Abstract
Agonists of the transmembrane intestinal receptor guanylyl cyclase C (GCC) have recently attracted interest as promising human therapeutics. Peptide ligands that can specifically induce GCC signaling in the intestine include endogenous hormones guanylin and uroguanylin, diarrheagenic bacterial enterotoxins (ST), and synthetic drugs linaclotide, plecanatide, and SP-333. These agonists bind to GCC at intestinal epithelial surfaces and activate the receptor’s intracellular catalytic domain, an event initiating discrete biological responses upon conversion of guanosine-5′-triphosphate to cyclic guanosine monophosphate. A principal action of GCC agonists in the colon is the promotion of mucosal homeostasis and its dependent barrier function. Herein, GCC agonists are being developed as new medications to treat inflammatory bowel diseases, pathological conditions characterized by mucosal barrier hyperpermeability, abnormal immune reactions, and chronic local inflammation. This review will present important concepts underlying the pharmacology and therapeutic utility of GCC agonists for patients with ulcerative colitis, one of the most prevalent inflammatory bowel disease disorders.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Ulcerative colitis (UC) is a major clinical syndrome of inflammatory bowel diseases (IBDs) and is characterized by chronic mucosal inflammation of the colon. Recurrent bloody diarrhea, tenesmus and rectal urgency are the most common symptoms for UC, but extraintestinal manifestations may also occur.Citation1 Diagnosis of UC is mainly based on intestinal mucosa examination and tissue histopathology following colonoscopy and biopsy.Citation1 The worldwide annual incidence of UC is 0.5–25 per 100,000 persons, with the highest rates in Western countries and the lowest in the developing world.Citation2 UC distributes equally among men and women, with a typical onset between 15 and 40 years of age.Citation1,Citation2 Although the exact pathogenetic mechanism remains unclear, it is now apparent that both environmental and genetic factors play relevant etiological roles in UC, comprising heterogeneous, multifactorial combinations giving rise to almost identical clinical syndromes.Citation3,Citation4 Specifically, any perturbation of the delicate balance between commensal bacteria, epithelial barrier functions, and host innate and adaptive immunity may result in chronic colonic inflammation.Citation5,Citation6 In the US, about 500,000 persons are affected by UC, with an annual incidence of 2–7 per 100,000 persons, representing a major clinical challenge for the absence of curative pharmacological therapies.Citation1
Selective ligands for guanylyl cyclase C (GCC) () include pathological agents (heat-stable bacterial enterotoxins; herein referred to as ST), endogenous hormones (guanylin and uroguanylin) and, most importantly for this review, therapeutic drugs (linaclotide, plecanatide, and SP-333).Citation7–Citation10 Mostly short peptides of 14–19 amino acids, these GCC agonists share close structural and functional similarities, including intrachain disulfide bonds required for biological activity, pH stability, protease resistance, and poor systemic bioavailability when administered orally. ST and its artificial analog, linaclotide, contain three disulfide bonds and are considered super-agonists for GCC, as they maximally activate the receptor-dependent signal transduction machinery.Citation9,Citation11,Citation12 Plecanatide and SP-333, in turn, are synthetic analogs of the endogenous agonist uroguanylin, and together with guanylin, exhibit only two intrachain disulfide bonds.Citation8,Citation10 Recently, the utility of GCC agonists as IBD therapeutics has been proposed,Citation13,Citation14 and SP-333 is currently being developed for the treatment of UC.Citation10 This review will discuss the pharmacological potential of GCC agonists as promising novel drugs for patients with UC.
Figure 1 The guanylyl cyclase C agonists.
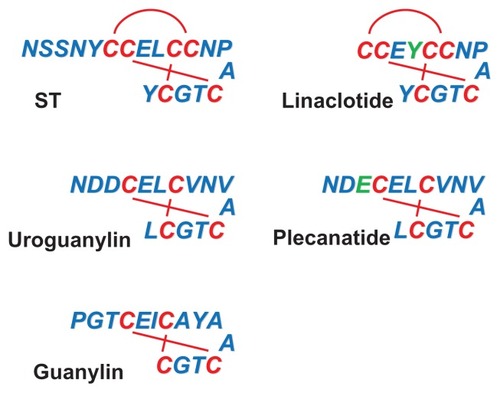
Pharmacological agonists of GCC
Bacterial enterotoxins
The first GCC ligand identified was the heat-stable enterotoxin ST (), a diarrheagenic agent produced by intestinal pathogens such as Escherichia coli, Klebsiella sp., and Yersinia enterocolitica.Citation15 ST comprises a family of small peptides (ranging from 17 to 53 amino acids) sharing a conserved carboxyl terminal region of 13 residues, with six cysteines forming three disulphide bridges that define the tertiary structure and physicochemical properties of the toxin.Citation7,Citation16 ST causes secretory diarrhea in exposed individuals by overactivating the signaling pathway of the intestinal receptor GCC, the only confirmed molecular target for ST in humans.Citation17 Accordingly, disruption of the gene encoding for GCC in mice resulted in resistance to ST-induced intestinal secretion and diarrhea.Citation18,Citation19 There is a general consensus that after colonizing the intestine of humans or animals for survival and growth, bacteria secrete ST to exploit the host GCC signaling and disseminate into the external environment. Infectious diarrhea caused by ST-producing bacteria is a major morbidity factor in areas of poor sanitation and crowded conditions, and remains a principal cause of travelers’ diarrhea and infant mortality in developing nations.Citation20 Despite improved mechanistic understanding, the frequency of these diarrheal diseases has not significantly changed during the past decades.Citation20 Provocatively, the worldwide risk of travelers’ diarrhea inversely correlates with the incidence of colon cancer, and developing countries appear to be protected from colorectal transformation.Citation21 This cancer resistance has been suggested to reflect, in part, longitudinal exposure of endemic populations to enterotoxigenic infectionsCitation22 and the ability of ST to regulate the cell cycle transition, and suppress proliferation of intestinal epithelial cells.Citation21,Citation23 In this model, ST and the host GCC represent an evolutionary-conserved symbiotic system conferring mutual beneficial effects to microbes and mammals.
Endogenous hormones
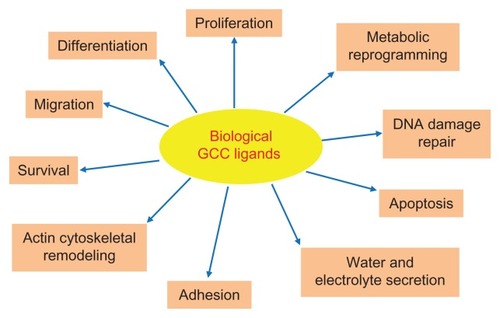
After ST, two endogenous peptide ligands for GCC with primary amino acid sequences similar to the enterotoxin were identified. First, a substance from rat intestinal extracts that stimulated GCC signaling in human colon adenocarcinoma cells was isolated and named guanylin.Citation24 Subsequently, a guanylin-like peptide was isolated from the urine and intestine of opossum and humans and named uroguanylin.Citation25,Citation26 Guanylin and uroguanylin are principally synthesized in the intestine, circulate in the bloodstream, are excreted in the urine, and induce diuresis, natriuresis, and kaliuresis, suggesting they act as endocrine hormones of an enteric-renal system regulating salt and water homeostasis.Citation27 Importantly, these peptides, exhibiting 15 (guanylin) or 16 (uroguanylin) residues and two intrachain disulfide bridges (), selectively bind and activate GCC in apical brush-border membranes of intestinal epithelial cells, although with less potency than ST.Citation8,Citation25,Citation26 They are encoded by genes organized in a tail-to-tail configuration on human chromosome 1p, and secreted by intestinal mucosal cells as proforms, which are activated upon carboxyl terminus cleavage.Citation8,Citation28 Intriguingly, ligand effects appear to depend on extracellular pH, and uroguanylin is 100-fold more potent than guanylin at acidic pH (5.0–5.5), while guanylin is fourfold more potent than uroguanylin at basic pH (8.0).Citation29 Both endogenous GCC ligands exhibit a crypt-to-villus gradient of expression, which is maximal in small intestinal villi and superficial epithelial cells of the colon.Citation28,Citation30,Citation31 However, uroguanylin is more abundant in the proximal tracts of the intestine, while guanylin is highly expressed in the colon and rectum.Citation28,Citation30,Citation31 Also, guanylin and uroguanylin expression undergoes circadian rhythm (highest at night),Citation32 is induced by osmotic stressCitation33 and zinc deficiency,Citation34 and is downregulated by low salt intake.Citation35 These regulatory mechanisms and distribution patterns of expression suggest a complex, spatially distinct role for these peptides in the intestine. In this context, guanylin and uroguanylin are considered autocrine/paracrine hormones, which control local fluid balance and the homeostasis of the intestinal mucosa, including crypt renewal dynamics, cell differentiation and metabolism, and epithelial barrier function ().Citation17,Citation36 Their crucial function as promoters of the normal intestinal epithelial cell phenotype is further underscored by the GCC ligandopenia characterizing early colorectal carcinogenesis,Citation37,Citation38 and the anticancer activity of oral replacement therapy with GCC agonists in the gut.Citation22
Figure 2 Physiological functions regulated by endogenous GCC agonists in the intestine.
Abbreviation: GCC, guanylyl cyclase C.
Therapeutic drugs
As our understanding of the number of critical physiological functions played by the biological ligands grows significantly (), it is becoming apparent that GCC agonists possess great translational potential for human intestinal diseases. Currently, three artificial GCC ligands are being exploited as oral therapeutics for chronic idiopathic constipation, constipation-predominant irritable bowel syndrome and IBD. The first synthetic GCC agonist entering the clinic has been linaclotide (Ironwood Pharmaceuticals Inc, Boston, MA, USA and Forest Laboratories Inc, New York, NY, USA), an ST analog cyclopeptide of 14 amino acids () which increases intestinal motility and fluid secretion, while decreasing visceral pain in preclinical models.Citation9 Linaclotide is converted in vivo by carboxypeptidase A into the active 13mer metabolite MM-419447, which contributes to linaclotide’s pharmacodynamics.Citation39 Recently, linaclotide has been approved in the US for the treatment of patients with chronic idiopathic constipation and irritable bowel syndrome with constipation, wherein this GCC agonist is behaving as a safe, reliable, and effective drug in improving disease-specific abdominal and bowel symptoms.Citation39–Citation42 In that context, a 26-week Phase III trial in 804 patients with constipation-predominant irritable bowel syndrome demonstrated that linaclotide (N of treated patients, 401) significantly ameliorated constipation and disease severity (clinical responders: linaclotide group, 33.7% versus placebo group, 13.9%), while inducing significant, sustained improvement of worst abdominal pain and abdominal discomfort, fullness, cramping, and bloating.Citation42 Of note, with the exception of diarrhea (mostly of mild/moderate intensity; linaclotide group, 19.7% versus placebo group, 2.5%), which is an expected extension of linaclotide pharmacology, the incidence of adverse events was not significantly different in linaclotide-treated patients compared with the placebo controls.Citation42 After linaclotide, two additional artificial GCC agonists, plecanatide (a 16mer) () and SP-333 (Synergy Pharmaceuticals Inc, New York, NY, USA), have entered the drug developmental stage.Citation10 Both synthetic analogs of uroguanylin, but of superior potency, plecanatide and SP-333 are exhibiting promises as gastrointestinal therapeutics.Citation10 Plecanatide is in clinical development for the treatment of chronic idiopathic constipation (Phase IIb/III studies ongoing) and constipation-predominant irritable bowel syndrome (Phase I study planned).Citation10 In the completed Phase I–II clinical trials, plecanatide significantly ameliorated patients’ bowel movements and symptoms.Citation10 In contrast, SP-333 is being investigated specifically for the treatment of IBD in patients with UC, and is currently in the preclinical stage of development.Citation10 In studies employing animal models of IBD, SP-333 attenuated colitis-associated events through the downregulation of locally released autacoids mediating the inflammatory response.Citation10
Signal transduction mechanisms
The receptor
The principal pharmacological target for ST, guanylin, uroguanylin, and their synthetic analogs is GCC. Discovered as the “heat stable enterotoxin receptor,”Citation15 GCC is encoded by the gene GUCY2C on human chromosome 12p12. GUCY2C homologs are widely conserved across species including mammals, reptiles, and birds, suggesting a fundamental role for GCC in organismal biology.Citation43–Citation45 Beyond discrete neuronal cells in the central nervous system,Citation46,Citation47 in mammals, GCC is uniquely expressed at apical, brush-border membranes of intestinal epithelial cells from the duodenum to the rectum, uniformly distributed in crypts, villi, and mucosal surfaces.Citation17 GCC, a member of membrane-bound guanylyl cyclases, is a homodimeric transmembrane enzyme exhibiting conserved functional domains (), including: (1) the extracellular domain for specific ligand binding; (2) a single transmembrane domain with an hydrophobic α-helix region; (3) a short juxtamembrane domain with a G-protein consensus sequence; (4) the kinase homology domain, which binds adenosine triphosphate and regulates ligand-receptor affinity; (5) a hinge region, probably mediating catalytic subunit dimerization; (6) the catalytic domain, which mediates the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP); and (7) a carboxyl terminal tail with key regulatory functions, including modulation of cyclase activity, cytoskeletal anchoring, and receptor internalization.Citation17 The extracellular domain of GCC possesses a unique amino acid sequence, and glycosylation and oligomerization sites, which affect the specificity, stability and efficacy of ligand-receptor binding.Citation17 In this context, upon agonist binding to the extracellular domain of GCC, an intramolecular conformational change is induced and transmitted along the transmembrane and cytoplasmic domains to the carboxyl terminal catalytic site, resulting in a manifold increase of intracellular cGMP concentration over the basal state.Citation15,Citation17
Figure 3 GCC and its downstream targets. (A) The domain structure of GCC. (B) Key proximal effectors activated by GCC in intestinal epithelial cells upon catalytic conversion of GTP to cGMP.
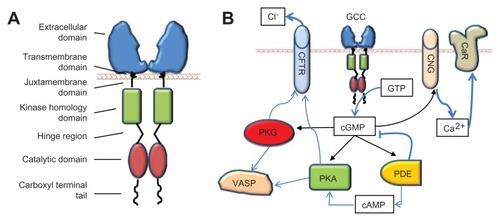
The downstream targets
Cyclic GMP represents the sole intracellular second messenger for GCC agonists. A variety of key cellular responses are mediated by cGMP,Citation17 which regulates virtually all major cytoplasmic signaling networks.Citation48 In intestinal epithelial cells, GCC is the principal source of cGMP, and ligand–GCC interactions in concert with distinct elimination mechanisms (phosphodiesterase-dependent hydrolysis; transporter-dependent efflux) define the type, intensity, and duration of cellular cGMP rises and effects. In this way, elegant spatio-terminal determinants regulate crucial physiological responses in intestine by imposing maximal cGMP signaling. Examples of these include the increased endogenous ligand expression at superficial epithelial compartments, which mediates maturation dynamics,Citation28,Citation31 and the GCC baso-apical expression gradient within cells, which ensures fluid regulation at luminal membrane borders.Citation17
The functional consequences of GCC agonist-induced cGMP elevations in intestinal epithelial cells reflect the selective targeting of downstream molecular effectors (), exhibiting two evolutionarily distinct allosteric binding sites for cGMP. One cGMP binding site is present in cGMP- and cAMP-dependent protein kinases (protein kinase G [PKG] and protein kinase A [PKA], respectively) and in cyclic nucleotide gated (CNG) cation channels, while the other is expressed in cGMP-regulated phosphodiesterases (PDEs). Differential tissue expression and intracellular compartmentalization of these cGMP targets enable selectivity and accuracy of signal transmission and execution. PKG and PKA principally mediate regulation of intestinal fluid homeostasis and cytoskeletal organization by GCC ligands at membrane regions (). PKG is a Ser/Thr protein kinase comprising two subtypes, the soluble PKG I, with two isoforms Iα and Iβ, widely expressed in tissues, and the particulate PKG II, abundant in the intestine.Citation49 PKA is a tetrameric kinase preferentially activated by cAMP.Citation17 PKG II and PKA activation by GCC agonists mediate water and electrolyte secretion by inducing phosphorylation and opening of the cystic fibrosis transmembrane conductance regulator (CFTR), with subsequent Cl− efflux into the intestinal lumen.Citation50–Citation52 In this way, endogenous GCC ligands act as “fluidity sensors” that provide optimal intestinal mucosa hydration through induction of net secretion of water, NaCl, and HCO3 −.Citation17 PKG I, in turn, mediates effects of GCC ligands on the cytoskeleton and contractile apparatus.Citation17 A particularly crucial PKG target in these actions is the vasodilator-stimulated phosphoprotein (VASP), an actin binding protein controlling cytoskeletal remodeling, cell shape, and adhesion contacts in intestinal epithelial cells.Citation53 Herein, GCC agonists induce rapid PKG I-mediated phosphorylation of Ser239 in the carboxyl terminal VASP domain,Citation54 thereby suppressing F-actin polymerization and membrane protrusion formation.Citation53,Citation55,Citation56
Further, CNG channels represent a principal intracellular effector for inhibition of proliferation by GCC agonists in intestine (). CNG channels are heterotetrameric proteins of α- and β-subunits, which mediate cGMP-dependent Na+ and Ca2+ influx.Citation57 GCC signaling through CNG channels slows intestinal cell cycle progression by inducing intracellular Ca2+ influx and cytosolic Ca2+ elevations.Citation21 An important mechanism by which intracellular Ca2+ by GCC agonists suppresses proliferation in intestinal cells is the translocation of calcium-sensing receptors to membrane compartments.Citation58 Calcium-sensing receptor is a key mediator of tumor inhibitory activities by luminal Ca2+, principally reflecting its role as a regulator of intestinal cell maturation dynamics. Thus, ligand–GCC signaling may act as a regulatory system promoting physiological actions by dietary Ca2+ in the gut, including cytostasis and the proliferation to differentiation transition along the crypt–villus axis.Citation21,Citation58 Finally, cGMP-regulated PDEs (eg, PDE2, PDE5, PDE6, and PDE10) are enzymes specialized in the cleavage of the cyclic nucleotide phosphodiester bond.Citation17 In intestinal epithelial cells, these PDEs are principally involved in the modulation or termination of biological signaling by GCC agonists, and in the cross-talk between cGMP- and cAMP-dependent pathways ().Citation52,Citation59
GCC signaling in colonic mucosal integrity
During the last decade, the appreciation of the biological significance of GCC and its ligands for intestinal mucosa homeostasis is increased substantially (), and from a mere fluidity sensor mechanism, the agonist/GCC pathway may now be considered a fundamental promoter of the intestinal mucosa integrity and its dependent barrier function. In this regard, the columnar epithelial cell monolayer covering the inner surface of the colorectum provides a complex chemical and physical barrier protecting the host from its harmful external environment, including pathogens, toxins, and food bioproducts.Citation60 This monolayer is sealed by tight junctions restricting barrier permeability and comprises three cell lineages arising from self-perpetuating stem cells located at colonic crypt bases, including absorptive colonocytes, mucus-producing goblet cells, and hormone-secreting enteroendocrine cells.Citation61 Maintenance of the balance between proliferation, migration, and differentiation along the colonic crypt-surface axis is central in preserving an intact epithelial barrier function, and alterations in those cell homeostatic dynamics compromise the bowel mucosal integrity, thereby favoring inflammatory responses and IBD by unchecked exposure of the sterile host compartment to dangerous luminal antigens.Citation62 Elimination of GCC signaling disrupts the epithelial lineage crypt-surface balance in the mouse colon, reflected by hyperplastic proliferative compartments with fast-cycling and fast-migrating progenitor cells,Citation63,Citation64 and poorly developed differentiated compartments, characterized by incomplete phenotypic and metabolic maturation and self-repair programs.Citation64–Citation66 In particular, loss of GCC resulted in a fewer number of colonic goblet cells, with reduced production of mucin and intestinal trefoil factor.Citation64 This is significant because UC patients exhibit fewer, malfunctioning goblet cells whose pathological recapitulation in mouse models results in loss of mucosal integrity, inflammation, and spontaneous colitis.Citation67 Moreover, mucin and intestinal trefoil factor are key components of the gut-coating mucus layer mediating epithelial barrier protection and post-injury restitution, and their defective production or activity in mice causes increased intestinal permeability and susceptibility to chronic colonic inflammation.Citation68 The contribution to optimal mucus barrier function is further underscored by the ability of ligand-dependent GCC signaling through CFTR to induce secretion of NaCl and HCO3 −,Citation17,Citation50 critical electrolytes controlling the rheological properties of the mucus layer and its proper interaction with the adjacent colonic microbiota.Citation69 Importantly, secretion of these electrolytes at colonic surfaces is often dysregulated in UC patients.Citation70
Beyond effects on lineage- and mucus-dependent functions, GCC signaling protects intestinal barrier integrity by promoting tight junction-mediated epithelial cell sealing. Elimination of GCC or uroguanylin in mice increased intestinal permeability and inflammation through myosin light chain kinase activation and tight junction disassembly, with reduced claudin-2 and JAM-A levels.Citation13 Accordingly, induction of GCC signaling with GCC agonists enhanced tight junction assembly, reduced intestinal barrier permeability and protected mice from chemical-induced colitis.Citation14 A key mechanism mediating regulation of epithelial cell tight junctions by GCC is the inhibition of v-akt murine thymoma viral oncogene homolog 1 (AKT-1) activity, coupled with increased expression of junctional proteins occludin and claudin-4.Citation14 In this context, it is reasonable to speculate that the likely candidate as the proximal molecular effector of GCC-mediated intestinal barrier functions is PKG. In support of this notion, PKG I-mediated VASP phosphorylation represents a well established paradigm underlying cell–cell junction integrity and tissue barrier protection.Citation71,Citation72 Notably, in intestinal epithelial cells, GCC agonists suppress cytoskeletal remodeling at dynamic membrane regions, the process driving epithelial junction disassembly,Citation73 by inducing PKG-dependent VASP phosphorylation.Citation53 Moreover, ligand-mediated GCC signaling through CFTR, which controls electrolyte secretion and intestinal barrier maintenance by the mucus layer, is a PKG II-dependent phenomenon.Citation17,Citation50 Together, these observations underscore the central role of the GCC pathway in support of intestinal barrier integrity, including its protection from or its restitution following injury. In one model, similarly to other intestinal regulatory peptides, GCC endogenous ligands guanylin and uroguanylin are to be considered “mucosal barrier guardians,” whose signal transduction dysregulation may contribute to inflammation and IBD.Citation74 Conversely, iatrogenic induction of colonic GCC signaling with administration of specific agonists as therapeutics may hold great promise for the prevention of further damage, or mucosal barrier restitution in patients with UC ().Citation10,Citation14
Figure 4 GCC agonists as ulcerative colitis therapeutics.
Notes: In ulcerative colitis, abnormal immune responses and colonic inflammation reflect inappropriate luminal antigen exposure for disruption of the mucosal barrier integrity (left panel). Pathogenetic mechanisms underlying loss of intestinal barrier function, in turn, include mutually reinforcing processes of cell lineage imbalance, defective mucus layer, tight junction disassembly and epithelial cell hyper-permeability (left panel). Administration of GCC agonists reconstitutes normal environment-immune interactions by restoring the colonic mucosal barrier integrity, in part, through the promotion of cell lineage-dependent homeostasis, optimal superficial mucus layer and epithelial cells’ tight junctions (right panel).
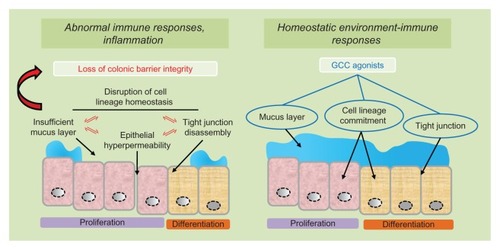
GCC agonists as UC therapeutics
Current pharmacological remedies for UC include 5-aminosalicylic acid, corticosteroids, cyclosporine, and infliximab, a monoclonal antibody blocking pro-inflammatory tumor necrosis factor-α.Citation1 Although these drugs may induce symptom remission and successfully prevent disease progression,Citation75 ~30% of patients with severe UC are resistant to pharmacological therapy and require colectomy.Citation76,Citation77 The global risk of colectomy for UC patients is ~9% over 10 years.Citation76 Common curative surgery consists of total proctocolectomy with ileal J-pouch anal anastomosis.Citation76 However, this procedure is associated with high rates (~20%) of postoperative complications,Citation76 including abscesses, sepsis, fistulas, infertility, and sexual dysfunction.Citation78,Citation79 Thus, innovative drug development programs for UC are warranted. In that context, GCC agonists could be exploited as a unique class of therapeutics for IBD and UC in particular. In the “leaky gut” hypothesis, intestinal hyper-permeability resulting from disruption of mucosal barrier integrity is a principal pathogenetic event underlying abnormal immune responses to luminal antigen exposure and IBD (, left panel).Citation60,Citation62,Citation80,Citation81 Reestablishment of epithelial barrier function, in turn, could arrest pathogenetic processes of inflammation thereby preventing or treating IBD.Citation81 As GCC signaling protects lineage-dependent homeostasis along the colonic crypt-surface axis,Citation36,Citation64 activation of that pathway by its specific ligands could restore normal environment-immune interactions regulated by the epithelial monolayer (, right panel).Citation60 Moreover, the ability of GCC agonists to specifically reinforce the colonic barrier through the regulation of the superficial mucus layerCitation17,Citation50 and epithelial tight junctionsCitation14 may predict their utility as novel drugs for the chemoprevention and treatment of patients with barrier-dependent intestinal inflammation ().Citation62,Citation81 Additionally, expression of endogenous GCC ligands guanylin and uroguanylin is significantly reduced in affected UC tissues compared with healthy subjects,Citation82 supporting the notion that restoration of the dormant GCC pathway with exogenous administration of GCC agonists may be an effective strategy to tissue restitution in UC patients.
In striking contrast with the above considerations, a nonsynonymous single nucleotide polymorphism in GUCY2C conferred a dominant phenotype on affected members of a Norwegian family, characterized by ligand-dependent hyperactivation of GCC signaling, chronic diarrhea, and susceptibility to intestinal inflammation and IBD.Citation83 Accordingly, ~10% of travelers experiencing acute diarrhea from exposure to Escherichia coli producing the GCC super-agonist ST develop post-infectious chronic intestinal symptoms and irritable bowel syndrome.Citation84 Further, the artificial ST analog linaclotide compared poorly with other drugs in protecting isolated pig jejunum from ischemia-induced intestinal barrier disruption,Citation85 and mice deficient in GUCY2C exhibited reduced mucosal damage and inflammation following chemical-induced colitis.Citation86 Beyond confounding variability in experimental models, apparent conflicting observations on the role of GCC in inflammatory gut disorders could be reconciled if one assumes that only physiological levels of ligand-dependent GCC activation mediate intestinal mucosa protection. In this model, activation of the wild-type GCC receptor with the endogenous ligands ensures beneficial GCC signaling underlying normal intestinal biology. In contrast, both dormancy and hyperactivation of the GCC pathway would disrupt mucosal homeostasis and result in bowel disease susceptibility. Thus, administration of adequate dosages of GCC agonists should mimic homeostatic functions of endogenous ligands and oppose intestinal barrier disruption, prevent inflammatory responses and treat IBD-associated pathological events. Of significance, SP-333, the GCC agonist currently under therapeutic development for the treatment of patients with UC, is an artificial analog of the endogenous GCC ligand uroguanylin.Citation10 SP-333 is behaving as a highly effective drug in preclinical mouse models of UC, wherein it is reducing colonic inflammatory damage induced with various chemicals, in part, through the downregulation of pro-inflammatory cytokines including interleukins 4, 5, and 23, and tumor necrosis factor.Citation10 Finally, prolonged stimulation of GCC with its ligands produces cellular refractoriness to biological effects of GCC agonists,Citation59 pointing toward selective temporal administration schedules as yet another critical variable, apart from drug concentrations, to consider for optimal translation of GCC-targeted strategies into UC patients’ therapeutics.
Conclusion
In UC, disruption of homeostatic balance between intestinal microflora, epithelial barrier permeability and host immune responses underlies colitis and tissue damage.Citation5,Citation6 It is suggested that unsupervised exposure of the sterile subepithelial compartment to luminal antigens promotes chronic inflammatory processes and recurrent symptoms.Citation62 There is a need for innovative drug development programs in UC, wherein ~9% of patients undergo colectomy as a result of severe disease that is resistant to current pharmacological therapy.Citation1,Citation76 GCC agonists are attractive novel UC therapeutics because of their unique mechanism of action, which would enable restoration of homeostatic signaling circuits promoting colonic mucosa integrity. In this way, GCC agonists will fill an unmet need in the pharmacological anti-UC armamentarium by targeting one causal mechanism of chronic colonic inflammation, abnormal mucosal permeability.Citation81
Beyond inflammation in the gut, intestinal barrier protection by GCC and its agonists also reduces local and systemic DNA damage and tumorigenesis.Citation14,Citation65 Not surprisingly, UC patients exhibit a higher incidence of colorectal cancer.Citation1 An inverse epidemiological association exists between colon cancer and the risk for enterotoxigenic infections,Citation21 and UC is less common in geographic areas where those infections are endemic.Citation2 Thus, it is tempting to speculate that intermittent longitudinal exposure to the exogenous GCC agonist ST may contribute to protect resident populations from both UC and colorectal cancer. These observations also suggest that GCC agonists might have a broad therapeutic utility, from local inflammation and tumorigenesis chemoprevention to systemic protective actions against genotoxicity and transformation.Citation14 Of relevance, oral administration of GCC agonists is a safe, effective, and well tolerated medication in patients with chronic constipation,Citation39–Citation42 underscoring the clinical utility for these agents as human therapeutics. Together, these considerations support the great significance and translational potential of GCC agonists for the management of UC patients, reflecting the emergent role of this new class of drugs as promoters and protectors of the mucosal barrier integrity in the colon.
Acknowledgment
This work was supported by a grant from the American Institute for Cancer Research.
Disclosure
The author has no conflicts of interest to declare in relation to the topics and content discussed in this review.
References
- Langan RC Gotsch PB Krafczyk MA Skillinge DD Ulcerative colitis: diagnosis and treatment Am Fam Physician 2007 76 9 1323 1330 18019875
- Lakatos PL Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol 2006 12 38 6102 6108 17036379
- Blumberg RS Strober W Prospects for research in inflammatory bowel disease JAMA 2001 285 5 643 647 11176874
- Podolsky DK Inflammatory bowel disease N Engl J Med 2002 347 6 417 429 12167685
- Xavier RJ Podolsky DK Unravelling the pathogenesis of inflammatory bowel disease Nature 2007 448 7152 427 434 17653185
- Sartor RB Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis Nat Clin Pract Gastroenterol Hepatol 2006 3 7 390 407 16819502
- Guerrant RL Hughes JM Chang B Robertson DC Murad F Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates J Infect Dis 1980 142 2 220 228 6106030
- Forte LR Guanylin regulatory peptides: structures, biological activities mediated by cyclic GMP and pathobiology Regul Pept 1999 81 1–3 25 39 10395405
- Harris LA Crowell MD Linaclotide, a new direction in the treatment of irritable bowel syndrome and chronic constipation Curr Opin Mol Ther 2007 9 4 403 410 17694454
- synergypharma.com [homepage on the Internet] New York, NY Synergy Pharmaceuticals, Inc. 2013 Available from: http://www.synergypharma.com Accessed January 14, 2013
- Guarino A Cohen MB Giannella RA Small and large intestinal guanylate cyclase activity in children: effect of age and stimulation by Escherichia coli heat-stable enterotoxin Pediatr Res 1987 21 6 551 555 2885801
- Waldman SA O’Hanley P Falkow S Schoolnik G Murad F A simple, sensitive, and specific assay for the heat-stable enterotoxin of Escherichia coli J Infect Dis 1984 149 1 83 89 6141207
- Han X Mann E Gilbert S Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier PLoS One 2011 6 1 e16139 21305056
- Lin JE Snook AE Li P GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity PLoS One 2012 7 2 e31686 22384056
- Schulz S Green CK Yuen PS Garbers DL Guanylyl cyclase is a heat-stable enterotoxin receptor Cell 1990 63 5 941 948 1701694
- Schmitt CK Meysick KC O’Brien AD Bacterial toxins: friends or foes? Emerg Infect Dis 1999 5 2 224 234 10221874
- Lucas KA Pitari GM Kazerounian S Guanylyl cyclases and signaling by cyclic GMP Pharmacol Rev 2000 52 3 375 414 10977868
- Mann EA Jump ML Wu J Yee E Giannella RA Mice lacking the guanylyl cyclase C receptor are resistant to STa-induced intestinal secretion Biochem Biophys Res Commun 1997 239 2 463 466 9344852
- Schulz S Lopez MJ Kuhn M Garbers DL Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice J Clin Invest 1997 100 6 1590 1595 9294128
- Pawlowski SW Warren CA Guerrant R Diagnosis and treatment of acute or persistent diarrhea Gastroenterology 2009 136 6 1874 1886 19457416
- Pitari GM Zingman LV Hodgson DM Bacterial enterotoxins are associated with resistance to colon cancer Proc Natl Acad Sci U S A 2003 100 5 2695 2699 12594332
- Shailubhai K Yu HH Karunanandaa K Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP Cancer Res 2000 60 18 5151 5157 11016642
- Pitari GM Di Guglielmo MD Park J Schulz S Waldman SA Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells Proc Natl Acad Sci U S A 2001 98 14 7846 7851 11438734
- Currie MG Fok KF Kato J Guanylin: an endogenous activator of intestinal guanylate cyclase Proc Natl Acad Sci U S A 1992 89 3 947 951 1346555
- Hamra FK Forte LR Eber SL Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase Proc Natl Acad Sci U S A 1993 90 22 10464 10468 7902563
- Kita T Smith CE Fok KF Characterization of human uroguanylin: a member of the guanylin peptide family Am J Physiol 1994 266 2 Pt 2 F342 F348 8141334
- Kuhn M Molecular physiology of natriuretic peptide signalling Basic Res Cardiol 2004 99 2 76 82 14963665
- Cohen MB Hawkins JA Witte DP Guanylin mRNA expression in human intestine and colorectal adenocarcinoma Lab Invest 1998 78 1 101 108 9461126
- Hamra FK Eber SL Chin DT Currie MG Forte LR Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity Proc Natl Acad Sci U S A 1997 94 6 2705 2710 9122260
- Cohen MB Witte DP Hawkins JA Currie MG Immunohistochemical localization of guanylin in the rat small intestine and colon Biochem Biophys Res Commun 1995 209 3 803 808 7733972
- Whitaker TL Witte DP Scott MC Cohen MB Uroguanylin and guanylin: distinct but overlapping patterns of messenger RNA expression in mouse intestine Gastroenterology 1997 113 3 1000 1006 9287995
- Scheving LA Jin WH Circadian regulation of uroguanylin and guanylin in the rat intestine Am J Physiol 1999 277 6 Pt 1 C1177 C1183 10600769
- Steinbrecher KA Rudolph JA Luo G Cohen MB Coordinate upregulation of guanylin and uroguanylin expression by hypertonicity in HT29-18-N2 cells Am J Physiol 2002 283 6 C1729 C1737
- Blanchard RK Cousins RJ Upregulation of rat intestinal uroguanylin mRNA by dietary zinc restriction Am J Physiol 1997 272 5 Pt 1 G972 G978 9176203
- Li Z Knowles JW Goyeau D Low salt intake down-regulates the guanylin signaling pathway in rat distal colon Gastroenterology 1996 111 6 1714 1721 8942754
- Pitari GM Li P Lin JE The paracrine hormone hypothesis of colorectal cancer Clin Pharmacol Ther 2007 82 4 441 447 17687268
- Steinbrecher KA Tuohy TM Heppner Goss K Expression of guanylin is downregulated in mouse and human intestinal adenomas Biochem Biophys Res Commun 2000 273 1 225 230 10873591
- Notterman DA Alon U Sierk AJ Levine AJ Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays Cancer Res 2001 61 7 3124 3130 11306497
- Busby RW Kessler MM Bartolini WP Pharmacologic properties, metabolism, and disposition of linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation J Pharmacol Exp Ther 2013 344 1 196 206 23090647
- Rao S Lembo AJ Shiff SJ A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation Am J Gastroenterol 2012 107 11 1714 1724 22986440
- Andresen V Camilleri M Busciglio IA Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome Gastroenterology 2007 133 3 761 768 17854590
- Chey WD Lembo AJ Lavins BJ Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety Am J Gastroenterol 2012 107 11 1702 1712 22986437
- Krause WJ Cullingford GL Freeman RH Distribution of heat-stable enterotoxin/guanylin receptors in the intestinal tract of man and other mammals J Anat 1994 184 Pt 2 407 417 8014132
- Krause WJ Freeman RH Eber SL Hamra FK Currie MG Forte LR Guanylyl cyclase receptors and guanylin-like peptides in reptilian intestine Gen Comp Endocrinol 1997 107 2 229 239 9245531
- Krause WJ Freeman RH Eber SL Distribution of Escherichia coli heat-stable enterotoxin/guanylin/uroguanylin receptors in the avian intestinal tract Acta Anat 1995 153 3 210 219 8984830
- Gong R Ding C Hu J Role for the membrane receptor guanylyl cyclase-C in attention deficiency and hyperactive behavior Science 2011 333 6049 1642 1646 21835979
- Valentino MA Lin JE Snook AE A uroguanylin-GUCY2C endocrine axis regulates feeding in mice J Clin Invest 2011 121 9 3578 3588 21865642
- Bryan NS Bian K Murad F Discovery of the nitric oxide signaling pathway and targets for drug development Front Biosci 2009 14 1 18 19273051
- Pfeifer A Ruth P Dostmann W Sausbier M Klatt P Hofmann F Structure and function of cGMP-dependent protein kinases Rev Physiol Biochem Pharmacol 1999 135 105 149 9932482
- Vaandrager AB Bot AG De Jonge HR Guanosine 3′,5′-cyclic monophosphate-dependent protein kinase II mediates heat-stable enterotoxin-provoked chloride secretion in rat intestine Gastroenterology 1997 112 2 437 443 9024297
- Chao AC de Sauvage FJ Dong YJ Wagner JA Goeddel DV Gardner P Activation of intestinal CFTR Cl− channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase EMBO J 1994 13 5 1065 1072 7510634
- Vaandrager AB Bot AG Ruth P Pfeifer A Hofmann F De Jonge HR Differential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colon Gastroenterology 2000 118 1 108 114 10611159
- Zuzga DS Pelta-Heller J Li P Bombonati A Waldman SA Pitari GM Phosphorylation of vasodilator-stimulated phosphoprotein Ser239 suppresses filopodia and invadopodia in colon cancer Int J Cancer 2012 130 11 2539 2548 21702043
- Deguchi A Soh JW Li H Pamukcu R Thompson WJ Weinstein IB Vasodilator-stimulated phosphoprotein (VASP) phosphorylation provides a biomarker for the action of exisulind and related agents that activate protein kinase G Mol Cancer Ther 2002 1 10 803 809 12492113
- Benz PM Blume C Seifert S Differential VASP phosphorylation controls remodeling of the actin cytoskeleton J Cell Sci 2009 122 Pt 21 3954 3965 19825941
- Lindsay SL Ramsey S Aitchison M Renne T Evans TJ Modulation of lamellipodial structure and dynamics by NO-dependent phosphorylation of VASP Ser239 J Cell Sci 2007 120 Pt 17 3011 3021 17684063
- Biel M Zong X Ludwig A Sautter A Hofmann F Structure and function of cyclic nucleotide-gated channels Rev Physiol Biochem Pharmacol 1999 135 151 171 9932483
- Pitari GM Lin JE Shah FJ Enterotoxin preconditioning restores calcium-sensing receptor-mediated cytostasis in colon cancer cells Carcinogenesis 2008 29 8 1601 1607 18566015
- Pitari GM Baksh RI Harris DM Li P Kazerounian S Waldman SA Interruption of homologous desensitization in cyclic guanosine 3′,5′-monophosphate signaling restores colon cancer cytostasis by bacterial enterotoxins Cancer Res 2005 65 23 11129 11135 16322263
- Moens E Veldhoen M Epithelial barrier biology: good fences make good neighbours Immunology 2012 135 1 1 8 22044254
- Potten CS Booth C Pritchard DM The intestinal epithelial stem cell: the mucosal governor Int J Exp Pathol 1997 78 4 219 243 9505935
- Sanders DS Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn’s disease J Clin Pathol 2005 58 6 568 572 15917403
- Steinbrecher KA Wowk SA Rudolph JA Witte DP Cohen MB Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation Am J Pathol 2002 161 6 2169 2178 12466132
- Li P Lin JE Chervoneva I Schulz S Waldman SA Pitari GM Homeostatic regulation of the crypt-to-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine Am J Pathol 2007 171 6 1847 1858 17974601
- Li P Schulz S Bombonati A Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity Gastroenterology 2007 133 2 599 607 17681179
- Lin JE Li P Snook AE The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling Gastroenterology 2010 138 1 241 254 19737566
- Heazlewood CK Cook MC Eri R Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis PLoS Med 2008 5 3 e54 18318598
- Kim YS Ho SB Intestinal goblet cells and mucins in health and disease: recent insights and progress Curr Gastroenterol Rep 2010 12 5 319 330 20703838
- Bachmann O Seidler U News from the end of the gut – how the highly segmental pattern of colonic HCO(3)(−) transport relates to absorptive function and mucosal integrity Biol Pharm Bull 2011 34 6 794 802 21628874
- Sandle GI Higgs N Crowe P Marsh MN Venkatesan S Peters TJ Cellular basis for defective electrolyte transport in inflamed human colon Gastroenterology 1990 99 1 97 105 2344946
- Wojciak-Stothard B Torondel B Zhao L Renne T Leiper JM Modulation of Rac1 activity by ADMA/DDAH regulates pulmonary endothelial barrier function Mol Biol Cell 2009 20 1 33 42 18923147
- Schlegel N Waschke J Vasodilator-stimulated phosphoprotein: crucial for activation of Rac1 in endothelial barrier maintenance Cardiovasc Res 2010 87 1 1 3 20308204
- Ivanov AI McCall IC Parkos CA Nusrat A Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex Mol Biol Cell 2004 15 6 2639 2651 15047870
- Sturm A Dignass AU Epithelial restitution and wound healing in inflammatory bowel disease World J Gastroenterol 2008 14 3 348 353 18200658
- Keyashian K Annunziata ML Sakuraba A Hanauer S Management of inflammatory bowel disease: past, present and future Expert Rev Clin Immunol 2012 8 4 303 305 22607175
- Meier J Sturm A Current treatment of ulcerative colitis World J Gastroenterol 2011 17 27 3204 3212 21912469
- Jenkins HR Inflammatory bowel disease Arch Dis Child 2001 85 5 435 437 11668114
- Loftus EVJr Delgado DJ Friedman HS Sandborn WJ Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States Am J Gastroenterol 2008 103 7 1737 1745 18564126
- Waljee A Waljee J Morris AM Higgins PD Threefold increased risk of infertility: a meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis Gut 2006 55 11 1575 1580 16772310
- Turner JR Intestinal mucosal barrier function in health and disease Nat Rev Immunol 2009 9 11 799 809 19855405
- Fasano A Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer Physiol Rev 2011 91 1 151 175 21248165
- Wu F Dassopoulos T Cope L Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis Inflamm Bowel Dis 2007 13 7 807 821 17262812
- Fiskerstrand T Arshad N Haukanes BI Familial diarrhea syndrome caused by an activating GUCY2C mutation N Engl J Med 2012 366 17 1586 1595 22436048
- Okhuysen PC Jiang ZD Carlin L Forbes C DuPont HL Post-diarrhea chronic intestinal symptoms and irritable bowel syndrome in North American travelers to Mexico Am J Gastroenterol 2004 99 9 1774 1778 15330917
- Cuppoletti J Blikslager AT Chakrabarti J Nighot PK Malinowska DH Contrasting effects of linaclotide and lubiprostone on restitution of epithelial cell barrier properties and cellular homeostasis after exposure to cell stressors BMC Pharmacol 2012 12 1 3 22553939
- Steinbrecher KA Harmel-Laws E Garin-Laflam MP Murine guanylate cyclase C regulates colonic injury and inflammation J Immunol 2011 186 12 7205 7214 21555532