. 2016;514(1):24–40. doi:10.1016/j.ijpharm.2016.06.00527863668
- BodmeierR, ChenH, PaeratakulO, NovelA. Approach to the oral delivery of micro- or nanoparticles. Pharm Res an off J Am Assoc Pharm Sci. 1989;6(5):413–417. doi:10.1023/A:1015987516796
- FattalE, YoussefM, CouvreurP, AndremontA. Treatment of experimental salmonellosis in mice with ampicillin-bound nanoparticles. Antimicrob Agents Chemother. 1989;33(9):1540–1543. doi:10.1128/AAC.33.9.15402684009
- CollnotEM, AliH, LehrCM. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release. 2012;161(2):235–246. doi:10.1016/j.jconrel.2012.01.02822306429
- KoziolekM, GrimmM, BeckerD, et al. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the IntelliCap® system. J Pharm Sci. 2015;104(9):2855–2863. doi:10.1002/jps.2427425411065
- ZhangS, LangerR, TraversoG. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today. 2019;176(1):82–96. doi:10.1016/j.physbeh.2017.03.040
- Al-GousousJ, TsumeY, FuM, SalemII, LangguthP. Unpredictable performance of pH-dependent coatings accentuates the need for improved predictive in vitro test systems. Mol Pharm. 2017;14(12):4209–4219. doi:10.1021/acs.molpharmaceut.6b0087728199791
- TranTT-D, TranPH-L, PhanML-N, VanTV. Colon specific delivery of fucoidan by incorporation of acidifier in enteric coating polymer. Int J Pharm Biosci Technol. 2013;9(13):14.
- KhobragadeDS, Trambak PatilA, Shamrao KhobragadeD, et al. Development and evaluation of a hot-melt coating technique for enteric coating. Brazilian J Pharm Sci. 2012;48:1.
- LiP, HaoJ, LiH, GuanH, LiC. Development of an enteric nanoparticle of marine sulfated polysaccharide propylene glycol alginate sodium sulfate for oral administration: formulation design, pharmacokinetics and efficacy. J Pharm Pharmacol. 2018;70(6):740–748. doi:10.1111/jphp.1290229532471
- ThiyagarajanV, LinS-X, LeeC-H, WengC-F. A focal adhesion kinase inhibitor 16-hydroxy-cleroda-3,13-dien-16,15-olide incorporated into enteric-coated nanoparticles for controlled anti-glioma drug delivery. Colloids Surfaces B Biointerfaces. 2016;141:120–131. doi:10.1016/j.colsurfb.2016.01.03826851441
- SampathkumarK, RiyajanS, TanCK, DemokritouP, ChudapongseN, LooSCJ. Small-intestine-specific delivery of antidiabetic extracts from withania coagulans using polysaccharide-based enteric-coated nanoparticles. ACS Omega. 2019;4(7):12049–12057. doi:10.1021/acsomega.9b0082331460318
- HeH, ZhangX, ShengY. Enteric-coated capsule containing β-galactosidase-loaded polylactic acid nanocapsules: enzyme stability and milk lactose hydrolysis under simulated gastrointestinal conditions. J Dairy Res. 2014;81(4):479–484. doi:10.1017/S002202991400049125263933
- YuF, LiY, LiuCS, et al. Enteric-coated capsules filled with mono-disperse micro-particles containing PLGA-lipid-PEG nanoparticles for oral delivery of insulin. Int J Pharm. 2015;484(1–2):181–191. doi:10.1016/j.ijpharm.2015.02.05525724135
- FanW, XiaD, ZhuQ, et al. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials. 2018;151:13–23. doi:10.1016/j.biomaterials.2017.10.02229055774
- NguyenDN, PalangeticL, ClasenC, Van den MooterG. One-step production of darunavir solid dispersion nanoparticles coated with enteric polymers using electrospraying. J Pharm Pharmacol. 2016;68(5):625–633. doi:10.1111/jphp.1245926272245
- SunH, LiuD, LiY, TangX, CongY. Preparation and in vitro/in vivo characterization of enteric-coated nanoparticles loaded with the antihypertensive peptide VLPVPR. Int J Nanomedicine. 2014;9(1):1709–1716. doi:10.2147/IJN.S5609224729706
- SladekS, McCartneyF, EskanderM, et al. An enteric-coated polyelectrolyte nanocomplex delivers insulin in rat intestinal instillations when combined with a permeation enhancer. Pharmaceutics. 2020;12(3):259. doi:10.3390/pharmaceutics12030259
- XuB, ZhangW, ChenY, XuY, WangB, ZongL. Eudragit® L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int J Biol Macromol. 2018;113:534–542. doi:10.1016/j.ijbiomac.2018.02.01629408613
- ChaturvediK, GangulyK, KulkarniAR, et al. Oral insulin delivery using deoxycholic acid conjugated PEGylated polyhydroxybutyrate co-polymeric nanoparticles. Nanomedicine. 2015;10(10):1569–1583. doi:10.2217/nnm.15.3626008194
- HeZ, LiuZ, TianH, et al. Scalable production of core–shell nanoparticles by flash nanocomplexation to enhance mucosal transport for oral delivery of insulin. Nanoscale. 2018;10(7):3307–3319. doi:10.1039/C7NR08047F29384554
- RayL, KarthikR, SrivastavaV, et al. Efficient antileishmanial activity of amphotericin B and piperine entrapped in enteric coated guar gum nanoparticles. Drug Deliv Transl Res. 2020. doi:10.1007/s13346-020-00712-9
- SinhmarGK, ShahNN, ChokshiNV, KhatriHN, PatelMM. Process, optimization, and characterization of budesonide-loaded nanostructured lipid carriers for the treatment of inflammatory bowel disease. Drug Dev Ind Pharm. 2018;44(7):1078–1089. doi:10.1080/03639045.2018.143419429376433
- KankalaRK, KuthatiY, SieH-W, et al. Multi-laminated metal hydroxide nanocontainers for oral-specific delivery for bioavailability improvement and treatment of inflammatory paw edema in mice. J Colloid Interface Sci. 2015;458:217–228. doi:10.1016/j.jcis.2015.07.04426225492
- TayelSA, El-NabarawiMA, TadrosMI, Abd-ElsalamWH. Duodenum-triggered delivery of pravastatin sodium: II. Design, appraisal and pharmacokinetic assessments of enteric surface-decorated nanocubosomal dispersions. Drug Deliv. 2016;23(9):3266–3278. doi:10.3109/10717544.2016.117236727094305
- JogR, UnachukwuK, BurgessDJ. Formulation design space for stable, pH sensitive crystalline nifedipine nanoparticles. Int J Pharm. 2016;514(1):81–92. doi:10.1016/j.ijpharm.2016.08.03927863686
- SunL, LiuZ, TianH, et al. Scalable manufacturing of enteric encapsulation systems for site-specific oral insulin delivery. Biomacromolecules. 2019;20(1):528–538. doi:10.1021/acs.biomac.8b0153030537806
- González-AlvarezM, CollC, Gonzalez-AlvarezI, et al. Gated mesoporous silica nanocarriers for a “two-step” targeted system to colonic tissue. Mol Pharm. 2017;14(12):4442–4453. doi:10.1021/acs.molpharmaceut.7b0056529064714
- ShiY, LiK, TianB, et al. Oral delivery of human growth hormone: preparation, characterization, and pharmacokinetics. J Biomater Appl. 2017;31(6):851–858. doi:10.1177/088532821667434727742865
- BendasER, AbdelbaryAA. Instantaneous enteric nano-encapsulation of omeprazole: pharmaceutical and pharmacological evaluation. Int J Pharm. 2014;468(1–2):97–104. doi:10.1016/j.ijpharm.2014.04.03024746414
- WuZM, ZhouL, GuoXD, et al. HP55-coated capsule containing PLGA/RS nanoparticles for oral delivery of insulin. Int J Pharm. 2012;425(1–2):1–8. doi:10.1016/j.ijpharm.2011.12.05522248666
- MüllerC, PereraG, KönigV, Bernkop-SchnürchA. Development and in vivo evaluation of papain-functionalized nanoparticles. Eur J Pharm Biopharm. 2014;87(1):125–131. doi:10.1016/j.ejpb.2013.12.01224373995
- SalvioniL, FiandraL, Del CurtoMD, et al. Oral delivery of insulin via polyethylene imine-based nanoparticles for colonic release allows glycemic control in diabetic rats. Pharmacol Res. 2016;110:122–130. doi:10.1016/j.phrs.2016.05.01627181095
- LuoS, HaoJ, GaoY, LiuD, CaiQ, YangX. Pore size effect on adsorption and release of metoprolol tartrate in mesoporous silica: experimental and molecular simulation studies. Mater Sci Eng C. 2019;100:789–797. doi:10.1016/j.msec.2019.03.050
- AlaiM, LinWJ. Novel lansoprazole-loaded nanoparticles for the treatment of gastric acid secretion-related ulcers: in vitro and in vivo pharmacokinetic pharmacodynamic evaluation. AAPS J. 2014;16(3):361–372. doi:10.1208/s12248-014-9564-024519468
- NguyenDN, ClasenC, Van den MooterG. Encapsulating darunavir nanocrystals within Eudragit L100 using coaxial electrospraying. Eur J Pharm Biopharm. 2017;113:50–59. doi:10.1016/j.ejpb.2016.12.00227993734
- KumarPV, MakiMAA, WeiYS, et al. Rabbit as an animal model for pharmacokinetics studies of enteric capsule contains recombinant human keratinocyte growth factor loaded chitosan nanoparticles. Curr Clin Pharmacol. 2019;14(2):132–140. doi:10.2174/157488471466618112010390730457053
- EskandariS, VaraminiP, TothI. Formulation, characterization and permeability study of nano particles of lipo-endomorphin-1 for oral delivery. J Liposome Res. 2013;23(4):311–317. doi:10.3109/08982104.2013.80533923931529
- ShahMK, MadanP, LinS. Preparation, in vitro evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticles for lymphatic absorption via oral administration. Pharm Dev Technol. 2014;19(4):475–485. doi:10.3109/10837450.2013.79516923697916
- NguyenH-N, WeyS-P, JuangJ-H, et al. The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in vivo. Biomaterials. 2011;32(10):2673–2682. doi:10.1016/j.biomaterials.2010.12.04421256586
- ChenK, ChangHHR, ShalviriA, et al. Investigation of a new pH-responsive nanoparticulate pore former for controlled release enteric coating with improved processability and stability. Eur J Pharm Biopharm. 2017;120:116–125. doi:10.1016/j.ejpb.2017.08.01428887098
- RoyU, DingH, Pilakka KanthikeelS, et al. Preparation and characterization of anti-HIV nanodrug targeted to microfold cell of gut-associated lymphoid tissue. Int J Nanomedicine. 2015:5819. doi:10.2147/IJN.S68348.26425084
- AnwerMK, Al-ShdefatR, EzzeldinE, AlshahraniSM, AlshetailiAS, IqbalM. Preparation, evaluation and bioavailability studies of eudragit coated PLGA nanoparticles for sustained release of eluxadoline for the treatment of irritable bowel syndrome. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00844.
- BiswasS, ChattopadhyayM, SenKK, SahaMK. Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice. Carbohydr Polym. 2015;121:403–410. doi:10.1016/j.carbpol.2014.12.04425659715
- ZhaoX, ShanC, ZuY, et al. Preparation, characterization, and evaluation in vivo of Ins-SiO2-HP55 (insulin-loaded silica coating HP55) for oral delivery of insulin. Int J Pharm. 2013;454(1):278–284. doi:10.1016/j.ijpharm.2013.06.05123830939
- SuF-Y, LinK-J, SonajeK, et al. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials. 2012;33(9):2801–2811. doi:10.1016/j.biomaterials.2011.12.03822243802
- LiP, YangZ, WangY, et al. Microencapsulation of coupled folate and chitosan nanoparticles for targeted delivery of combination drugs to colon. J Microencapsul. 2015;32(1):40–45. doi:10.3109/02652048.2014.94494725198909
- NassarT, Attili-QadriS, Harush-FrenkelO, et al. High plasma levels and effective lymphatic uptake of docetaxel in an orally available nanotransporter formulation. Cancer Res. 2011;71(8):3018–3028. doi:10.1158/0008-5472.CAN-10-311821363913
- MahjubR, NajafabadiFK, DehkhodaeiN, et al. Eudragit L-100 capsules/aromatize and quaternerize chitosan for insulin nanoparticle oral delivery on toxic oxidative stress in rat liver and kidney. Pharm Nanotechnol. 2020;8. doi:10.2174/2211738508666200628033442
- YusC, IrustaS, SebastianV, ArrueboM. Controlling particle size and release kinetics in the sustained delivery of oral antibiotics using pH-independent mucoadhesive polymers. Mol Pharm. 2020;acs.molpharmaceut.0c00408. doi:10.1021/acs.molpharmaceut.0c00408
- ZhouK, YanY, ChenD, et al. Solid lipid nanoparticles for duodenum targeted oral delivery of tilmicosin. Pharmaceutics. 2020;12(8):731. doi:10.3390/pharmaceutics12080731
- SahuKK, KauravM, PandeyRS. Chylomicron mimicking solid lipid nanoemulsions encapsulated enteric minicapsules targeted to colon for immunization against hepatitis B. Int Immunopharmacol. 2019;66:317–329. doi:10.1016/j.intimp.2018.11.04130503974
- TummalaS, KuppusamyG, Satish KumarMN, PraveenTK, WadhwaniA. 5-Fluorouracil enteric-coated nanoparticles for improved apoptotic activity and therapeutic index in treating colorectal cancer. Drug Deliv. 2016;23(8):2902–2910. doi:10.3109/10717544.2015.111602626634385
- HosnyKM. Alendronate sodium as enteric coated solid lipid nanoparticles; preparation, optimization, and in vivo evaluation to enhance its oral bioavailability. Santos HA, ed. PLoS One. 2016;11(5):e0154926. doi:10.1371/journal.pone.015492627148747
- ValatMT. Mechanistic study of NVP-CGM097: A potent, selective and species specific inhibitor of p53-Mdm2. Drug Des Open Access. 2015;04:02. doi:10.4172/2169-0138.S1.008
- Amini-FazlMS, MohammadiR, KheiriK. 5‑Fluorouracil loaded chitosan/polyacrylic acid/Fe 3 O 4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int J Biol Macromol. 2019;132:506–513. doi:10.1016/j.ijbiomac.2019.04.00530951773
- ChoiJS, CaoJ, NaeemM, et al. Size-controlled biodegradable nanoparticles: preparation and size-dependent cellular uptake and tumor cell growth inhibition. Colloids Surfaces B Biointerfaces. 2014;122:545–551. doi:10.1016/j.colsurfb.2014.07.03025108477
- ChoiYH, HanHK. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig. 2018;48(1):43–60. doi:10.1007/s40005-017-0370-4
- BanerjeeA, QiJ, GogoiR, WongJ, MitragotriS. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–185. doi:10.1016/j.jconrel.2016.07.05127480450
- XiaoB, SiX, HanM, ViennoisE, ZhangM, MerlinD. Codelivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J Mater Chem B. 2015;3:7724–7733. doi:10.1039/c5tb01245g26617985
- AkincA, BattagliaG. Exploiting endocytosis for nanomedicines. Cold Spring Harb Perspect Biol. 2013;5(11):a016980–a016980. doi:10.1101/cshperspect.a01698024186069
- ChenS, GuoF, DengT, et al. Eudragit S100-coated chitosan nanoparticles co-loading tat for enhanced oral colon absorption of insulin. AAPS Pharm Sci Tech. 2017;18(4):1277–1287. doi:10.1208/s12249-016-0594-z
- KarnPR, VanićZ, PepićI, Škalko-BasnetN. Mucoadhesive liposomal delivery systems: the choice of coating material. Drug Dev Ind Pharm. 2011;37(4):482–488. doi:10.3109/03639045.2010.52342520961263
- NikamV, KotadeK, GawareV, et al. Eudragit a versatile polymer: a review. Pharmacologyonline. 2011;1:152–164.
- SunL, ChenY, ZhouY, et al. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J Pharm Sci. 2017;12(5):418–423. doi:10.1016/J.AJPS.2017.04.00232104354
- KhatikR, MishraR, VermaA, et al. Colon-specific delivery of curcumin by exploiting Eudragit-decorated chitosan nanoparticles in vitro and in vivo. J Nanoparticle Res. 2013;15:9. doi:10.1007/s11051-013-1893-x
- LiCF, LiYC, ChenLB, WangY, SunLB. Doxorubicin-loaded Eudragit-coated chitosan nanoparticles in the treatment of colon cancers. J Nanosci Nanotechnol. 2016;16(7):6773–6780. doi:10.1166/jnn.2016.11374
- JainA, JainS, JainR, KohliDV. Coated chitosan nanoparticles encapsulating caspase 3 activator for effective treatment of colorectral cancer. Drug Deliv Transl Res. 2015;5(6):596–610. doi:10.1007/s13346-015-0255-x26334865
- SubudhiMB, JainA, JainA, et al. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials (Basel). 2015;8(3):832–849. doi:10.3390/ma803083228787974
- MaY, FuchsAV, BoaseNRB, RolfeBE, CoombesAGA, ThurechtKJ. The in vivo fate of nanoparticles and nanoparticle-loaded microcapsules after oral administration in mice: evaluation of their potential for colon-specific delivery. Eur J Pharm Biopharm. 2015;94:393–403. doi:10.1016/j.ejpb.2015.06.01426117186
- KanthamneniN, ChaudharyA, WangJ, PhabhuS. Nanoparticulate delivery of novel drug combination regimens for the chemoprevention of colon cancer. Int J Oncol. 2010;37:177–185. doi:10.3892/ijo20514409
- MaY, CoombesAGA. Designing colon-specific delivery systems for anticancer drug-loaded nanoparticles: an evaluation of alginate carriers. J Biomed Mater Res. 2014;102(9):3167–3176. doi:10.1002/jbm.a.34988
- RajpootK, JainSK. Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: a dual-targeted approach. Int J Biol Macromol. 2020;151:830–844. doi:10.1016/j.ijbiomac.2020.02.13232061847
- CerchiaraT, BigucciF, CoraceG, ZecchiV, LuppiB. Eudragit-coated albumin nanospheres carrying inclusion complexes for oral administration of indomethacin. J Incl Phenom Macrocycl Chem. 2011;71(1–2):129–136. doi:10.1007/s10847-010-9916-z
- JainA, JainSK. Optimization of chitosan nanoparticles for colon tumors using experimental design methodology. Artif Cells Nanomedicine Biotechnol. 2016;44(8):1917–1926. doi:10.3109/21691401.2015.1111236
- ThakralS, ThakralNK, MajumdarDK. Eudragit®: a technology evaluation. Expert Opin Drug Deliv. 2013;10(1):131–149. doi:10.1517/17425247.2013.73696223102011
- NassarT, Attili-QadriS, Harush-FrenkelO, et al. High plasma levels and effective lymphatic uptake of docetaxel in an orally available nanotransporter formulation. Cancer Res. 2011;71(8):3018–3028. doi:10.1158/0008-5472.CAN-10-311821363913
- SinhaVR, KumriaR. Coating polymers for colon specific drug delivery: a comparative in vitro evaluation. Acta Pharm. 2003;53(1):41–47.14769251
- ThakralS, ThakralNK, MajumdarDK. Eudragit®: a technology evaluation. Expert Opin Drug Deliv. 2013;10(1):131–149. doi:10.1517/17425247.2013.73696223102011
- MohammedMA, SyedaJTM, WasanKM, WasanEK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9:4. doi:10.3390/pharmaceutics9040053
- SonajeK, ChenYJ, ChenHL, et al. Enteric-coated capsules filled with freeze-dried chitosan/poly(γ-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31(12):3384–3394. doi:10.1016/j.biomaterials.2010.01.04220149435
- AlaiMS, LinWJ, PingaleSS. Application of polymeric nanoparticles and micelles in insulin oral delivery. J Food Drug Anal. 2015;23(3):351–358. doi:10.1016/j.jfda.2015.01.00728911691
- ThiyagarajanV, LinSX, LeeCH, WengCF. A focal adhesion kinase inhibitor 16-hydroxy-cleroda-3,13-dien-16,15-olide incorporated into enteric-coated nanoparticles for controlled anti-glioma drug delivery. Colloids Surfaces B Biointerfaces. 2016;141:120–131. doi:10.1016/j.colsurfb.2016.01.03826851441