Abstract
Background
The formation of hypertrophic scars (HS) can result in the failure of glaucoma surgery, and fibrosis is known to be closely associated with the progression of HS. Dihydroartemisinin (DHA) has been reported to inhibit the progression of fibrosis; however, whether DHA can alleviate the formation of HS remains unclear.
Methods
In the present study, in order to examine the effects of DHA on the progression of HS, human Tenon’s capsule fibroblasts (HTFs) were isolated from patients who underwent glaucoma surgery. In addition, Western blot analysis, microtubule associated protein 1 light chain 3 α staining and reverse transcription-quantitative PCR were performed to detect protein and mRNA expression levels in the HTFs, respectively. Cell proliferation was detected by Ki67 staining. Flow cytometry was used to examine apoptosis and reactive oxygen species (ROS) levels in the HTFs.
Results
The results revealed that TGF-β promoted the proliferation and fibrosis of HTFs; however, DHA significantly reversed the effects of TGF-β by increasing cell autophagy. In addition, DHA notably induced the apoptosis of TGF-β-stimulated HTFs by increasing the ROS levels, while these increases were partially reversed by 3-methyladenine. Furthermore, DHA notably increased the expression of microRNA (miR)-145-5p in HTFs in a dose-dependent manner.
Conclusion
The present study demonstrated that DHA inhibits the TGF-β-induced fibrosis of HTFs by inducing autophagy. These findings may aid in the development of novel agents for the prevention of the formation of HS following glaucoma surgery.
Introduction
The formation of hypertrophic scars (HS) following glaucoma filtering surgery is the primary cause of surgical failure.Citation1 Moreover, it has been confirmed that fibrosis is closely associated with the formation of HS.Citation2,Citation3 At present, anti-metabolite drugs can inhibit scar formation following surgery; however, the use of these drugs may result in certain complications, including the formation of bubble fistula.Citation4 Therefore, it is necessary to explore safe and effective methods for the prevention of the formation of HS following glaucoma surgery.
Dihydroartemisinin (DHA) is a natural product originating from artemisinin, which exerts antitumor and anti-inflammatory effects in various diseases.Citation5–Citation7 It has previously been demonstrated that DHA inhibited the progression of fibrosis;Citation8,Citation9 however, whether DHA can alleviate the formation of HS remains unclear.
Autophagy is an important cellular process, which enables cytoplasmic components to move to the lysosome for degradation.Citation10 The aberrant occurrence of autophagy can lead to the dysfunction of cellular organelles.Citation11,Citation12 On the other hand, autophagy protects organisms from inflammatory injuries via the modulation of the apoptotic cascade.Citation13 Recent studies have revealed that autophagy plays a crucial role in the formation of HS;Citation13–Citation15 however, the association between DHA and cell autophagy in HS remains unclear. 3-methyladenine (3-MA) is an effective autophagy inhibitor,Citation16 which functions by inhibiting the formation of the autophagosome.Citation17 In the present study, 3-MA was used to investigate the association between DHA and cell autophagy. The findings of the present study may aid in the development of novel agents for the prevention of the formation of HS following glaucoma surgery.
Materials and Methods
Isolation of Tenon’s Capsule Fibroblasts
Tenon’s capsule fibroblasts (HTFs) and corneal epithelial cells (control cells) were isolated from patients with primary glaucoma (PG) who had undergone glaucoma surgery at The First Affiliated Hospital of Harbin Medical University (Harbin, China). The procedure of cell isolation was performed as previously described.Citation18 The morphology of the HTFs and corneal epithelium cells was observed under a microscope. To mimic the formation of HS in vitro, HTFs were stimulated with 10 ng/mL TGF-β (Sigma-Aldrich; Merck KGaA) as previously described.Citation19,Citation20 The present study was approved by the Ethics Committee of Harbin Medical University. Written informed consent was obtained from all patients.
Reagents
3-MA and DHA were purchased from MedChemExpress.
Cell Culture
HTFs were cultured in 90% RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, 1% penicillin (Thermo Fisher Scientific, Inc.) and 1% streptomycin (Thermo Fisher Scientific, Inc.), and maintained in an incubator with 5% CO2 at 37°C.
Reverse Transcription-Quantitative PCR
Total RNA was extracted from the HTFs using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized using a PrimeScript RT reagent kit (Takara Bio, Inc.). qPCR was performed on an ABI 7500 Real-Time PCR system using SYBR-Green methods. qPCR experiments were performed in triplicate using the following thermocycling conditions: Initial denaturation for 2 min at 95°C; followed by 35 cycles for 30 sec at 95°C and 45 sec at 60°C. The primers were obtained from Shanghai GenePharma Co., Ltd. and were as follows: MicroRNA (miRNA/miR)-145-5p forward, 5ʹ-CAGTTTTCCCAGGAATCCCT-3ʹ and reverse, 5ʹ-CTCAACTGGTGTCGTGGAGTC-3ʹ; miR-205-5p forward, 5ʹ-TTAATCCTTCATTCCACCGG-3ʹ and reverse, 5ʹ-CTCAACTGGTGTCGTGGAGTC-3ʹ; miR-486-5p forward, 5ʹ-ATCGTCCTGTACTGAGCTGCC-3ʹ and reverse, 5ʹ-CTCAACTGGTGTCGTGGAGTC-3ʹ; and U6 forward, 5ʹ-CTCGCTTCGGCAGCACAT-3ʹ and reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ. The 2−ΔΔCq method was used to quantify the results. The internal reference gene (U6) was used for normalization.
Western Blot Analysis
Total protein was isolated from the HTFs using RIPA lysis buffer (Thermo Fisher Scientific, Inc.). Protein was quantified using a BCA protein kit (Thermo Fisher Scientific, Inc.). Proteins were then separated via 10% SDS-PAGE and transferred onto PVDF membranes. Subsequently, the membranes were blocked with 3% non-fat milk for 1 h at room temperature and incubated with primary antibodies overnight at 4°C. Subsequently, membranes were incubated with an anti-rabbit secondary antibody (1:5000) for 1 h at room temperature. Finally, the membranes were visualized with an enhanced chemiluminescence (Thermo Fisher Scientific, Inc.) and the density of blots was analyzed using ImageJ software (National Institutes of Health). The primary antibodies used in the present study were as follows: Anti-α-smooth muscle actin (SMA; Abcam; 1:1000), anti-collagen I (Abcam; 1:1000), anti-survivin (Abcam; 1:1000), anti-autophagy related (ATG)5 (Abcam; 1:1000), anti-Beclin-1 (Abcam; 1:1000), anti-Bax (Abcam; 1:1000), anti-cleaved caspase 3 (Abcam; 1:1000) and anti-β-actin (Abcam; 1:1000). β-actin was used as an internal control. Moreover, the Western blot raw data are presented in the supplementary material.
Cell Counting Kit-8 Assay
Briefly, following treatment with DHA or/and TGF-β for 72 h, the HTFs were incubated with 10 μL cell counting kit-8 (CCK-8) reagent (Beyotime Institute of Biotechnology) for a further 2 h at 37°C. Finally, the absorbance of HTFs was measured at 450 nm using a microplate reader.
Immunofluorescence
HTFs were fixed with 4% paraformaldehyde, permeabilized and blocked at room temperature. The HTFs were then incubated with anti-Ki67, anti-vimentin, anti-keratin or anti-microtubule associated protein 1 light chain 3 α (LC3) antibody (Abcam; 1:1000) at 4°C overnight. Following the primary antibody incubation, cells were incubated with secondary antibody (IgG, Abcam; 1:5000) for 1 h. The nuclei were stained with DAPI (Beyotime Institute of Biotechnology). Finally, cells were observed under a fluorescence microscope.
Cell Apoptosis Assay
HTFs were seeded into a 6-well plate (1x106/well). The pellet was re-suspended with 100 μL binding buffer after centrifugation at 1000 rpm/min for 5 min. The cells were then incubated with 5 μL Annexin V-FITC and propidium (PI) for 15 min in the dark. The cell apoptotic rate was measured using a flow cytometer (BD Biosciences) and the results were analyzed using Fluorescence Activated Cell Sorting (FACS, BD Biosciences).
Reactive Oxygen Species Detection
Reactive oxygen species (ROS) detection was performed using the ROS detection kit (Beyotime Institute of Biotechnology). Cell suspensions were collected and stained with the ROS probe 2,7-dichlorofluorescein diacetate (DCFDA; Beyotime Institute of Biotechnology) as previously described.Citation21 Following 20 min of incubation, cells were centrifuged at 300 x g, washed with PBS and resuspended. Finally, the relative ROS levels were measured by flow cytometry (BD Biosciences), and the data were analyzed using FlowJo 10.0 software (FlowJo LLC).
ELISAs
The levels of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) in the supernatants of HTFs were detected using ELISA kits [Multisciences (Lianke) Biotech Co., Ltd.] according to the manufacturer’s instructions.
Statistical Analysis
All data are expressed as the mean ± standard error (SD). CCK-8 assay, immunofluorescence staining, reverse transcription-quantitative PCR (RT-qPCR), ROS detection, ELISA, flow cytometry and Western blot experiments were repeated in triplicate. Graphs were generated using GraphPad Prism software (version 7.0; GraphPad Software, Inc.). One-way ANOVAs and Tukey’s tests were performed for multiple group comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Successful Isolation of HTFs from Patients with PG
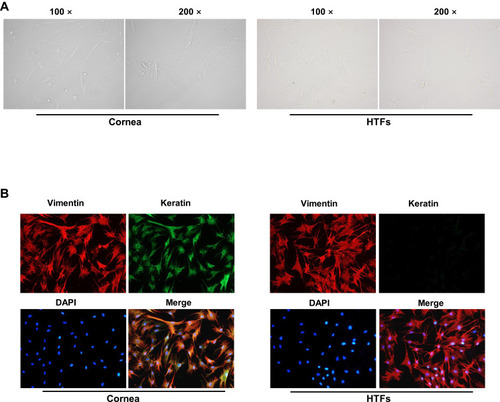
HTFs and corneal epithelial cells were isolated from patients with PG who underwent surgery, and the morphology of the cells was observed under a microscope (). In addition, immunofluorescence staining revealed that vimentin was extensively distributed, and keratin was rarely expressed in the HTFs, compared with the cornea epithelial cells (). These findings were consistent with the characteristic of HTFs,Citation22 indicating that the HTFs were successfully isolated from the patients.
DHA Significantly Reverses the TGF-β-Induced Proliferation of HTFs
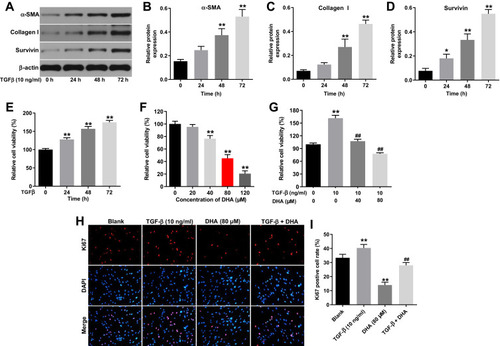
To mimic the formation of HS in vitro, HTFs were stimulated with 10 ng/mL TGF-β. As shown in –, TGF-β upregulated the expression levels of α-SMA, collagen I and survivin in the HTFs in a time-dependent manner. Moreover, TGF-β increased the viability of HTFs in a time-dependent manner (). By contrast, DHA inhibited the viability of the HTFs in a dose-dependent manner (). In addition, the TGF-β-induced increase in the viability of HTFs was significantly reversed by treatment with 40 or 80 μM DHA (). Since the TGF-β-stimulated HTFs were more susceptible to 80 μM DHA than 40 μM DHA, 80 μM DHA was selected for use in the following experiments. Notably, the TGF-β-induced proliferation of HTFs was notably reversed by DHA ().
Figure 2 DHA significantly reversed TGF-β-induced proliferation in HTFs.
DHA Inhibits the TGF-β-Induced Fibrosis of HTFs by Inducing Autophagy
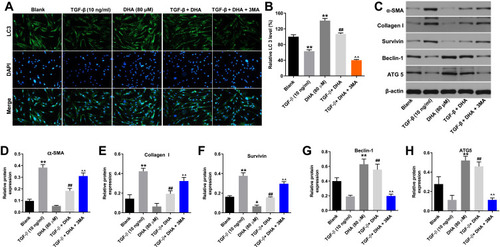
To investigate the association between DHA and cell autophagy, immunofluorescence staining was performed to determine LC3 expression. The results indicated that the expression of LC3 in HTFs was notably decreased by TGF-β, while this decrease was significantly reversed by DHA (). Moreover, the effect of DHA was partially reversed by 3-MA (), and DHA alone also significantly increased the level of LC3 in HTFs (). In addition, DHA markedly reversed the TGF-β-induced activation of α-SMA, collagen I and survivin in HTFs, while the effect of DHA on these proteins was partially reversed by 3-MA (–). By contrast, the expression levels of autophagy-related proteins (Beclin-1 and ATG5) in the TGF-β-stimulated HTFs were significantly increased by DHA, while this trend was significantly reversed by 3-MA (). Altogether, these results demonstrated that DHA inhibited the TGF-β-induced growth of HTFs by inducing autophagy.
Figure 3 DHA inhibited TGF-β-induced fibrosis in HTFs via inducing autophagy.
Autophagy Inhibitor Partially Reverses DHA-Induced Oxidative Stress in TGF-β-Stimulated HTFs
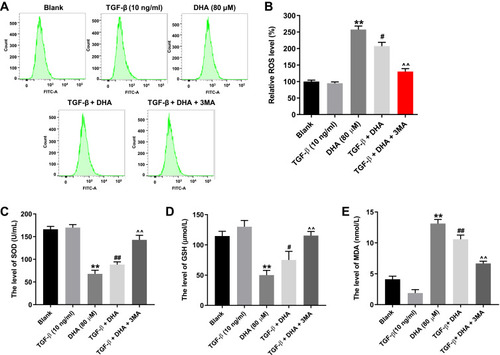
To further verify the association between DHA and cell autophagy in HTFs, flow cytometry was performed to detect the ROS levels. As shown in , DHA significantly increased the ROS levels in TGF-β-stimulated HTFs, while this increase was notably reversed in the presence of 3-MA. Moreover, DHA notably decreased the levels of SOD and GSH, and increased the levels of MDA in the supernatants of TGF-β-stimulated HTFs, while the effect of DHA on SOD, GSH and MDA levels was reversed in the presence of 3-MA (–). Furthermore, DHA alone also increased the ROS levels in HTFs via the mediation of SOD, GSH and MDA levels (–). Taken together, the autophagy inhibitor partially reversed DHA-induced oxidative stress in TGF-β-stimulated HTFs.
Figure 4 Autophagy inhibitor partially reversed DHA-induced oxidative stress in TGF-β-treated HTFs.
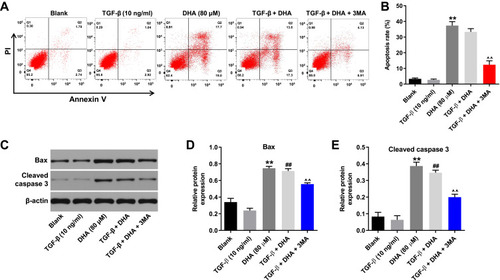
DHA-Induced Apoptosis of TGF-β-Stimulated HTFs is Partially Reversed by 3-MA
To investigate the association between cell apoptosis and autophagy in the formation of HS, flow cytometry was used. As shown in , DHA notably induced the apoptosis in TGF-β-stimulated HTFs, while this increase was markedly attenuated by 3-MA. Moreover, the DHA-induced upregulation of Bax and cleaved caspase 3 in TGF-β-stimulated HTFs was partially inhibited by 3-MA (–). Moreover, DHA alone induced the apoptosis pf HTFs via the activation of Bax and cleaved caspase 3 (–). Taken together, these results demonstrated that the DHA-induced apoptosis of TGF-β-stimulated HTFs was partially reversed by 3-MA.
Figure 5 DHA-induced apoptosis in TGF-β-treated HTFs was partially reversed by 3-MA.
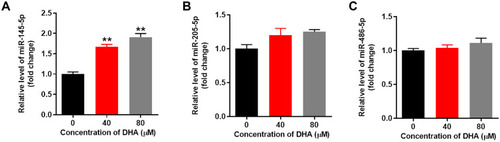
DHA Notably Upregulates the Expression of miR-145-5p in HTFs
Since miR-145-5p, miR-205-5p and miR-486-5p are involved in the formation of HS,Citation23–Citation25 RT-qPCR was performed to detect the effect of DHA on the expression of these miRNAs. The results discovered that DHA notably upregulated the expression of miR-145-5p in a dose-dependent manner (). However, DHA exerted minimal effects on miR-205-5p and miR-486-5p expression (). Thus, DHA notably upregulated the expression of miR-145-5p in HTFs.
Figure 6 DHA notably upregulated the expression of miR-145-5p in HTFs.
Discussion
It was previously reported that DHA exerted an effect in multiple diseases, including fibrosis.Citation8,Citation26,Citation27 In the present study, DHA inhibited the TGF-β-induced fibrosis of HTFs following glaucoma surgery by inducing autophagy. To the best of our knowledge, the present study was the first to determine the function of DHA in the formation of HS.
It is well-established that apoptosis is an important cellular process.Citation28,Citation29 In the present study, it was found that DHA inhibited the proliferation of TGF-β-stimulated HTFs by inducing apoptosis. Bax and cleaved caspase-3 are proapoptotic proteins.Citation30,Citation31 The present data revealed that DHA notably induced the apoptosis of HTFs via the upregulation of Bax and cleaved caspase-3. Importantly, autophagy is necessary for the onset of apoptosis, which usually affects the progression of apoptosis.Citation13,Citation32 Thus, it can be concluded that DHA induced the apoptosis of TGF-β-stimulated HTFs by inducing autophagy.
The present study found that DHA inhibited the fibrosis in HTFs via the inactivation of α-SMA, survivin and collagenI. A previous study indicated that α-SMA plays an important role in fibrosis.Citation12 In addition, collagen I and survivin are known to be positively associated with the progression of fibrosis.Citation33,Citation34 Consistent with these previous findings, in the present study, DHA inhibited the formation of HS via the inhibition of α-SMA, survivin and collagen I. Additionally, it has been reported that the activation of α-SMA promotes the epithelial-mesenchymal transition (EMT) process.Citation35,Citation36 Based on the previous research, it can be hypothesized that DHA may act as an inhibitor of the EMT process.
LC3, Beclin 1 and ATG5 have been confirmed to play regulatory roles in cell autophagy.Citation37,Citation38 Consistent with these data, the present study confirmed that DHA induced autophagy in HTFs via the mediation of these three proteins. In addition, Chen et alCitation5 indicated that DHA inhibited esophageal cancer cell migration via inducing autophagy. Thus, based on the aforementioned data, DHA may be used as a promoter of cell autophagy.
It has been reported that miR-145-5p participates in the formation of HS.Citation23 In the present study, it was found that DHA upregulated the expression of miR-145-5p in HTFs, and the present data were consistent with this previous finding. However, whether DHA mediates miR-145-5p to prevent the formation of HS warrants further investigation. Moreover, since Zhu et al found that DHA ameliorated asthma in mice via the mediation of miR-183c, the role of other miRNAs that may be regulated by DHA in the formation of HS should be investigated.
It should be stated that there are some limitations to the present study as follows: i) The association between miR-145-5p and cell autophagy remains unclear; ii) the association between DHA and miR-145-5p in HS warrants further investigation. Therefore, further investigations are required in the future.
In conclusion, the present study demonstrates that DHA inhibits the TGF-β-induced fibrosis and growth of HTFs by inducing autophagy. These findings may shed new light into the exploration of novel agents for the prevention of the formation of HS following glaucoma surgery.
Disclosure
The authors declared no competing interests in this work.
Additional information
Funding
References
- LiN, ZhangR. Silencing of p53 reduces cell migration in human tenon’s fibroblasts induced by TGF-beta. Int Ophthalmol. 2020;40(6):1509–1516. doi:10.1007/s10792-020-01320-932124130
- MarchesiniA, De FrancescoF, Mattioli-BelmonteM, et al. A new animal model for pathological subcutaneous fibrosis: surgical technique and in vitro analysis. Front Cell Dev Biol. 2020;8:542. doi:10.3389/fcell.2020.0054232850775
- WietechaMS, PensalfiniM, CangkramaM, et al. Activin-mediated alterations of the fibroblast transcriptome and matrisome control the biomechanical properties of skin wounds. Nat Commun. 2020;11(1):2604. doi:10.1038/s41467-020-16409-z32451392
- ZahorecP, SarkozyovaN, FerancikovaN, et al. Autologous mesenchymal stem cells application in post-burn scars treatment: a preliminary study. Cell Tissue Bank. 2021;22(1):39–46. doi:10.1007/s10561-020-09862-z32862394
- ChenX, HeLY, LaiS, HeY. Dihydroartemisinin inhibits the migration of esophageal cancer cells by inducing autophagy. Oncol Lett. 2020;20(4):94. doi:10.3892/ol.2020.1195532831913
- YangB, GaoX, SunY, et al. Dihydroartemisinin alleviates high glucose-induced vascular smooth muscle cells proliferation and inflammation by depressing the miR-376b-3p/KLF15 pathway. Biochem Biophys Res Commun. 2020;530(3):574–580. doi:10.1016/j.bbrc.2020.07.09532753318
- LeiZ, YangY, LiuS, et al. Dihydroartemisinin ameliorates dextran sulfate sodium induced inflammatory bowel diseases in mice. Bioorg Chem. 2020;100:103915. doi:10.1016/j.bioorg.2020.10391532450383
- ZhangB, LiuP, ZhouY, et al. Dihydroartemisinin attenuates renal fibrosis through regulation of fibroblast proliferation and differentiation. Life Sci. 2019;223:29–37. doi:10.1016/j.lfs.2019.03.02030862567
- YangDX, QiuJ, ZhouHH, et al. Dihydroartemisinin alleviates oxidative stress in bleomycin-induced pulmonary fibrosis. Life Sci. 2018;205:176–183. doi:10.1016/j.lfs.2018.05.02229752961
- LiuQ, LuoL, RenC, et al. The opposing roles of the mTOR signaling pathway in different phases of human umbilical cord blood-derived CD34(+) cell erythropoiesis. Stem Cells. 2020. doi:10.1002/stem.3268
- PantazopoulouM, BrembatiV, KanellidiA, et al. Distinct alpha-synuclein species induced by seeding are selectively cleared by the lysosome or the proteasome in neuronally differentiated SH-SY5Y cells. J Neurochem. 2020. doi:10.1111/jnc.15174
- MiyakeT, SakaiN, TamaiA, et al. Trehalose ameliorates peritoneal fibrosis by promoting snail degradation and inhibiting mesothelial-to-mesenchymal transition in mesothelial cells. Sci Rep. 2020;10(1):14292. doi:10.1038/s41598-020-71230-432868830
- XuY, SunQ, YuanF, et al. RND2 attenuates apoptosis and autophagy in glioblastoma cells by targeting the p38 MAPK signalling pathway. J Exp Clin Cancer Res. 2020;39(1):174. doi:10.1186/s13046-020-01671-232867814
- ShiW, WuY, BianD. p75NTR silencing inhibits proliferation, migration and extracellular matrix deposition of hypertrophic scar fibroblasts by activating autophagy through inhibiting PI3K/Akt/mTOR pathway. Can J Physiol Pharmacol. 2020. doi:10.1139/cjpp-2020-0219
- ShiJ, XiaoH, LiJ, et al. Wild-type p53-modulated autophagy and autophagic fibroblast apoptosis inhibit hypertrophic scar formation. Lab Invest. 2018;98(11):1423–1437. doi:10.1038/s41374-018-0099-330089855
- FangS, HuC, XuL, et al. All-trans-retinoic acid inhibits the malignant behaviors of hepatocarcinoma cells by regulating autophagy. Am J Transl Res. 2020;12(10):6793–6810.33194073
- LuYT, XiaoYF, LiYF, et al. Sulfuretin protects hepatic cells through regulation of ROS levels and autophagic flux. Acta Pharmacol Sin. 2019;40(7):908–918. doi:10.1038/s41401-018-0193-530560904
- TrelfordCB, DenstedtJT, ArmstrongJJ, HutnikCML. The pro-fibrotic behavior of human tenon’s capsule fibroblasts in medically treated glaucoma patients. Clin Ophthalmol. 2020;14:1391–1402. doi:10.2147/OPTH.S24591532546947
- TongJ, ChenF, DuW, ZhuJ, XieZ. TGF-beta1 induces human tenon’s fibroblasts fibrosis via miR-200b and its suppression of PTEN signaling. Curr Eye Res. 2019;44(4):360–367. doi:10.1080/02713683.2018.154926130512998
- LinLT, ChenJT, LuDW, et al. Antifibrotic role of low-dose mitomycin-c-induced cellular senescence in trabeculectomy models. PLoS One. 2020;15(6):e0234706. doi:10.1371/journal.pone.023470632574191
- FengL, SunZG, LiuQW, et al. Propofol inhibits the expression of Abelson nonreceptor tyrosine kinase without affecting learning or memory function in neonatal rats. Brain Behav. 2020;e01810.32869521
- QinX, WuK, ZuoC, LinM. The expression and role of hypoxia-induced factor-1alpha in human tenon’s capsule fibroblasts under hypoxia. Curr Eye Res. 2020;1–9. doi:10.1080/02713683.2020.1805470
- ShenW, WangY, WangD, et al. miR-145-5p attenuates hypertrophic scar via reducing Smad2/Smad3 expression. Biochem Biophys Res Commun. 2020;521(4):1042–1048. doi:10.1016/j.bbrc.2019.11.04031732152
- QiJ, LiuY, HuK, et al. MicroRNA-205-5p regulates extracellular matrix production in hyperplastic scars by targeting Smad2. Exp Ther Med. 2019;17(3):2284–2290. doi:10.3892/etm.2019.718730867712
- XiaoY. MiR-486-5p inhibits the hyperproliferation and production of collagen in hypertrophic scar fibroblasts via IGF1/PI3K/AKT pathway. J Dermatolog Treat. 2020;1–10. doi:10.1080/09546634.2020.1728210
- ShiX, LiS, WangL, et al. RalB degradation by dihydroartemisinin induces autophagy and IFI16/caspase-1 inflammasome depression in the human laryngeal squamous cell carcinoma. Chin Med. 2020;15(1):64. doi:10.1186/s13020-020-00340-y32577124
- TangM, WangR, FengP, et al. Dihydroartemisinin attenuates pulmonary hypertension via inhibition of pulmonary vascular remodeling in rats. J Cardiovasc Pharmacol. 2020;76(3):337–348. doi:10.1097/FJC.000000000000086232569012
- DuX, WangL, LiQ, et al. miR-130a/TGF-beta1 axis is involved in sow fertility by controlling granulosa cell apoptosis. Theriogenology. 2020;157:407–417. doi:10.1016/j.theriogenology.2020.08.01532871445
- QiC, LiuX, XiongT, WangD. Tempol prevents isoprenaline-induced takotsubo syndrome via the reactive oxygen species/mitochondrial/anti-apoptosis/p38 MAPK pathway. Eur J Pharmacol. 2020;886:173439. doi:10.1016/j.ejphar.2020.17343932871175
- RenP, XingL, HongX, ChangL, ZhangH. LncRNA PITPNA-AS1 boosts the proliferation and migration of lung squamous cell carcinoma cells by recruiting TAF15 to stabilize HMGB3 mRNA. Cancer Med. 2020;9(20):7706–7716. doi:10.1002/cam4.326832871048
- ShababS, GholamnezhadZ, MahmoudabadyM. Protective effects of medicinal plant against diabetes induced cardiac disorder: a review. J Ethnopharmacol. 2021;265:113328. doi:10.1016/j.jep.2020.11332832871233
- LvW, JiangJ, LiY, et al. MiR-302a-3p aggravates myocardial ischemia-reperfusion injury by suppressing mitophagy via targeting FOXO3. Exp Mol Pathol. 2020;117:104522. doi:10.1016/j.yexmp.2020.10452232866521
- YinF, WangWY, MaoLC, CaiQQ, JiangWH. Effect of human umbilical cord mesenchymal stem cells transfected with HGF on TGF-beta1/Smad signaling pathway in CCl4-induced liver fibrosis rats. Stem Cells Dev. 2020;29(21):1395–1406. doi:10.1089/scd.2020.006032867602
- CheM, KweonSM, TeoJL, et al. Targeting the CBP/beta-catenin interaction to suppress activation of cancer-promoting pancreatic stellate cells. Cancers (Basel). 2020;12(6):1476. doi:10.3390/cancers12061476
- LouH, LianC, ShiF, et al. The petri dish-N2B27 culture condition maintains rpe phenotype by inhibiting cell proliferation and mTOR activation. J Ophthalmol. 2020;2020:4892978. doi:10.1155/2020/489297832855817
- CavalleroS, Neves GranitoR, StockholmD, et al. Exposure of human skin organoids to low genotoxic stress can promote epithelial-to-mesenchymal transition in regenerating keratinocyte precursor cells. Cells. 2020;9(8):1912. doi:10.3390/cells9081912
- CaoC, WangW, LuL, et al. Inactivation of beclin-1-dependent autophagy promotes ursolic acid-induced apoptosis in hypertrophic scar fibroblasts. Exp Dermatol. 2018;27(1):58–63. doi:10.1111/exd.1341028767174
- ShiJH, HuDH, ZhangZF, et al. Reduced expression of microtubule-associated protein 1 light chain 3 in hypertrophic scars. Arch Dermatol Res. 2012;304(3):209–215. doi:10.1007/s00403-012-1204-x22237724