Abstract
Psoriatic arthritis (PsA) is a chronic inflammatory arthropathy associated with psoriasis (PsO). PsA could be considered an enthesal disease because of the link between mechanical stress (entheses) and immunologically active tissue (synovium). Evidence of efficacy of anti-tumor necrosis factor alpha (TNF-α) is supported by reduction of histological vascularity and immune cell infiltrates in synovial tissue after treatment. Certolizumab pegol (CZP) is a polyethylene glycolylated (PEGylated) Fab’ fragment of a humanized monoclonal antibody that binds and neutralizes human TNF-α. The PEG moiety of the Fab fragment, markedly increases the half-life of CZP and confers to the drug a unique structure that differs from the other anti-TNF-α agents tested for the treatment of Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, axial spondyloarthritis, nonradiographic spondyloarthritis, PsO, and PsA. In contrast to other anti-TNF-α agents, CZP did not mediate increased levels of apoptosis, suggesting that these mechanisms are not essential for the anti-TNF-α efficacy in Crohn’s disease. As CZP, infliximab, and adalimumab, but not etanercept, almost completely inhibited lipopolysaccharide-induced interleukin-1 beta release from monocytes, this cytokine-production inhibition may be relevant for drug efficacy. Due to these characteristics, it has been demonstrated in clinical studies that CZP effectively improves signs and symptoms of arthritis and physical function and skin manifestations of PsO, with a safety profile similar to rheumatoid arthritis. This drug can be considered as a valid treatment in patients affected by PsA. The efficacy and tolerability profiles suggest CZP as a suitable antipsoriatic drug in the treatment of PsA.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory arthropathy commonly associated with psoriasis (PsO). Several epidemiologic studies have examined the association between PsO and inflammatory arthritis. In studies performed in outpatients suffering from PsO, arthritis prevalence varied from 6% to 39%.Citation1 Joint disease is characterized by systemic inflammation and extensive synovitis, resulting in erosions of articular cartilage leading to joint destruction. Progressive damage begins early in the course of the disease as a consequence of the active inflammation, and results in radiological damage in up to 47% of patients at a median interval of 2 years, causing irreversible disability.Citation2 PsA belongs to the spondyloarthritis (SpA) group and affects primarily the peripheral joints, the spine, and the entheses. The pathogenesis of PsA is linked to innate immune response that generates high concentrations of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), which promotes effector function of a variety of tissue cells and thereby sustains the chronic inflammation leading to synovitis in PsA.Citation3 Overexpression of TNF-α is believed to play a key role in the pathogenic mechanisms linking PsO and arthritis.Citation4 This cytokine has been implicated in a number of inflammatory diseases, including PsA, by inducing the production of other inflammatory cytokines such as interleukin (IL)-1 and IL-6, chemokines like IL-8, and degradative enzymes, including several matrix metalloproteinases.Citation5 Moreover, it mediates a number of biological processes that can result in joint damage characterized by stimulation of bone resorption and inhibition of bone formation and of synthesis of proteoglycans. TNF-α may also contribute to vascular proliferation, which is probably one of the key and earlier observable changes in psoriasis and PsA.Citation6 Therapies neutralizing pivotal cytokines might not only limit joint damage and skin inflammation but also reduce the incidence of adverse metabolic and cardiovascular events in affected patients.Citation7 Several trials have shown excellent clinical results with anti-TNF-α agents, etanercept,Citation8 infliximab,Citation9 adalimumab,Citation10 and golimumab.Citation11 These agents have been proven to be effective in different aspects of the disease, including skin lesions, joint pain and swelling, enthesitis, and dactylitis, resulting in a significant improvement both in mobility and in radiographic progression and quality of life (QoL) parameters.Citation12 Recently, certolizumab pegol (CZP) has been tested and evaluated for several inflammatory diseases, and for its safety and efficacy profiles. This drug can also be considered for the treatment of PsA. In this review, we investigated the pathogenetic roles of TNFα in inflammatory arthritis as PsA, and the mechanisms of action of CZP. In particular, the evidence of efficacy of CZP in PsA will be explored.
Pathogenesis of PsA: link with TNF-α
Although the biological function of TNF-α is not completely clear in both normal and pathological conditions, elevated levels of TNF-α have been found in patients experiencing inflammatory diseases, and clinical data on the efficacy of TNF-α-blocking agents suggest that it plays a key role in the pathogenesis of various inflammatory disorders such as Crohn’s disease (CD), rheumatoid arthritis (RA), and SpA, as well as PsO and PsA.Citation13 PsA pathogenesis is incompletely understood, and a pathophysiological role of the synovium has been recently suggested. Some authors consider PsA as an enthesal disease linking mechanical stress (entheses) to immunologically active tissue (synovium).Citation4 A dermatological perspective brings an alternative view of autoimmunity that could partly explain the role of innate immunity. The skin may likely secrete proinflammatory mediators, such as TNF-α, to the affected proximal or distant joints, and the interruption of this pathological communication may represent a therapeutic targetCitation14 (). TNF-α produced by macrophage infiltration in synovial tissue may constitute the basis of synovium hypersensitization to endogenous ligands of the innate immune system.Citation15 Synovial tissue analysis has been used in order to distinguish PsA from other inflammatory arthritis, particularly in terms of pathological aspects and response to synthetic or biological disease-modifying antirheumatic drugs (DMARDs).Citation16 Recent findings suggest an important feature of PsA synovium: the presence of lymphoid aggregates of variable size and organization, previously considered highly specific of RA while recently observed in other inflammatory arthritis as PsA. Two major cytokines are involved in the pathogenesis of lymphoid aggregates: TNF-α and lymphotoxin beta. They play a potential role in the development of the high endothelial venule phenotype and in the expression of homing chemokines. Moreover, increased levels of these cytokines have been demonstrated in PsA synovial fluid.Citation17 It has been suggested that these structures linked to inflammatory cytokines may play a role in multiple processes, including antigen presentation, T-cell costimulation, and synthesis of soluble mediators.Citation18 For this reason, TNF-α-targeting agents represent a valuable therapeutic approach in treating such disorders as PsA. There are five marketed agents targeting TNF-α that possibly have a complex mechanism of action beyond the plain TNF-α neutralization. They differ in structure, pharmacokinetic, and in vitro properties, all of which may have relevant therapeutic implications.
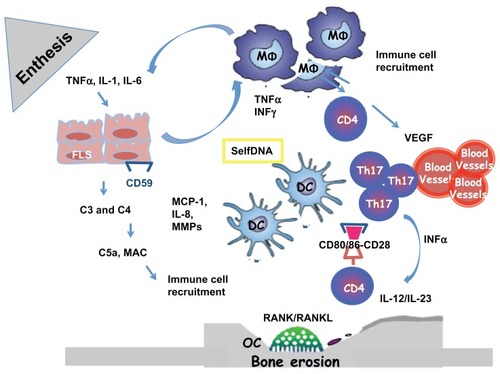
Figure 1 Enthesal and Joint pathology in Psoriatic Arthritis. The origin of Inflammation in the enthesal complex in PsA is multifactorial. When stimulated by stress, infections and trauma (Koebner phenomenon), in a genetic background, fibroblast-like synoviocytes express selfDNA or/ and secrete inflammatory cytokines and MMPs. They produce complement system factors as C3 and C4 and express less CD59 during inflammation, who is an inhibitor of complement system activation. The activation of the complement system lead to the release of C5a and production of MAC with the lysis of cells and recruitment of immune cells. FLS secrete MCP-1 and IL-8 necessary for recruitment and activation of MΦ. Polyclonal activation of CD4+Tcells and Th17 is the consequence of the immunological synapse with DC linked to the binding with CD80/86 and CD28. When activate CD4+ Tcells produce: RANKL with activation of OC with the consequence of bone erosions and VEGF with the activation of endothelial cells and the formation of HEV
Treatment of PsA: evidence of anti-TNF-α efficacy
Few studies have focused on the correlation between clinical composite scores and changes in PsA synovium. Treatment with DMARDs can modify synovial cell populations and infiltrates, correlating with the clinical improvement observed in treated patients.Citation19 One of the first studies establishing the efficacy of anti-TNF-α in modifying synovial tissue in PsA showed reduced vascularity and suppression of immune cells after treatment with infliximab.Citation20 In particular, the authors of this study evaluated the effects of anti-TNF-α treatment on histologic abnormalities of the synovium in patients with SpA in order to confirm a potential benefit on peripheral synovitis and to investigate the mechanism of action of anti-TNF-α agents. Histologic and immunohistochemical data showed a significant decrease in vascular cell adhesion molecule 1 expression on the synovial endothelium, a reduction in neutrophil and macrophage infiltration of the synovial sublining layer, and diminished presence of macrophage-like synoviocytes. The aforementioned properties suggest that primarily anti-TNF-α agents act in deactivating the endothelium, with a decrease in vascularity, which in turn reduces migration and homing of inflammatory cells into the synovial tissue. Additionally, the thickness of the synovial lining was normalized after only 12 weeks of treatment. This effect is possibly mediated by a reduction in TNF-α-induced proliferation and/or restoration of Fas-mediated apoptotic cell death of synovial fibroblasts.Citation20 Moreover, the number of CD55-positive synovial lining fibroblasts was significantly reduced at week 12. The most striking immunohistopathological changes included a reduction in lining layer thickness and in downregulation of hypervascularity and in endothelial activation, resulting in a decrease of the inflammatory cell infiltrate with differential effects on T and B cells.Citation21 Kruithof et al demonstrated a significant reduction in CD3-positive cells in a cohort of PsA patients treated with adalimumab. Interestingly, a significant reduction in the number of CD3-positive cells was observed after only 4 weeks of treatment.Citation22 Systemic modifications of the inflammatory process during anti-TNF-α treatment concern mediators of inflammation. In fact, the evaluation of complement-system fragments in patients with PsA treated with anti-TNF-α agents showed a significant decrease in plasma C3 and C4 levels, independently from the anti-TNF-α used. Patients with good response showed a more pronounced reduction in serum complement C3 levels as well as low baseline C3 levels. These findings underline that a reduction in complement native components may be considered as an improvement of a preexisting proinflammatory status reverted by anti-TNF-α drugs. It could be postulated that persistently elevated C3 levels may represent a negative predictive factor influencing the outcome of anti-TNF-α therapy in RA and PsA. As a consequence, the detection of blood C3 levels may provide an additional tool in monitoring disease activity during treatment with anti-TNF-α in PsA patients.Citation23 Recently, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) reported recommendations for PsA treatment.Citation12 An excellent therapeutic improvement has been obtained by the introduction of biologic treatments designed to modify and regulate pivotal and specific mechanisms involved in PsO and PsA immunopathogenesis. To date, anti-TNF-α has been suggested to have a more favorable side-effect profile than synthetic DMARDs.Citation24
Profile of certolizumab
Structural function
CZP (Cimzia; UCB, Brussels, Belgium) is a PEGylated (polyethylene glycol) Fragment antigen binding (Fab) of a humanized monoclonal antibody that binds and neutralizes human TNF-α. The structure of CZP differs significantly from the other agents that have undergone clinical evaluation. In vivo, the pharmacokinetic properties of Fab are limited. However, attachment of a 40 kDa PEG moiety to the Fab fragment markedly increases the half-life of CZP to a value comparable with that of a whole-antibody product, conferring to the drug a fast and lasting effect on the inhibition of joint damage and inflammation. The Fab fragment was engineered with a single free-cysteine residue in the hinge region, which enables site-specific attachment of PEG without affecting the ability of the Fab fragment to bind and neutralize TNF-α.Citation26,Citation27 The unique structure of CZP differs from that of other anti-TNF-α agents that have been tested for the treatment of CD, RA, ankylosing spondylitis (AS), axial SpA, nonradiographic SpA, PsO, and PsA. In fact, clinical efficacy of the drug reported in clinical trials highlighted the rapid improvement of CZP in signs and symptoms of inflammatory arthritis.
Mechanisms of action of CZP
Concerning the mechanism of action of this drug, it is not clear why CZP does not induce apoptosis of cells bearing TNF-α, conversely to other anti-TNF-α agents. Hypothetically, in contrast to the other TNF-α-targeting drugs, CZP may bind to a different epitope compared to the other agents, which leads to a different intracellular signaling pattern. Increased apoptosis has been reported in tissue sections from affected bowel after 24 hours and 6 and 28 days of infliximab treatment.Citation28 It is possible that the anti-inflammatory effect of infliximab indirectly induces apoptosis of activated cells. In this context, a series of comparative in vitro studies were conducted in order to explore the mechanism of action of CZP in CD. The mechanisms, including induction of apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC), may not be required for clinical efficacy of an anti-TNF-α agent in CD, however, inhibition of bacterially stimulated cytokine production from macrophages may be needed for the function of an anti-TNF-α agent to produce efficacy. In fact, in contrast to the other anti-TNF-α agents tested, CZP did not mediate increased levels of apoptosis in any of the in vitro assays used, suggesting that these mechanisms are not essential for the efficacy of anti-TNF-α agents in CD. As CZP, infliximab, and adalimumab, but not etanercept, almost completely inhibited lipopolysaccharide-induced IL-1-β release from monocytes, this mechanism of inhibition of cytokine production may be important for efficacy of anti-TNF-α agents.Citation29 Recently, Ueda et al investigated the cytotoxic effects of the anti-TNF-α agents CZP and golimumab.Citation30 Upon evaluation of their ability in binding to transmembrane TNF-α (tmTNF-α), and in inducing ADCC and CDC, both agents were proven effective in neutralizing tmTNF-α. In contrast to CZP, golimumab was also able to induce ADCC and CDC, similarly to infliximab and adalimumab. While CZP directly provoked nonapoptotic cell death in tmTNF-α-expressing cells, golimumab generated a weaker apoptotic effect compared to infliximab and adalimumab. Accounting for these properties, the cytotoxic effects of anti-TNF-α agents on TNF-α-expressing cells underline their efficacy in treating granulomatous diseases.Citation30 The direct cytotoxic effect of CZP on TNF-α-producing cells may contribute to the clinical effectiveness in the treatment of CD, whereas golimumab may be less effective for the treatment of granulomatous diseases.Citation28,Citation30
Intracellular signal transduction of CZP
Another important concern about the mechanism of action of CZP is the evidence of the intracellular signal transduction triggered by the drug. All available anti-TNF-α biologics have in common the capability to effectively neutralize TNF-α as a major pharmacological mechanism of action. However, there are distinctions with respect to the effect of engagement with tmTNF-α. In all cases, anti-TNF-α biologics can act as antagonists by blocking interaction between tmTNF-α and TNFR1/2 (Tumor necrosis factor receptor 1/2) expressed on a responsive cell. A recent study reported similar ability of CZP, adalimumab, and infliximab in neutralizing mTNF-a-mediated (membrane Tumor necrosis factor-α mediated) signaling, whereas etanercept appeared to be about twofold less potent.Citation31 However, in certain instances, an anti-TNF-α may also act as an agonist through the phenomenon known as “reverse signaling,” in which the engagement of tmTNF-α leads to phosphorylation of specific serine residues in the cytoplasmic tail of tmTNF-α and a signal is transduced in the cell expressing tmTNF-α.Citation32,Citation33 Furthermore, regulated intramembrane proteolysis of tmTNF-α by signal peptide peptidase-like proteases is reported to release a TNF-α intracellular domain mediating reverse signaling in dendritic cells. The full significance of reverse signaling is unknown. One consequence of reverse signaling through tmTNF-α in activated human T cells by both etanercept and infliximab is the induction of E-selectin.Citation34,Citation35 However, by binding only a single TNF-α homotrimer, etanercept does not cross-link tmTNF-α (in the absence of rheumatoid factor), contrary to bivalent mAbs (monoclonal antibodies) such as infliximab. In this regard, it is interesting to highlight that infliximab, but not etanercept, suppresses T-cell proliferation by inducing G0/G1 cell-cycle arrest. Although the rheumatoid factor positively interferes in cross-linking etanercept to tmTNF-α, the suppression of cell proliferation is weakly observed, approximately half the level occurring with infliximab.Citation36 Another possible consequence of reverse signaling induced by biologic anti-TNF-α relates to intracellular events and potential competition for shared signaling pathway molecules, in particular those induced by endotoxin-mediated TLR (Toll-like receptor) signaling, given the observation that in vitro reverse signaling through tmTNF-α induces endotoxin resistance and suppression of cytokines, including TNF-α, IL-1β, IL-10, and IL-12.Citation37 Regarding cytokines, as mentioned above, various in vitro studies report that infliximab and/or adalimumab markedly suppress lipopolysaccharide-induced production of cytokines, including TNF-α, IL-1β, IL-10, and IL-12, as well as CZP whereas this does not occur with etanercept.Citation28,Citation38
First data on the efficacy of CZP
An interesting study performed by Nesbitt et al compared the biological effects of CZP to other anti-TNF-α agents.Citation28 The different spectrum of biologic effects mediated by TNF-α has hampered efforts to define the mechanisms of action of these agents. Although CZP can bind to human polymorphonuclear cells no increase in cell death or release of myeloperoxidase was observed, possibly because of a difference in the way in which it signals through membrane mTNF. In contrast, the other three anti-TNF-α agents all induced both cell death and release of myeloperoxidase. In contrast with these biological differences between the drugs, no clinical studies were performed to compare the clinical efficacy between the five anti-TNF-α agents available. Actually, positive clinical outcomes from the PRECISE study on CZP in CD provided an opportunity to reassess available data from infliximab, adalimumab, and etanercept clinical trials.Citation39,Citation40 CZP, infliximab, and adalimumab appear to be similar in terms of induction, maintenance of response, and remission, as reported in the PRECiSECitation40, ACCENT I (A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-term Treatment Regimen) and CHARM (Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance) trials.Citation41,Citation42
Use of certolizumab in inflammatory arthritis: is there a rationale in psoriatic disease?
Distribution of CZP in inflamed tissues
The main feature of CZP is the distribution of the drug in inflamed tissues due to the effect of PEGylation. This aspect has been investigated using a noninvasive biofluorescence labeling methodology in murine arthritis.Citation43 CZP, adalimumab, and infliximab distributed more effectively into inflamed tissue rather than noninflamed tissue. The penetration of CZP into arthritic paws was greater compared with adalimumab and infliximab, as was the duration of drug exposure in the inflamed tissue. These important features, characterizing CZP activity, may be attributed to the PEGylation and the smaller molecular weight. Exposure to a drug at the site of inflammation may be relevant in terms of efficacy for the treatment of inflammatory disorders such as inflammatory arthritis, namely RA and PsA. In this context, Palframan et al developed an in vivo methodology, enabled to measure antibody-type reagents in normal and inflamed tissue by detecting the distribution of TNF-α, using a novel noninvasive biofluorescence method.Citation44 This technique indicated the ratio of penetration of CZP into inflamed arthritic paws compared with normal tissue was greater than that observed with adalimumab and infliximab. Furthermore, the duration of exposure in the inflamed versus normal tissue was more prolonged for CZP than for both adalimumab and infliximab, and the accumulation of CZP in diseased tissue was more responsive to the severity of inflammation when compared with adalimumab and infliximab. These distinct structural features may affect efficacy, tolerability, rapidity, and/or sustainability of effect of CZP.Citation45 In support of these findings, CZP was tested in inflammatory noninfectious diseases.
Clinical efficacy of CZP in active RA
Clinical efficacy of CZP in inflammatory arthritis – RA,Citation46 PsA,Citation47 and SpACitation48 – was evaluated in several clinical trials (). However, the first clinical evidence comes from the use of CZP in the treatment of CD.Citation39 Actually, in Europe, CZP in combination with methotrexate (MTX) is indicated for the treatment of moderate to severe active RA in adult patients when the response to DMARDs including MTX has been inadequate. CZP can be given as monotherapy in case of intolerance to MTX or when continued treatment with MTX is contraindicated.Citation49 The drug has been shown to reduce the rate of progression of joint damage, as measured by X-ray, and to improve physical function when given in combination with MTX.Citation50 Moreover, CZP was associated with a rapid, consistent clinical response in a diverse group of RA patients, including those with prior TNF-α-inhibitor exposure in the Dose Flex dose-comparison trial.Citation51 Dose Flex was a 34-week, phase IIIb, open-label, run-in, double-blind, placebo (PBO)-controlled randomized study in patients with active RA on stable-dose MTX. Of 333 patients who entered the run-in, 53.5% had prior TNF-α-inhibitor use. CZP demonstrated similar efficacy in RA patients with or without prior exposure to TNF-α inhibitors over 34 weeks of treatment. When CZP was withdrawn at week 16 in American College of Rheumatology 20 (ACR20) responders, a greater maintenance of response in patients who had not previously been exposed to TNF-α inhibitors was observed. Moreover, a long-term safety study of 400 mg CZP for the treatment of RA was made with a period of observation of over 5 years.Citation51 Patients in the FAST4 WARD trialCitation52 were monitored for safety. The retention rates were reported up to week 280 (5.4 years) and safety results up to week 364. Adverse events (AEs) and serious AEs, as well as the number of serious infections, were similar between CZP and PBO; these findings were in the range of those reported for anti-TNF-α. Therefore the use of CZP 400 mg combination or monotherapy has been confirmed to have an acceptable long-term safety profile in line with what would be expected from an anti-TNF-α agent.Citation52,Citation53
Table 1 Clinical indications of certolizumab pegol
Clinical efficacy of CZP and quality of life in SpA
Following the excellent results observed in patients affected by RA, as well as in patients affected by CD treated with CZP, the drug was tested in randomized clinical trialsCitation48,Citation54 in SpA patients. Axial SpA (axSpA) is a form of SpA that includes both AS and nonradiographic axial SpA (nr-axSpA), as defined by the Assessment of Spondyloarthritis International Society criteria.Citation55 Both subgroups of patients have been shown to have a similar burden on QoL. In particular, the effect of CZP on signs and symptoms of AS and nr-axSpA was evaluated in a 24-week, double-blind, randomized, PBO-controlled phase III trial;Citation54 325 patients were randomized. Baseline characteristics were similar between groups.Citation54 Improvements in the CZP-treated groups were observed in pain, fatigue, Bath Ankylosing Spondylitis Functional Index, and AS-QoL from the first measurement at week 1 through to week 24 compared to PBO. Patients in the CZP-treated arm had greater improvements in all the parameters studied compared to PBO. Improvements were also seen in the Medical Outcomes Study, the Short Form (36) Health Survey Mental Component Summary, and domains. CZP effectively improved patient-relevant outcomes in the broad population of axSpA patients classified using the Assessment of Spondyloarthritis International Society criteria.Citation55 One of the main characteristics of SpA is the bone marrow edema of sacroiliac joints (SIJs) and spine, leading to chronic back pain. axSpACitation54 was the first report of the effect of CZP on inflammation of spine and SIJs in axSpA patients, including both AS and nr-axSpA patient populations, using magnetic resonance imaging (MRI). Moreover, improvements in Spondyloarthritis Research Consortium of Canada MRI, SIJ scores, and ankylosing spondylitis spine MRI score for activity, Berlin modifications, were observed in both CZP-dose arms compared to PBO overall and in both AS and nr-axSpA populations. Greater reductions in SIJ inflammation were observed for patient subgroups with <5 years’ symptom duration, age < 45 years, and in males. In this clinical trial, CZP was effective in reducing inflammation in the SIJs and spine, as assessed by MRI in patients with axSpA, and in both AS and nr-axSpa populations.Citation54
The application of CZP in PsO
Concerning PsO, CZP at an initial dose of 400 mg followed by 200 or 400 mg every 2 weeks was evaluated in a randomized, PBO-controlled, double-blind study.Citation56 The drug results were significantly more effective than PBO in the treatment of patients with moderate to severe plaque psoriasis. Coprimary end points were ≥75% improvement from baseline in the Psoriasis Area and Severity Index (PASI 75) and a Physician’s Global Assessment (PGA) of clear–almost clear at week 12. A 75% decrease in PASI score was observed as early as the initial observation at week 2 in some CZP-treated patients. Therefore, CZP was well tolerated in patients with moderate to severe PsO, with a low incidence of injection-site pain or treatment discontinuation due to AEs.Citation51 A retreatment extension study was conducted in 71 CZP PASI 75 responders who relapsed during a 12- to 24-week observation period without treatment. PASI 75 was achieved by 75%, 83%, and 47% of patients in the CZP 200 mg, CZP 400 mg, and PBO groups, respectively (P < 0.001 for both treatment arms vs PBO). A PGA score of clear–almost clear was achieved by 53%, 72%, and 2%, respectively (P < 0.001 for both treatment arms vs PBO). In the retreatment study, median PASI scores were similar at week 12 in the first treatment and retreatment periods for both CZP groups. Serious AEs occurred in 3%, 5%, and 2% of CZP 200 mg, CZP 400 mg, and PBO patients, respectively. Treatment with CZP significantly improved psoriasis at week 12. Similar efficacy was observed at week 12 in patients receiving retreatment for loss of response after drug withdrawal.Citation56
Efficacy of CZP in PsA: rational use in psoriatic arthritis
Clinical and experimental findings suggest that CZP has a unique property of distribution in inflamed tissues. Moreover, in a recent paper of Shu et al, CZP was effective in inhibiting human dermal microvascular endothelial cell expression of angiogenic adhesion molecules and decreased human dermal microvascular endothelial cell angiogenic chemokine secretion.Citation57 At the same time, CZP downregulated TNF-α-induced myeloid cell adhesion to endothelial cells and blocked leukocyte–endothelial cell adhesive interactions in RA synovial tissue, suggesting a novel role for CZP in blocking monocyte adhesion to inflamed synovial vasculature.Citation57 In this regard, PsA can be considered as a systemic disease that involves not only skin and joints but also such other organs as enthesis, vascular endothelium, and adipocyte tissue. PsA synovial tissue is typically characterized by the presence of high endothelial venules associated with immune cell infiltrates.Citation16 Concerning only joint and enthesis involvement, Mease et al experienced for the first time the clinical efficacy and safety of CZP in PsA (RAPID-PsA).Citation47 Patients with active PsA who had failed ≥ 1 DMARD and could have failed ≤ 1 anti-TNF-α were randomized PBO or CZP 400mg at week 0, 2 and 4 followed by either 200 mg CZP or 400 mg CZP. Patients receiving PBO who failed to achieve ≥10% decrease in tender-joint count and swollen-joint count at both weeks 14 and 16 were rescued and randomized at week 16 to receive CZP 200 mg or CZP 400 mg. The clinical primary end point was ACR20 response at week 12. A total of 409 patients were randomized with similar baseline demographic characteristics, and 20% of patients had previously failed an anti-TNF-α treatment. ACR20 response at week 12 was significantly higher in both CZP arms vs PBO. The majority of the overall response rate observed at week 24 was achieved by week 12. Response with CZP was rapid, with a greater ACR20 response as early as week 1 (7.4% for PBO vs 21.0% for CZP 200 mg [P = 0.001] and vs 23.0% for CZP 400 mg [P < 0.001]). At weeks 12 and 24, both CZP arms showed significantly greater improvements than PBO in ACR50 and in ACR70. Greater improvements were also observed for both CZP arms in PASI 75, as well as in the Health Assessment Questionnaire Disability Index at week 24. AEs occurred at the rates of 68% vs 62% and serious AEs at 4% vs 7% in PBO vs CZP, respectively. The safety profile was similar to that observed with CZP in RA.Citation47 The authors concluded that CZP effectively improved the signs and symptoms of arthritis, physical function, and skin manifestations of PsO in patients with PsA, with a safety profile similar to RA.Citation53 Other clinical values, like enthesitis and nail psoriasis, were considered in the ongoing 158-week RAPID-PsA trial (double-blind and PBO controlled to week 24, dose-blind to week 48, and then open-label to week 158). In patients with enthesitis (64.3%), the Leeds Enthesis Index change from baseline at week 24 was −2.0 with CZP 200 mg (P < 0.001) and −1.8 with CZP 400 mg (P < 0.003) vs −1.1 PBO. For patients with baseline nail disease (73.3%), Nail Psoriasis Severity Index change from baseline at week 24 was −1.6 with CZP 200 mg and −2.0 with CZP 400 mg vs −1.1 PBO. No differences in Leeds Dactylitis Index change from baseline were observed in patients with baseline dactylitis.Citation58
Efficacy of CZP in radiographic progression in PsA
Furthermore, the efficacy of CZP was also evaluated in radiographic progression in PsA patients. Gladman and colleagues performed a 24-week patient-reported outcome, phase III, double-blind, randomized, PBO-controlled study. Effect of CZP on the multiple facets of PsA included an analysis of changes from baseline of modified Total Sharp Score. CZP was efficacious in inhibiting radiographic progression compared to PBO. Conventional radiographic imputation methods showed that CZP effectively inhibited radiographic progression in PsA patients. Significantly fewer patients had progression with either CZP dose compared to PBO. CZP was shown to be effective also in improvements in productivity at paid work and within the household, and increased participation in daily activities in patients with PsA.Citation56 Compared to the other anti-TNF agents, CZP is characterized by a different mechanism of action, possibly due to both structure and signal transduction. These features could be related to exact epitopes to which the anti-TNF agents bound the anti-TNF.
Conclusion
Psoriatic arthritis should be rather considered as a systemic disease but the major clinical characteristics are involvement of joints, enthesis and skin.Citation1 Pathogenetic and clinical evidence suggests the role of a complex interplay between chronic inflammatory processes and bone remodeling.Citation14 Clinical guidelines and consensus statements on anti-TNF-α treatment are under constant revision, as data from long-term studies are becoming continuously available. CZP is effective and safe for the treatment of such inflammatory diseases as CD38, RA46, AS, SpA48 and PsO56 and has been evaluated for the treatment of PsA47, with various interesting results. Moreover, the properties of the drug have several advantages for a rapid remission of the diseases. Subcutaneous administration confers good compliance in treated patients for procedures and time of administration. Furthermore, considering PsA as a systemic disease, with major involvement of both skin and joints, clinical trials demonstrated that CZP is efficacious in PsO, PsA, and SpA, as well as in radiographic progression.Citation54,Citation59 Moreover, CZP treatment should be taken into consideration not only in patients unresponsive to synthetic DMARDs but also in those patients who have failed to respond to previous anti-TNF-α treatments, as has been demonstrated in several clinical trials.Citation51,Citation52
Acknowledgments
We would like to thank the rheumatology and dermatology unit health professionals of the University of Rome Tor Vergata. We gratefully acknowledge Dr Simone Emanuele Auteri, UCB Pharma, for scientific support in manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
- Christophers E Psoriasis – epidemiology and clinical spectrum Clin Exp Dermatol 2001 26 4 314 320 11422182
- Kane D Stafford L Bresnihan B FitzGerald O A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience Rheumatology (Oxford) 2003 42 12 1460 1468 14523223
- Partsch G Steiner G Leeb BF Dunky A Broll H Smolen JS Highly increased levels of tumor necrosis factor-alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid J Rheumatol 1997 24 3 518 523 9058659
- Gladman DD Brockbank J Psoriatic arthritis Expert Opin Investig Drugs 2000 9 7 1511 1522
- Laloux L Voisin MC Allain J Immunohistological study of entheses in spondyloarthropathies: comparison in rheumatoid arthritis and osteoarthritis Ann Rheum Dis 2001 60 4 316 321 11247858
- Saad AA Ashcroft DM Watson KD Symmons DP Noyce PR Hyrich KL Efficacy and safety of anti-TNF therapies in psoriatic arthritis: an observational study from the British Society for Rheumatology Biologics Register Rheumatology (Oxford) 2010 49 4 697 705 20056769
- Punzi L Podswiadek M Sfriso P Oliviero F Fiocco U Todesco S Pathogenetic and clinical rationale for TNF-blocking therapy in psoriatic arthritis Autoimmun Rev 2007 6 8 524 528 17854743
- Strohal R Chimenti S Vena GA Girolomoni G Etanercept provides an effective, safe and flexible short- and long-term treatment regimen for moderate-to-severe psoriasis: a systematic review of current evidence J Dermatolog Treat Epub November 10, 2012
- van der Heijde D Kavanaugh A Gladman DD Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: results from the induction and maintenance psoriatic arthritis clinical trial 2 Arthritis Rheum 2007 56 8 2698 2707 17665424
- Papoutsaki M Costanzo A Chimenti MS Chimenti S Adalimumab for the treatment of severe psoriasis and psoriatic arthritis Expert Opin Biol Ther 2008 8 3 363 370 18294106
- Tracey D Klareskog L Sasso EH Salfeld JG Tak PP Tumor necrosis factor antagonist mechanisms of action: a comprehensive review Pharmacol Ther 2008 117 2 244 279 18155297
- Ritchlin CT Kavanaugh A Gladman DD Treatment recommendations for psoriatic arthritis Ann Rheum Dis 2009 68 9 1387 1394 18952643
- FitzGerald O McInnes I Spondyloarthropathy: disease at the crossroads of immunity Best Pract Res Clin Rheumatol 2006 20 5 949 967 16980217
- Chimenti MS Ballanti E Perricone C Cipriani P Giacomelli R Perricone R Immunomodulation in psoriatic arthritis: focus on cellular and molecular pathways Aut Rev 2013 3 12 5 599 606
- Hueber AJ McInnes IB Immune regulation in psoriasis and psoriatic arthritis-recent developments Immunol Lett 2007 114 2 59 65 17928070
- Codullo V McInnes IB Synovial tissue response to treatment in psoriatic arthritis Open Rheumatol J 2011 5 133 137 22279513
- Gerhard N Krenn V Magalhães R Morawietz L Brändlein S König A IgVH-genes analysis from psoriatic arthritis shows involvement of antigen-activated synovial B-lymphocytes Z Rheumatol 2002 61 6 718 727 12491138
- Cañete JD Santiago B Cantaert T Ectopic lymphoid neogenesis in psoriatic arthritis Ann Rheum Dis 2007 66 6 720 726 17223654
- Valesini G Iannuccelli C Marocchi E Pascoli L Scalzi V Di Franco M Biological and clinical effects of anti-TNFalpha treatment Autoimmun Rev 2007 7 1 35 41 17967723
- Baeten D Kruithof E Van den Bosch F Immunomodulatory effects of anti-tumor necrosis factor alpha therapy on synovium in spondylarthropathy: histologic findings in eight patients from an open-label pilot study Arthritis Rheum 2001 44 1 186 195 11212159
- Ohshima S Mima T Sasai M Tumour necrosis factor alpha (TNF-a) interferes with Fas-mediated apoptotic cell death on rheumatoid arthritis (RA) synovial cells: a possible mechanism of rheumatoid synovial hyperplasia and a clinical benefit of anti-TNF therapy for RA Cytokine 2000 12 3 281 288 10704256
- Kruithof E Baeten D Van den Bosch F Mielants H Veys EM De Keyser F Histological evidence that infliximab treatment leads to downregulation of inflammation and tissue remodelling of the synovial membrane in spondyloarthropathy Ann Rheum Dis 2005 64 4 529 536 15388510
- Chimenti MS Perricone C Graceffa D Complement system in psoriatic arthritis: a useful marker in response prediction and monitoring of anti-TNF treatment Clin Exp Rheumatol 2012 30 1 23 30 22260811
- Gossec L Smolen JS Gaujoux-Viala C European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies Ann Rheum Dis 2012 71 1 4 12 21953336
- Statkute L Ruderman EM Novel TNF antagonists for the treatment of rheumatoid arthritis Expert Opin Investig Drugs 2010 19 1 105 115
- Choy EH Hazleman B Smith M Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial Rheumatology (Oxford) 2002 41 10 1133 1137 12364632
- Harris JM Martin NE Modi M Pegylation: a novel process for modifying pharmacokinetics Clin Pharmacokinet 2001 40 7 539 551 11510630
- Nesbitt A Fossati G Bergin M Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor agents Inflamm Bowel Dis 2007 13 11 1323 1332 17636564
- Cominelli F Cytokine-based therapies for Crohn’s disease – new paradigms N Engl J Med 2004 351 20 2045 2048 15537904
- Ueda N Tsukamoto H Mitoma H The cytotoxic effects of certolizumab pegol and golimumab mediated by transmembrane tumor necrosis factor α Ann Rheum Dis 2012 71 Suppl 3 665
- Ohshima S Saeki Y Mima T Long-term follow-up of the changes in circulating cytokines, soluble cytokine receptors, and white blood cell subset counts in patients with rheumatoid arthritis (RA) after monoclonal anti-TNF alpha antibody therapy J Clin Immunol 1999 19 5 305 313 10535607
- Taylor PC Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases Curr Opin Pharmacol 2010 10 3 308 315 20172761
- Kirchner S Holler E Haffner S Andreesen R Eissner G Effect of different tumor necrosis factor (TNF) reactive agents on reverse signalling of membrane integrated TNF in monocytes Cytokine 2004 28 2 67 74 15381183
- Watts AD Hunt NH Wanigasekara Y A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in ‘reverse signalling.’ EMBO J 1999 18 8 2119 2126 10205166
- Harashima S Horiuchi T Hatta N Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells J Immunol 2001 166 1 130 136 11123285
- Mitoma H Horiuchi T Hatta N Infliximab induces potent anti-inflammatory responses by outside-to- inside signals through transmembrane TNF-[alpha] Gastroenterology 2005 128 2 376 392 15685549
- Eissner G Kolch W Scheurich P Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system Cytokine Growth Factor Rev 2004 15 5 353 366 15450251
- van Lent PL Blom AB Grevers L Sloetjes A van den Berg WB Toll-like receptor 4 induced FcgR expression potentiates early onset of joint inflammation and cartilage destruction during immune complex arthritis: Toll-like receptor 4 largely regulates FcgR expression by IL-10 Ann Rheum Dis 2007 66 3 334 340 17068066
- Schreiber S Rutgeerts P Fedorak RN A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn‘s disease Gastroenterology 2005 129 3 807 818 Erratum in: Gastroenterology 2005;129(5):1808 16143120
- Sandborn WJ Feagan BG Stoinov S Certolizumab pegol administered subcutaneously is effective and well tolerated in patients with active Crohn’s disease: results from a 26-week, placebo-controlled phase III study (PRECiSE 1) Gastroenterology 2006 130 Suppl 2 A1 A912 16642574
- Kozuch PL Hanauer SB General principles and pharmacology of biologics in inflammatory bowel disease Gastroenterol Clin North Am 2006 35 4 757 773 17129812
- Colombel J Sandborn WJ Rutgeerts P Adalimumab induces and maintains clinical response and remission in patients with active Crohn’s disease: results of the CHARM trial Gastroenterology 2006 130 Suppl 2 A1 A912 16642574
- Shen C Assche GV Colpaert S Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanercept Aliment Pharmacol Ther 2005 21 3 251 258 15691299
- Jazayeri JA Carroll GJ Fc-based cytokines: prospects for engineering superior therapeutics Bio Drugs 2008 22 1 11 26
- Palframan R Airey M Moore A Vugler A Nesbitt A Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis J Immunol Methods 2009 348 1–2 36 41 19567252
- Fleischmann R The clinical efficacy and safety of certolizumab pegol in rheumatoid arthritis Expert Opin Biol Ther 2010 10 5 773 786 20230188
- Mease PJ Fleischmann RM Deodhar A Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24 week results of a phase 3 double blind randomized placebo-controlled study (RAPID-PsA) Ann Rheum Dis 2012 71 Suppl 3 LB0001
- Sieper J Kivitz AJ Van Tubergen AM Rapid Improvements in Patient Reported Outcomes with Certolizumab Pegol in Patients with Axial Spondyloarthritis, Including Ankylosing Spondyltitis and Non-Radiographic Axial Spondyloarthritis: 24 Week Results of a Phase 3 Double Blind Randomized Placebo-Controlled Study Ann Rheum Disease 2012 64 Suppl10 558
- Deeks ED Certolizumab Pegol: A Review of Its Use in the Management of Rheumatoid Arthritis Drugs 2013 22 [Epub ahead of print]
- Furst DE Keystone EC Fleischmann R Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2009 Ann Rheum Dis 2010 69 Suppl 1 i2 29 Erratum in: Ann Rheum Dis 2011;70(8)1519 19995740
- Furst DE Shaikh SA Greenwald M Evaluation of two dosing regimens of certolizumab pegol for maintenace of clinical response in patients with active rheumatoid arthritis: primary results from Doseflex, a Phase IIIB study Ann Rheum Dis 2012 71 Suppl3 SAT0126
- Fleischmann R Vencovsky J van Vollenhoven RF Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4 WARD study Ann Rheum Dis 2009 68 6 805 811 19015206
- Ramiro S van Tubergen AM Landewé RB RAPID and FAST4 WARD trials: certolizumab pegol for rheumatoid arthritis Expert Rev Clin Immunol 2010 6 5 713 720 20828279
- Van der Heijde D Maksymowych W Landewé R Effect of certolizumab pegol on inflammation of spine and sacroiliac joints in patients with axial spondyloarthritis: 12 week magnetic resonance imaging results of a phase 3 double blind randomized placebo-controlled study Arthritis Rheum 2012 64 Suppl10 1705
- Sieper J Rudwaleit M Baraliakos X The Assessment of Spondyloarthritis International Society (ASAS) handbook: a guide to assess spondyloarthritis Ann Rheum Dis 2009 68 Suppl 2 ii1 ii44 19433414
- Reich K Ortonne JP Gottlieb AB Successful treatment of moderate to severe plaque psoriasis with the pegylated fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension Br J Dermatol 2012 167 1 180 190 22413944
- Shu Q Amin MA Ruth JH Suppression of endothelial cell activity by inhibition of TNFα Arthritis Res Ther 2012 14 2 R88 22534470
- Mease PJ Fleischmann RM Wollenhaupt J Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis with and without prior anti-TNF exposure: 24 week results of a phase 3 double-blind randomized placebo-controlled study Ann Rheum Dis 2012 71 Suppl 3 150 22039170
- Gladman DD Fleischmann RM Coteur G Woltering F Mease P Effect of certolizumab pegol on the multiple facets of psoriatic arthritis as reported by patients: 24 week patient reported outcome results of a phase 3 double blind randomized placebo-controlled study Arthritis Rheum 2012 64 Suppl 10 557 21953497