Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) directly binds to the epidermal growth factor-like repeat A domain of low-density lipoprotein receptor and induces its degradation, thereby controlling circulating low-density lipoprotein cholesterol (LDL-C) concentration. Heterozygous loss-of-function mutations in PCSK9 can decrease the incidence of coronary heart disease by up to 88%, owing to lifelong reduction of LDL-C. Moreover, two subjects with PCSK9 loss-of-function mutations on both alleles, resulting in a total absence of functional PCSK9, were found to have extremely low circulating LDL-C levels without other apparent abnormalities. Accordingly, PCSK9 could represent a safe and effective pharmacological target to increase clearance of LDL-C and to reduce the risk of coronary heart disease. Recent clinical trials using anti-PCSK9 monoclonal antibodies that block the PCSK9:low-density lipoprotein receptor interaction were shown to considerably reduce LDL-C levels by up to 65% when given alone and by up to 72% in patients already receiving statin therapy. In this review, we will discuss how major scientific breakthroughs in PCSK9 cell biology have led to the development of new and forthcoming LDL-C-lowering pharmacological agents.
Introduction
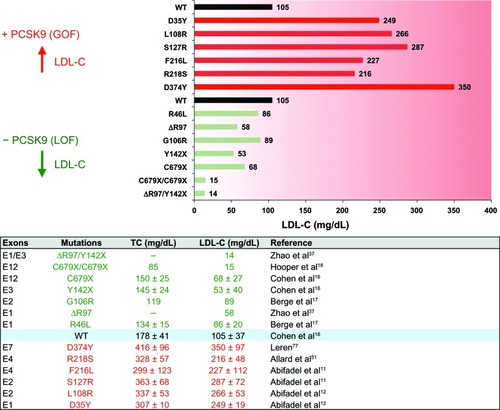
The worldwide prevalence of cardiovascular diseases (CVDs) is a major public health problem that is expected to increase in the next decades.Citation1,Citation2 Elevated circulating low-density lipoprotein cholesterol (LDL-C) is one of the major risk factors positively correlated with premature development of CVD.Citation3–Citation5 Subendothelial retention of LDL particles within the arterial walls is an important initiating event in atherosclerosis, leading to pathological accumulation of lipids and cell debris and chronic inflammation, often culminating in coronary events and stroke.Citation1,Citation6 Through binding of apolipoprotein B100 (ApoB), plasma LDL particles are mainly cleared by hepatic LDL receptor (LDLR)-mediated endocytosis.Citation7 Heterozygous familial hypercholesterolemia (HeFH), characterized by elevated levels of circulating LDL-C, occurs in about one in 500 people who inherit genetic mutations mostly in LDLR but also in APOB, ARH, and APOE loci.Citation8,Citation9 In 2003, proprotein convertase subtilisin/kexin type 9 (PCSK9),Citation10 which has been identified as the third locus associated with familial hypercholesterolemia (FH) (),Citation11,Citation12 was shown to code for a natural inducer of LDLR degradation.Citation13–Citation15 Loss-of-functionCitation16–Citation18 (LOF) mutations or genetic invalidationCitation19 at the PCSK9 locus robustly lower circulating LDL-C () and reduce cardiovascular events by up to ~88% in humans.Citation20 So far, >1,700 LDLR and >160 PCSK9 allelic variants have been identified.Citation21–Citation23 Based on human genetic studies, PCSK9 inhibition should represent a new potent approach to lower LDL-C with the aim to reduce progression of atherosclerosis and CVD risk.
Figure 1 Effect of proprotein convertase subtilisin/kexin type 9 (PCSK9) human mutations on plasma low-density lipoprotein cholesterol (LDL-C) levels. Selected PCSK9 gain-of-function (GOF, red) and loss-of-function (LOF; green) mutations and their impact on circulating LDL-C and total cholesterol (TC; lower panel) are shown. Subjects with wild-type alleles (WT) are used as a reference. An exhaustive list of PCSK9 mutations can be found at http://www.ucl.ac.uk/ldlr/Current/.
Regulation of PCSK9 gene expression
In adult mice, PCSK9 is almost exclusively expressed in the liver and to a lesser extent in other tissues such as the intestine and kidney.Citation10 In functional genomics studies, PCSK9 has been identified as a direct sterol regulatory element-binding protein-2 (SREBP-2) target coregulated with the rate-limiting enzyme for cholesterol synthesis 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and LDLR.Citation24,Citation25 This regulation is of importance, given that statins (HMG-CoA reductase inhibitors), the most important class of LDL-lowering drugs currently used in the clinic,Citation26 also increase the expression of PCSK9,Citation27 which significantly attenuates their potency at increasing LDLR levels.Citation19 This mechanism could explain why for many patients at high risk for CVD, LDL-C levels are not reaching the therapeutic goals with statin therapy alone. Expression of PCSK9 is also regulated by the bile acid-regulated hepatocyte nuclear factor 1 (HNF1),Citation28 which is crucial for the full transcriptional activity of SREBP-2 at the PCSK9 promoter.Citation29 The lipid-lowering compound berberine,Citation30 which is an alkaloid isolated from a Chinese herb used in traditional medicine, was shown to strongly lower PCSK9 gene expression by reducing HNF1α mRNA levels.Citation29,Citation31 In addition, berberine also increases LDLR mRNA stability,Citation32 and based on these properties it has been proposed that it could be used as a monotherapy or in combination with statins to treat hypercholesterolemic patients.Citation30,Citation31
Autocatalytic activation and PCSK9 exit from the endoplasmic reticulum
Human PCSK9 encodes a 692 amino acid protein composed of a signal peptide (aa 1–30), a prosegment (aa 31–152), a catalytic domain (aa 153–404), a hinge region (HR; aa 405–454), and a C-terminal cysteine- and histidine-rich domain (CHRD; aa 455–692; ).Citation10,Citation33 The newly synthesized ~72 kDa proPCSK9 is translocated in the endoplasmic reticulum (ER) and undergoes autocatalytic processing of its prosegment at the VFAQ152↓SIP site.Citation34 Crystallographic studies confirmed that mature PCSK9 has three distinct domains with the prosegment noncovalently bound to the catalytic domain and the CHRD, resulting in a triangular pyramid shape ().Citation33 Similar to other proprotein convertases,Citation35,Citation36 the cleaved prosegment is an inhibitor and an intramolecular chaperone of the catalytic domain required for proper folding and ER exit of PCSK9.Citation10 Indeed, LOF mutations in the prosegment can result in lower circulating PCSK9 due to impaired autocatalytic processing and secretion.Citation37 Moreover, misfolded precursors in the ER act in a dominant negative manner by strongly decreasing secretion of PCSK9 from the wild-type allele.Citation38,Citation39 Therefore, it is considered that inhibition of PCSK9 autoactivation would be a suitable approach to lower LDL-C. However, the exact mechanism by which PCSK9 exits the ER remains largely unknown. A recent study identified the COPII-coated vesicle component Sec24ACitation40 as a selective cytosolic factor for vesicular packaging and ER-to-Golgi trafficking of PCSK9 ().Citation41 Sec24A deficiency was shown to significantly lower circulating PCSK9 and LDL-C in mice. Selective ER export of soluble PCSK9 would involve its binding to a transmembrane cargo receptor that interacts with Sec24A through its cytosolic tail, thereby initiating packaging into COPII vesicles and transport to the Golgi apparatus. Thus, Sec24A and the putative cargo receptor may also represent interesting targets to reduce circulating LDL-C.
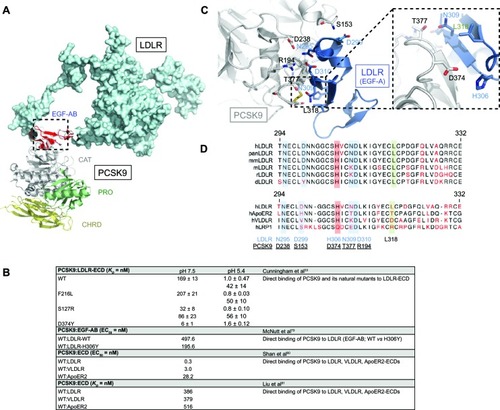
Figure 2 Proprotein convertase subtilisin/kexin type 9 (PCSK9) structure and importance of the cysteine- and histidine-rich domain (CHRD) in low-density lipoprotein receptor (LDLR) degradation. (A) After removal of the signal peptide (SP; aa 1–30, light blue), human proPCSK9 is autocatalytically cleaved at position Q152 within the endoplasmic reticulum, resulting in mature PCSK9 comprising the prosegment (PRO; aa 31–152, green), catalytic domain (aa 153–404, gray), hinge region (HR; aa 405–454), and a C-terminal CHRD (aa 455–692, yellow). (B) Crystal structure of PCSK9 was visualized using MacPymol (Protein Data Bank ID code PDB 2P4E).Citation33 PCSK9 residues (R194, D238, T377, and D374) interacting with LDLR are emphasized (inset).Citation55 PCSK9 residue D374, highlighted in red, is the site of D374Y gain-of-function (GOF) mutation causing severe hypercholesterolemia.Citation76,Citation77 (C) Superposition of PCSK9:epidermal growth factor-like repeat A (EGF-A) complexes with (gray:yellow; PDB 3BPS)Citation55 or without PCSK9-CHRD domain (blue:light blue; PDB 2W2M).Citation69 (D) western blot of media from HEK293 cells transfected with an empty internal ribosome entry site expression vector (IRES) or with plasmids encoding full-length (WT) or truncated human V5-tagged PCSK9 constructs (deltaPRO, Δ33–58; deltaCHRD, L455X, or CHRD alone).Citation74 (E) HepG2 cells were incubated with HEK293-derived conditioned media (shown in [D]), and LDLR, PCSK9, and β-actin protein levels were analyzed by western blotting.
![Figure 2 Proprotein convertase subtilisin/kexin type 9 (PCSK9) structure and importance of the cysteine- and histidine-rich domain (CHRD) in low-density lipoprotein receptor (LDLR) degradation. (A) After removal of the signal peptide (SP; aa 1–30, light blue), human proPCSK9 is autocatalytically cleaved at position Q152 within the endoplasmic reticulum, resulting in mature PCSK9 comprising the prosegment (PRO; aa 31–152, green), catalytic domain (aa 153–404, gray), hinge region (HR; aa 405–454), and a C-terminal CHRD (aa 455–692, yellow). (B) Crystal structure of PCSK9 was visualized using MacPymol (Protein Data Bank ID code PDB 2P4E).Citation33 PCSK9 residues (R194, D238, T377, and D374) interacting with LDLR are emphasized (inset).Citation55 PCSK9 residue D374, highlighted in red, is the site of D374Y gain-of-function (GOF) mutation causing severe hypercholesterolemia.Citation76,Citation77 (C) Superposition of PCSK9:epidermal growth factor-like repeat A (EGF-A) complexes with (gray:yellow; PDB 3BPS)Citation55 or without PCSK9-CHRD domain (blue:light blue; PDB 2W2M).Citation69 (D) western blot of media from HEK293 cells transfected with an empty internal ribosome entry site expression vector (IRES) or with plasmids encoding full-length (WT) or truncated human V5-tagged PCSK9 constructs (deltaPRO, Δ33–58; deltaCHRD, L455X, or CHRD alone).Citation74 (E) HepG2 cells were incubated with HEK293-derived conditioned media (shown in [D]), and LDLR, PCSK9, and β-actin protein levels were analyzed by western blotting.](/cms/asset/ccf09cc6-77c4-476f-8429-e766368895ab/dddt_a_36984_f0002_c.jpg)
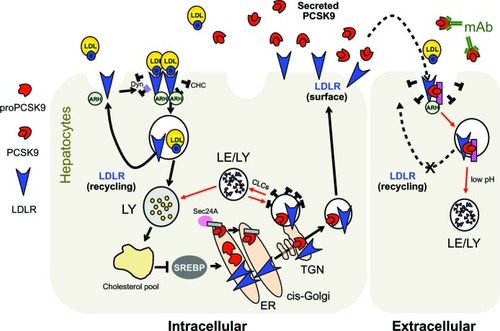
Figure 3 Current cellular model for PCSK9-assisted LDLR degradation. The pink rectangle at the cell surface and in endosomes denotes a putative PCSK9 cofactor needed for LDLR degradation. The gray rectangle in the ER denotes a putative ER cargo receptor.
Unlike other convertases, the N-terminal prosegment of PCSK9 is not released after a second autocatalytic cleavage but remains tightly associated with the catalytic domain, rendering PCSK9 enzymatically inactive.Citation33,Citation42 In hepatocytes, PCSK9 is also prone to proteolytic cleavage by proprotein convertases, which can be localized in the Golgi, endosomes, and at the plasma membrane.Citation43–Citation46 Studies demonstrated that furin cleaves PCSK9 at position R218, generating a truncated form with lower affinity to LDLR.Citation47–Citation50 Human PCSK9 gain-of-function (GOF) mutations R218SCitation51 and F216LCitation11 associated with severe hypercholesterolemia () presumably lead to an increased pool of “active” PCSK9 by preventing the cleavage by furin. However, furin-cleaved PCSK9 may still be active, as it was found to bind LDLR and to reduce its levels in vitro and in vivo.Citation50 Interestingly, although R218 is not in direct contact with the epidermal growth factor-like repeat A (EGF-A) domain, PCSK9-R218A mutation showed a ~ten-fold weaker binding to LDLR.Citation52 This finding suggests that R218 is involved in intramolecular PCSK9 interactions or binds to other LDLR residues.
PCSK9 targets LDLR towards lysosomes for degradation
PCSK9 induces intracellular degradation of LDLR in acidic compartmentsCitation53 independently of its catalytic activity,Citation42 thereby causing LDL-C levels to rise.Citation13–Citation15,Citation19 So far, the exact mechanism by which PCSK9 induces LDLR degradation remains elusive. The prevailing hypothesis is that PCSK9 directly interacts with the EGF-A domain of LDLR (),Citation54,Citation55 forming a complex that is internalized in endosomes via clathrin-coated pits and the cytosolic adaptor protein ARH ().Citation56–Citation58 Interestingly, the EGF-A domain is not directly involved in apolipoprotein binding but rather is important for their release in endosomes.Citation59 In the acidic environment of endosomes, the affinity of PCSK9 for the LDLR increases considerably (),Citation33 which might create additional sites of interaction.Citation60 This two-step binding model would explain how PCSK9 hinders recycling of LDLR to the cell surface,Citation61 thereby promoting its degradation by lysosomal hydrolases independently of ubiquitination, autophagy, and the endosomal sorting complex.Citation57 Based on a study showing that PCSK9 can degrade the LDLR in the absence of ARH,Citation15 an intracellular pathway of LDLR degradation was also demonstrated (). Indeed, clathrin light chains small interfering RNAs (siRNA), which block intracellular trafficking from the trans-Golgi network to lysosomes, rapidly prevented LDLR degradation in human liver-derived HepG2 cells in a PCSK9-dependent fashion without affecting the ability of exogenous PCSK9 to enhance LDLR degradation.Citation62
Figure 4 PCSK9:LDLR binding interface and comparative analysis with other LDLR family members. (A) Structure of PCSK9:EGF-AB (PDB 3BPS)Citation55 complex superposed to LDLR ECD (PDB 1N7D)Citation131 was determined using MacPymol. (B) Residues mainly involved in PCSK9 (gray) and LDLR (EGF-A, blue) binding are represented: S153:D299, D238:N295, R194:D310, T377:N309, and L318. Critical interacting residues PCSK9-D374 and LDLR-H306 implicated in PCSK9-D374Y and LDLR-H306Y GOF mutations are emphasized (inset).Citation79 (C) Primary sequence alignments of LDLR-EGF-A domain from selected species and human LDLR family members (ApoER2, VLDLR, and LRP1) were performed using the CLC workbench. Of note is the high sequence homology between EGF-A domains and PCSK9 interacting residues. (D) Binding affinity of PCSK9 to LDLR family members at neutral or acidic pH.
Although PCSK9 primarily interacts with LDLR via its catalytic domain (),Citation55 over 60% of human mutations in PCSK9 are localized in the prosegment (~34%) or the HR-CHRD (~29%).Citation22 Intriguingly, it was shown that removal of the prosegment N-terminal acidic stretch (aa 31–53) results in increased PCSK9 binding to LDLR by ~ten-fold,Citation55 in a four-fold increased activity on LDLR degradation and faster endocytosis in lysosomes-like compartments.Citation63,Citation64 It was recently proposed that LDL particles, possibly through their ApoB molecule,Citation65 interact with the N-terminal acidic stretch of PCSK9 and, by an allo steric mechanism, inhibit its binding to LDLR and depress its degradation.Citation66,Citation67 The crystal structure of LDLR bound to PCSK9 at neutral pH revealed a low-affinity interaction between residue L108 of the prosegment and residue L626 of the LDLR β-propeller that seemed to stabilize the receptor in an extended conformation.Citation68 On the other hand, although PCSK9-ΔC (lacking its CHRD) cocrystallizes with EGF-A (),Citation69 it is unable to direct the PCSK9:LDLR complex for lysosomal degradation ().Citation61 Recent in vitro studies showed that the CHRD could interact with (1) the ligand-binding domain of LDLR, allowing PCSK9 to remain bound to the receptor at low pH,Citation60,Citation70 and (2) its own prosegment in an intramolecular interaction that could regulate PCSK9 secretion and function.Citation71 Importantly, it was found that a PCSK9 C-terminal domain antibody, which does not affect the PCSK9:LDLR interaction, inhibited PCSK9 internalization and LDLR degradation, revealing the importance of the CHRD for the cellular uptake of PCSK9.Citation72 In addition, it was reported that the cytosolic tail of LDLR is not required for its PCSK9-dependent degradation.Citation73 This suggests that a putative transmembrane protein would bind the CHRD and connect PCSK9 to cytosolic adaptors in order to sort the PCSK9:LDLR complex to lysosomes (). This is in agreement with the C-terminal domain being solvent-exposed, enabling cofactor binding.Citation68 Annexin A2 (AnxA2) was found to bind the CHRD,Citation74 but in wild-type mice the almost complete absence of AnxA2 expression in liver precludes it from being required for PCSK9 activity in hepatocytes.Citation75 However, analyses of AnxA2 −/− mice revealed an increase in circulating PCSK9 and in LDL-C and a decrease of LDLR levels in extrahepatic tissues.Citation75 A 73-aa peptide derived from AnxA2 R1 domain (aa 25–97) inhibited PCSK9 binding to LDLR with a half maximal inhibitory concentration (IC50) of 0.6 μM (). Moreover, AnxA2 prevented PCSK9-induced LDLR degradation in hepatic cell lines and in vivo in mice livers following ectopic over-expression.Citation75 Therefore, peptides derived from AnxA2 or synthetic peptidomimetic derivatives are attractive strategies to inhibit PCSK9.
Table 1 Peptidomimetic inhibition of PCSK9
Direct interaction of PCSK9 to LDLR
At neutral pH, PCSK9 associates to LDLR with a K D of ~169 nM ().Citation33 At endosomal acidic pH, PCSK9 binding to LDLR is increased by 150–170-fold (K D ~1 nM)Citation33,Citation66 and in vitro data also revealed another form of PCSK9 having a K D of 42 nM (),Citation33 possibly due to a second processing event in PCSK9.Citation63 PCSK9-D374Y GOF mutationCitation76,Citation77 has a much greater affinity for the LDLR extracellular domain ()Citation58 and leads to an extremely severe familial hypercholesterolemia phenotype.Citation78 PCSK9-D374 forms a salt bridge with the LDLR EGF-A-H306 residue, and the mutation D374Y results in a more favorable distance, increasing the affinity for LDLR by ~25-fold at neutral pH and its LDLR degrading activity by ~ten-fold.Citation33,Citation58,Citation66 In counterpart, the FH mutation LDLR-H306Y was also shown to increase binding affinity to PCSK9 by ~2.5-fold ().Citation79 Thus, interacting residues PCSK9-D374 and LDLR-H306 act as a pH switch important to further weld complex formation to be subsequently degraded by lysosomal hydrolases.Citation53,Citation69,Citation79
The crystal structure of PCSK9 in complex with the EGF-A domain revealed that the binding site resides on the surface of PCSK9’s catalytic domain but at more than 20 Å away from the catalytic site ().Citation55 Autocatalysis at position Q152 releases a newly available amine group (−NH2) at a distance of 27 Å, forming a hydrogen bond with D299 of the LDLR ().Citation55 Other residues directly involved in the avidity of PCSK9:LDLR interface are R194:D310, D238:N295, D374:H306, and T377:N309 ().Citation55 Remarkably, it was shown that PCSK9 binds similarly to other LDLR family members (very LDLR [VLDLR] and ApoER2; )Citation80 and that EGF-A peptides antagonize PCSK9 binding to LDLR and VLDLR ( and ).Citation79–Citation81 Although EGF-A is a relatively weak antagonist, a phage-display approach has revealed peptidic combinations with greatly improved affinity to PCSK9 ().Citation82 These pioneering studies have clearly demonstrated that targeting the PCSK9:LDLR surface of interaction is a valuable therapeutic strategy to increase cell surface LDLR expression. However, orally active small-molecule inhibitors that would disrupt this interaction or interfere with the autocatalytic processing of PCSK9 in the ER have not yet been reported, possibly due to cytotoxicity and/or less than optimal potency and stability.Citation36
Monoclonal therapy to PCSK9
Several clinical trials have shown a positive correlation between greater levels of LDL-C lowering and greater reductions in coronary heart disease risk.Citation83,Citation84 Statins, currently the most powerful class of lipid-lowering drugs, can help decrease LDL-C levels by 20%–55%, depending on the statin molecule and dosage.Citation85 In addition, combination of statins with ezetimibe, bile-acid sequestrants, or niacin produces an additional 10%–20% decrease in LDL-C.Citation86 However, even if these therapies can help achieve strong reductions in LDL-C, more efficient LDL-C-lowering therapies are still needed, especially for patients with very high initial LDL-C levels. Many of these patients (10%–20%) have undesirable side effects with high-dose statins and/or fail to achieve recommended LDL-C targets.Citation87 In order to fill these important clinical needs, monoclonal antibodies against PCSK9, which inhibit its binding to LDLR and mimic the effect of LOF mutations, are presently being tested in Phase II and III clinical trials.
Pharmacokinetic and pharmacodynamic data
Preclinical studies
Studies using antibodies that disrupt the interaction between PCSK9 and LDLR and inhibit the PCSK9-mediated LDLR degradation have first been performed in cell culture systemsCitation88 and in vivo in mice and nonhuman primates.Citation89 It has been reported that PCSK9 binding to LDLR can be blocked by >80% using anti-PCSK9 polyclonal antibodies.Citation88 Amgen Inc (Thousand Oaks, CA, USA) developed a humanized anti-PCSK9 monoclonal antibody (mAb1) that blocked the PCSK9:LDLR interaction with an IC50 of 2.08 ± 1.21 nM and prevented LDLR degradation in HepG2 cells and in vivo in mice and nonhuman primates.Citation89 In cynomolgus monkeys, a single 3 mg/kg intravenous injection of mAb1 reduced circulating LDL-C by 80%. Pharmacokinetic data demonstrated that circulating mAb1 had a half-life of 61 ± 9 hours. Notably, within 15 minutes and up to 3 days after mAb1 administration, more than 97% of free circulating PCSK9 was complexed with the antibody. These positive preclinical data prompted a Phase I clinical trial to evaluate the safety and tolerability of multiple doses of mAb1 (now named AMG145) when given as an add-on to stable statin therapy in subjects with hyperlipidemia.
Merck Research Laboratories (Whitehouse Station, NJ, USA) developed a neutralizing anti-PCSK9 monoclonal antibody (1D05-IgG2) that structurally mimics the LDLR EGF-A domain.Citation90 1D05 disrupted the PCSK9:LDLR interaction with an IC50 of 3.7 ± 0.1 nM,Citation90 inhibited PCSK9 internalization, and completely restored LDL-C uptake in cells treated with wild-type PCSK9 or GOF mutants.Citation91 In in vivo experiments, one single intravenous injection of 1D05 reduced plasma LDL-C by 40% in a humanized transgenic mouse model (CETP/LDLr-hemi) and by up to 50% in healthy rhesus monkeys. This effect could be maintained for over 2 weeks, even though the antibody displayed a relatively short half-life of 3.2 days. Researchers at Merck reported the use of a similar antibody named 1B20, which blocks PCSK9 uptake in human primary hepatocytes and inhibits PCSK9:LDLR interaction with a calculated IC50 of 11.4 ± 1.5 nM.Citation92 Intravenous injections of 1B20 reduced LDL-C by 50%–70% in CETP/LDLr-hemi mice and in healthy rhesus monkeys (half-life of 1B20 was ~39 hours). Importantly, this study demonstrated that subcutaneous administration of 1B20 in rhesus monkeys robustly lowered LDL-C by up to ~70%, and LDL-C reduction lasted longer, returning to baseline levels after ~28 days compared with ~12 days for the intravenously injected antibody. Moreover, this study showed that dyslipidemic monkeys treated with a combination of 1B20 and simvastatin additively reduced LDL-C levels.
Pfizer-Rinat (New York, NY, USA) has reported a humanized monoclonal antibody directed against PCSK9. The J16 antibody binds to a three-dimensional epitope mapping to the catalytic domain of PCSK9 and also, in part, to the C-terminus of the prosegment.Citation93 The antibody completely disrupts PCSK9:LDLR interaction (IC50 1.4 nM). When injected intravenously in cynomolgus monkeys, J16 (one 3 mg/kg dose, half-life of 2.3 days)Citation94 reduced LDL-C levels by 70%, an effect that was maintained for 10 days. Similar results were observed when the antibody was administered to monkeys fed a high-fat diet (64% reduction in LDL-C at the 3 mg/kg dose). In addition, when hypercholesterolemic monkeys receiving 50 mg/kg/day simvastatin, which reduced LDL-C by 43%, were injected with a single dose of J16 at 3 mg/kg, LDL-C levels were reduced by an additional 65%, emphasizing the beneficial effect of an anti-PCSK9 antibody/statin combination therapy. However, as for mAb1 and 1D05, J16 was reported to have a short half-life (~2.3 days). It was shown that J16 exhibits a dose-dependent half-life that is PCSK9 dependent.Citation94 Opposite to mAb1 and 1D05, which inhibit the endocytosis of PCSK9, J16 is internalized in complex with PCSK9 and is degraded in lysosomes by a target-mediated clearance pathway, which usually occurs for antibodies targeting membrane-bound receptors. To enhance its pharmacokinetic and pharmacodynamic properties, J16 was modified to bind PCSK9 in a pH-sensitive manner by introducing histidines into complementarity determining regions.Citation94 The resultant J17 antibody escapes degradation by dissociating from PCSK9 at acidic pH in endosomes and is recycled to the cell surface by neonatal Fc receptors. J17 was found to reduce LDL-C levels as well as the J16 antibody, but its effect lasted for two to three times as long, due to its prolonged half-life, in mice (12.9 days; 1 mg/kg) and monkeys (7.4 days; 1.5 mg/kg).
Eli Lilly (Indianapolis, IN, USA) recently developed a monoclonal antibody against PCSK9 catalytic domain (aa 160–181)Citation95 that inhibits PCSK9 binding to LDLR and its degradation in HepG2 cells with an IC50 of 104 nM. Following a single intravenous or subcutaneous dose of 5 mg/kg in healthy cynomolgus monkeys, half-life serum concentration of the antibody was 7.3 days and 5.4 days, respectively. A maximal LDL-C decrease of 60% was observed, and LDL-C reduction was maintained below that of baseline levels for approximately 8 weeks. When administered subcutaneously, this antibody was as effective in reducing LDL-C levels as that observed after intravenous injection.Citation95
Human clinical studies
Recent results from Phase I and II clinical trials represent a major step in the quest to develop new and potent lipid-lowering therapy based on PCSK9-neutralizing antibodies. The first-in-human Phase I studies were conducted by Regeneron Pharmaceuticals, Inc (Tarrytown, NY, USA)/Sanofi SA (Paris, France) with their REGN727/SAR236553 antibody, now named alirocumab.Citation96 Single-dose escalation of the antibody given intravenously (0.3–12.0 mg/kg) or subcutaneously (50–250 mg) in healthy subjects significantly reduced LDL-C levels by 28%–65% and 33%–46%, respectively. Importantly, the duration of LDL-C lowering lasted up to day 64 at higher doses injected subcutaneously and up to day 106 by intravenous injection. In a multiple subcutaneous dose study in hypercholesterolemic subjects on atorvastatin treatment, alirocumab dose dependently reduced LDL-C levels by up to 65%. The combination of alirocumab and atorvastatin was shown to be additive on LDL-C lowering, and the antibody was shown to be as potent with or without atorvastatin. Subsequently, a Phase II trial in subjects with LDL-C ≥100 mg/dL receiving stable atorvastatin therapy demonstrated that alirocumab at 150 mg administered subcutaneously every 2 weeks resulted in dose-related LDL-C reductions of up to 72% plus reduced ApoB and lipoprotein(a) up to 56% and 29%, respectively.Citation97 In combination with atorvastatin, this dosing schedule allowed 100% of patients to achieve the treatment goals of LDL-C <70 mg/dL and ApoB <80 mg/dL. Similar results were reported in a larger multicenter study in patients with HeFH, including >40% with coronary artery disease and elevated LDL-C, despite aggressive treatment with high-dose statins (atorvastatin 80 mg/day). Alirocumab at 150 mg every 2 weeks resulted in a decrease of LDL-C by 73% compared with a reduction of 17% with high-dose atorvastatin alone.Citation98
In Phase I studies, Amgen’s antibody (AMG145) lowered LDL-C by up to 64% (maximum single dose of 420 mg) in healthy subjects and by up to 81% in hypercholesterolemic statin-treated subjects with or without HeFH (multiple subcutaneous dose of 140 mg every 2 weeks). As reported for alirocumab, AMG145 also significantly reduced ApoB (≥55%) and lipoprotein(a) (up to 50% in HeFH).Citation99 Thereafter, AMG145 was also tested in several 12-week Phase II studies:
The Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-intolerant Subjects (GAUSS) trial assessed the efficacy and tolerability of the antibody as a monotherapy in statin-intolerant hypercholesterolemic patients.Citation100
The Reduction of LDL-C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) trial tested AMG145 in patients with HeFH with LDL-C ≥ 100 mg/dL despite statin therapy with or without ezetimibe.Citation101
The Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Patients Currently Not Receiving Drug Therapy for Easing Lipid Levels (MENDEL) trial tested AMG145 as a monotherapy in patients with hypercholesterolemia.Citation102
The LDL-C Assessment With PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy-Thrombolysis in Myocardial Infarction 57 (LAPLACE-TIMI 57) trial assessed the antibody in combination with a statin in patients with hypercholesterolemia.Citation103
All four trials demonstrated that subcutaneous injections of AMG145 could reduce LDL-C by >50% either alone or in addition to other LDL-C-lowering therapies. Overall, the antibody was well tolerated, and there were no major adverse safety signals compared with placebo.
Pfizer/Rinat RN316 (PF04950615) is a humanized monoclonal antibody that binds to PCSK9 and prevents LDLR degradation. In Phase II clinical studies, RN316 was administered intravenously in 136 hypercholesterolemic patients already on high doses of statins at 3 mg/kg or 6 mg/kg every 4 weeks for 12 weeks with an 8-week follow-up period. Preliminary data presented at the 2012 American Heart Association Scientific Sessions demonstrated, for the two doses, significant reduction in LDL-C by ~46% and 56%, respectively. In addition, several patients had LDL-C reduction of >75%, and some had treatment interrupted because of LDL-C levels below 25 mg/dL. The effect of the antibody on LDL-C lasted for 4 weeks post-treatment without major adverse events. Phase IIb (NCT01592240) results on the subcutaneous formulation of the antibody given monthly or every 2 weeks (200–300 mg) in hypercholesterolemic subjects on a statin should be available soon, as the study was completed in May 2013.Citation104
Other PCSK9 monoclonal antibodies in clinical trials include LGT209 from Novartis International AG (Basel, Switzerland)/KaloBios Pharmaceuticals, Inc (South San Francisco, CA, USA) and Roche/Genentech RG7652 (F Hoffmann-La Roche Ltd, Basel, Switzerland). LGT209, a humanized antibody derived from a mouse precursor that binds to the C-terminal residues 680–692 of PCSK9 and poorly disrupts the PCSK9 and LDLR interaction but inhibits LDLR degradation and restores LDL-C uptake in HepG2 cells.Citation105 A Phase I study evaluating subcutaneous injections of LGT209 in healthy volunteers with elevated cholesterol and in hypercholesterolemic patients treated with statins was completed in May 2013, but no results have yet been released (NCT01859455).Citation106 Roche/Genentech RG7652 (MPSK3169A), an antibody against the catalytic domain of PCSK9, is being tested in a Phase II trial by subcutaneous injections every 4 weeks for 24 weeks to patients with a high risk of cardiovascular events and elevated LDL-C levels (NCT01609140).Citation107 The results of this study are expected by the end of 2013.
Safety and tolerability
As opposed to drugs such as anticancer therapeutic antibodies, PCSK9 antibodies may be restricted for long-term chronic use. Results of short-term Phase I and II clinical trials using AMG145 or alirocumab indicate that the PCSK9 monoclonal antibody approach is safe and well tolerated. No evidence of drug-related serious adverse events was noted when compared with all treatment groups, including placebo. No binding or neutralizing antibodies to AMG145 have been detected to date.Citation100–Citation103 However, antibodies against alirocumab were detected at low titer in seven out of 56 atorvastatin-treated hypercholesterolemic patients after 8 weeks of combination treatment.Citation98 Thus, although humanized monoclonal antibodies should reduce the risk of eliciting an immune response, it is not known whether the immunogenicity of PCSK9 antibodies will increase after several months or years of treatment. This could result in a loss of responsiveness to treatment and/or to antibody-mediated hypersensitivity reactions such as chronic systemic immunologic diseases (eg, vasculitis, arthritis, and nephritis). The answer to the issues of drug-related adverse events and immunogenicity of anti-PCSK9 antibodies may be available only after completion of Phase III clinical trials and long-term follow-up.
Human genetic studies showed that individuals with PCSK9 LOF mutations, which result in low circulating levels of functional PCSK9 and very low LDL-C levels, are healthy and have reduced cardiovascular risks.Citation20 Thus, it is expected that long-term PCSK9 inhibition will be a safe approach either as a monotherapy or as an add-on to statin therapy to even further reduce cardiovascular events in hypercholesterolemic patients. However, PCSK9 also induces the degradation of closely related LDLR family members ApoER2, VLDLR,Citation108 and LRP1Citation109 and of other transmembrane proteins such as BACE1,Citation110 ENaC,Citation111 and CD81.Citation112 Whereas the in vivo significance of the regulation of multiple proteins by PCSK9 remains to be demonstrated, it cannot be excluded that large population studies could reveal new phenotypes related to PCSK9 inhibition. Another important aspect that needs to be taken into account in clinical trials will be the effect of the very strong increase of LDLR stemming from the additive effect of statins (transcriptional mechanism) and PCSK9 inhibitors (post-translational mechanism). LDLR can act as a receptor for viruses such as human rhinovirus responsible for the common cold,Citation113 vesicular stomatitis virus causing a flu-like illness in infected humans,Citation114 and hepatitis C virus.Citation115 In addition, other targets of PCSK9 such as CD81Citation116 (hepatitis C virus receptor), VLDLR, and LRP1Citation113,Citation117 (rhinovirus receptors) have been found to act as virus receptors. Consequently, inhibition of PCSK9 activity may increase some viral infections, and the number and type of infections should be carefully monitored in Phase III clinical trials.
Clinical trial updates
Two large Phase III clinical trials are currently ongoing and recruiting volunteers. The Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab SAR236553 (REGN727) (ODYSSEY Outcomes) trial (NCT01663402)Citation118 will enroll ~18,000 patients worldwide who experienced an acute coronary syndrome, in order to compare the effect of alirocumab with placebo on the occurrence of cardiovascular events over a 5-year period. Patients will be on lipid-lowering therapy and dietary management but not at their LDL-C goal. The Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial (NCT01764633)Citation119 will study cardiovascular outcomes of AMG145 over ~5 years in ~22,500 patients at increased risk of cardiovascular events. The major goal of these studies is to find out whether additional LDL-C reduction achieved with PCSK9 antibodies in patients on stable statin therapy with clinically evident CVD will significantly decrease major cardiovascular events better than statin therapy alone. Several other smaller Phase III trials with AMG145 and alirocumab are ongoing to collect long-term data on projects already initiated in Phase II trials. These studies will provide additional information about the efficacy, safety, and tolerability of anti-PCSK9 monoclonal therapy. Interestingly, a trial Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV; NCT01813422)Citation120 is set to evaluate plaque regression with AMG145 in subjects with coronary artery disease on lipid-lowering therapy. Plaque size will be evaluated by intravascular ultrasound following treatment with AMG145 or placebo for 78 weeks. The therapeutic potential of neutralizing anti-PCSK9 antibodies will also be tested in the Trial Evaluating PCSK9 Antibody in Subjects With LDL Receptor Abnormalities (TESLA) (NCT01588496)Citation121 designed to assess LDL-C reduction achieved by AMG145 in patients with homozygous FH due to LDLR mutations. Results of this trial will reveal whether increasing the level of dysfunctional LDLR, which may still have some LDL uptake activity, can be beneficial and significantly reduce LDL-C.
Conclusion
Place in therapy
In secondary prevention in high-risk patients with coronary artery disease, intensive lipid lowering with statins results in additional reduction of vascular events and mortality.Citation122,Citation123 Indeed, it is estimated that for every 1 mmol/L (39 mg/dL) reduction in LDL-C, the risk of annual rate of major vascular events is decreased by 21%.Citation124 However, the clinical benefits of high-dose statin therapy may not solely depend on lowering LDL-C level. Statins also have anti-inflammatory effects and reduce the inflammatory biomarker high-sensitivity C-reactive protein, which is associated with better cardiovascular outcomes.Citation125 Additionally, achieving lower concentration of both LDL-C and high-sensitivity C-reactive protein is predictive of better outcomes.Citation125 Yet it is not feasible to establish whether the clinical benefits of statins are due to LDL-C reduction alone, to inflammation inhibition, or to a combination of both activities.Citation126
It is expected that aggressive LDL-C reduction alone, achieved through PCSK9 inhibition, will be atheroprotective. This is exemplified by genetic studies in humansCitation20,Citation77,Citation78,Citation127 and in mouseCitation128,Citation129 and minipig modelsCitation130 showing that development of clinical signs of hypercholesterolemia and atherosclerotic lesions are directly associated with PCSK9 expression and LDL-C levels. Large Phase III clinical trials should establish whether anti-PCSK9 antibodies, which could help more than 80% of hypercholesterolemic patients to achieve an LDL-C concentration lower than or down to optimal target level (~70 mg/dL), have potential for further reduction of the occurrence of major vascular events in humans better than that achieved through HMG-CoA reductase inhibitors.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (Institute of Nutrition, Metabolism, and Diabetes), Fonds de recherche du Québec-Santé, the Heart and Stroke Foundation of Canada, and the Montreal Heart Institute Foundation.
Disclosure
The authors of this publication have no conflicts of interest in this work.
References
- Mackay J Mensah GA The atlas of heart disease and stroke Geneva World Health Organization 2004
- Heidenreich PA Trogdon JG Khavjou OA Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association Circulation 2011 123 8 933 944 21262990
- Kannel WB Dawber TR Kagan A Revotskie N Stokes J3rd Factors of risk in the development of coronary heart disease – six year follow-up experience. The Framingham Study Ann Intern Med 1961 55 33 50 13751193
- Müller C Xanthoma, hypercholesterolemia, angina pectoris Acta Med Scand Suppl 1938 89 75 84
- Yusuf S Hawken S Ounpuu S Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study Lancet 2004 364 9438 937 952 15364185
- Lusis AJ Atherosclerosis Nature 2000 407 6801 233 241 11001066
- Brown MS Goldstein JL A receptor-mediated pathway for cholesterol homeostasis Science 1986 232 4746 34 47 3513311
- Marduel M Ouguerram K Serre V Description of a large family with autosomal dominant hypercholesterolemia associated with the APOE p.Leu167del mutation Hum Mutat 2013 34 1 83 87 22949395
- Rader DJ Cohen J Hobbs HH Monogenic hypercholesterolemia: new insights in pathogenesis and treatment J Clin Invest 2003 111 12 1795 1803 12813012
- Seidah NG Benjannet S Wickham L The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation Proc Natl Acad Sci U S A 2003 100 3 928 933 12552133
- Abifadel M Varret M Rabès JP Mutations in PCSK9 cause autosomal dominant hypercholesterolemia Nat Genet 2003 34 2 154 156 12730697
- Abifadel M Guerin M Benjannet S Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia Atherosclerosis 2012 223 2 394 400 22683120
- Maxwell KN Breslow JL Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype Proc Natl Acad Sci U S A 2004 101 18 7100 7105 15118091
- Benjannet S Rhainds D Essalmani R NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol J Biol Chem 2004 279 47 48865 48875 15358785
- Park SW Moon YA Horton JD Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver J Bio Chem 2004 279 48 50630 50638 15385538
- Cohen J Pertsemlidis A Kotowski IK Graham R Garcia CK Hobbs HH Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9 Nat Genet 2005 37 2 161 165 15654334
- Berge KE Ose L Leren TP Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy Arterioscler Thromb Vasc Biol 2006 26 5 1094 1100 16424354
- Hooper AJ Marais AD Tanyanyiwa DM Burnett JR The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population Atherosclerosis 2007 193 2 445 448 16989838
- Rashid S Curtis DE Garuti R Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9 Proc Natl Acad Sci U S A 2005 102 15 5374 5379 15805190
- Cohen JC Boerwinkle E Mosley THJr Hobbs HH Sequence variations in PCSK9, low LDL, and protection against coronary heart disease N Engl J Med 2006 354 12 1264 1272 16554528
- Leigh SE Foster AH Whittall RA Hubbart CS Humphries SE Update and analysis of the University College London low density lipoprotein receptor familial hypercholesterolemia database Ann Hum Genet 2008 72 Pt 4 485 498 18325082
- Leigh SE Leren TP Humphries SE Commentary PCSK9 variants: a new database Atherosclerosis 2009 203 1 32 33 19249440
- Abifadel M Rabès JP Devillers M Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease Hum Mutat 2009 30 4 520 529 19191301
- Horton JD Shah NA Warrington JA Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes Proc Natl Acad Sci U S A 2003 100 21 12027 12032 14512514
- Maxwell KN Soccio RE Duncan EM Sehayek E Breslow JL Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice J Lipid Res 2003 44 11 2109 2119 12897189
- Wenner Moyer M The search beyond statins Nat Med 2010 16 2 150 153 20134459
- Dubuc G Chamberland A Wassef H Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia Arterioscler Thromb Vasc Biol 2004 24 8 1454 1459 15178557
- Jung D Kullak-Ublick GA Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression Hepatology 2003 37 3 622 631 12601360
- Li H Dong B Park SW Lee HS Chen W Liu J Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine J Biol Chem 2009 284 42 28885 28895 19687008
- Kong W Wei J Abidi P Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins Nat Med 2004 10 12 1344 1351 15531889
- Cameron J Ranheim T Kulseth MA Leren TP Berge KE Berberine decreases PCSK9 expression in HepG2 cells Atherosclerosis 2008 201 2 266 273 18355829
- Abidi P Zhou Y Jiang JD Liu J Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine Arterioscler Thromb Vasc Biol 2005 25 10 2170 2176 16100034
- Cunningham D Danley DE Geoghegan KF Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia Nat Struct Mol Biol 2007 14 5 413 419 17435765
- Naureckiene S Ma L Sreekumar K Functional characterization of Narc 1, a novel proteinase related to proteinase K Arch Biochem Biophys 2003 420 1 55 67 14622975
- Seidah NG Mayer G Zaid A The activation and physiological functions of the proprotein convertases Int J Biochem Cell Biol 2008 40 6–7 1111 1125 18343183
- Seidah NG Prat A The biology and therapeutic targeting of the proprotein convertases Nat Rev Drug Discov 2012 11 5 367 383 22679642
- Zhao Z Tuakli-Wosornu Y Lagace TA Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote Am J Hum Genet 2006 79 3 514 523 16909389
- Mayne J Dewpura T Raymond A Novel loss-of-function PCSK9 variant is associated with low plasma LDL cholesterol in a French-Canadian family and with impaired processing and secretion in cell culture Clin Chem 2011 57 10 1415 1423 21813713
- Cariou B Ouguerram K Zaïr Y PCSK9 dominant negative mutant results in increased LDL catabolic rate and familial hypobetalipoproteinemia Arterioscler Thromb Vasc Biol 2009 29 12 2191 2197 19762784
- Mancias JD Goldberg J Structural basis of cargo membrane protein discrimination by the human COPII coat machinery EMBO J 2008 27 21 2918 2928 18843296
- Chen XW Wang H Bajaj K SEC24A deficiency lowers plasma cholesterol through reduced PCSK9 secretion eLife 2013 2 e00444 23580231
- McNutt MC Lagace TA Horton JD Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells J Biol Chem 2007 282 29 20799 20803 17537735
- Thomas G Furin at the cutting edge: from protein traffic to embryogenesis and disease Nat Rev Mol Cell Biol 2002 3 10 753 766 12360192
- Mayer G Boileau G Bendayan M The proprotein convertase furin colocalizes with caveolin-1 in the Golgi apparatus and endosomes of hepatocytes Cell Tissue Res 2004 316 1 55 63 14986103
- Mayer G Boileau G Bendayan M Sorting of furin in polarized epithelial and endothelial cells: expression beyond the Golgi apparatus J Histochem Cytochem 2004 52 5 567 579 15100235
- Boucher E Mayer G Londono I Bendayan M Expression and localization of MT1-MMP and furin in the glomerular wall of short- and long-term diabetic rats Kidney Int 2006 69 9 1570 1577 16541018
- Benjannet S Rhainds D Hamelin J Nassoury N Seidah NG The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications J Biol Chem 2006 281 41 30561 30572 16912035
- Essalmani R Susan-Resiga D Chamberland A In vivo evidence that furin from hepatocytes inactivates PCSK9 J Biol Chem 2011 286 6 4257 4263 21147780
- Mayer G Hamelin J Asselin MC The regulated cell surface zymogen activation of the proprotein convertase PC5A directs the processing of its secretory substrates J Biol Chem 2008 283 4 2373 2384 18039650
- Lipari MT Li W Moran P Furin-cleaved proprotein convertase subtilisin/kexin type 9 (PCSK9) is active and modulates low density lipoprotein receptor and serum cholesterol levels J Biol Chem 2012 287 52 43482 43491 23135270
- Allard D Amsellem S Abifadel M Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia Hum Mutat 2005 26 5 497 16211558
- Abdiche Y Riggers J Gomes Binventors Pfizer, Rinat Neuroscience Corp, assignee PCSK9 antagonists United States patent US 20100068199A1 3 18 2010
- Maxwell KN Fisher EA Breslow JL Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment Proc Natl Acad Sci U S A 2005 102 6 2069 2074 15677715
- Zhang DW Lagace TA Garuti R Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation J Biol Chem 2007 282 25 18602 18612 17452316
- Kwon HJ Lagace TA McNutt MC Horton JD Deisenhofer J Molecular basis for LDL receptor recognition by PCSK9 Proc Natl Acad Sci U S A 2008 105 6 1820 1825 18250299
- Nassoury N Blasiole DA Tebon Oler A The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR Traffic 2007 8 6 718 732 17461796
- Wang Y Huang Y Hobbs HH Cohen JC Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR J Lipid Res 2012 53 9 1932 1943 22764087
- Lagace TA Curtis DE Garuti R Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice J Clin Invest 2006 116 11 2995 3005 17080197
- Malby S Pickering R Saha S Smallridge R Linse S Downing AK The first epidermal growth factor-like domain of the low-density lipoprotein receptor contains a noncanonical calcium binding site Biochemistry (Mosc) 2001 40 8 2555 2563
- Yamamoto T Lu C Ryan RO A two-step binding model of PCSK9 interaction with the low density lipoprotein receptor J Biol Chem 2011 286 7 5464 5470 21149300
- Zhang DW Garuti R Tang WJ Cohen JC Hobbs HH Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor Proc Natl Acad Sci U S A 2008 105 35 13045 13050 18753623
- Poirier S Mayer G Poupon V Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route J Biol Chem 2009 284 42 28856 28864 19635789
- Benjannet S Saavedra YG Hamelin J Effects of the prosegment and pH on the activity of PCSK9: evidence for additional processing events J Biol Chem 2010 285 52 40965 40978 20937814
- Holla ØL Laerdahl JK Strøm TB Removal of acidic residues of the prodomain of PCSK9 increases its activity towards the LDL receptor Biochem Biophys Res Commun 2011 406 2 234 238 21324305
- Sun H Samarghandi A Zhang N Yao Z Xiong M Teng BB Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor Arterioscler Thromb Vasc Biol 2012 32 7 1585 1595 22580899
- Fisher TS Lo Surdo P Pandit S Effects of pH and low density lipoprotein (LDL) on PCSK9-dependent LDL receptor regulation J Biol Chem 2007 282 28 20502 20512 17493938
- Kosenko T Golder M Leblond G Weng W Lagace TA Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation J Biol Chem 2013 288 12 8279 8288 23400816
- Lo Surdo P Bottomley MJ Calzetta A Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH EMBO Reports 2011 12 12 1300 1305 22081141
- Bottomley MJ Cirillo A Orsatti L Structural and biochemical characterization of the wild type PCSK9-EGF(AB) complex and natural familial hypercholesterolemia mutants J Biol Chem 2009 284 2 1313 1323 19001363
- Tveten K Holla ØL Cameron J Interaction between the ligand-binding domain of the LDL receptor and the C-terminal domain of PCSK9 is required for PCSK9 to remain bound to the LDL receptor during endosomal acidification Hum Mol Genet 2012 21 6 1402 1409 22156580
- Du F Hui Y Zhang M Linton MF Fazio S Fan D Novel domain interaction regulates secretion of proprotein convertase subtilisin/kexin type 9 (PCSK9) protein J Biol Chem 2011 286 50 43054 43061 22027821
- Ni YG Condra JH Orsatti L A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake J Biol Chem 2010 285 17 12882 12891 20172854
- Strøm TB Holla ØL Tveten K Cameron J Berge KE Leren TP Disrupted recycling of the low density lipoprotein receptor by PCSK9 is not mediated by residues of the cytoplasmic domain Mol Genet Metab 2010 101 1 76 80 20659812
- Mayer G Poirier S Seidah NG Annexin A2 is a C-terminal PCSK9-binding protein that regulates endogenous low density lipoprotein receptor levels J Biol Chem 2008 283 46 31791 31801 18799458
- Seidah NG Poirier S Denis M Annexin A2 is a natural extrahepatic inhibitor of the PCSK9-induced LDL receptor degradation PLoS One 2012 7 7 e41865 22848640
- Timms KM Wagner S Samuels ME A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree Hum Genet 2004 114 4 349 353 14727179
- Leren TP Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia Clin Genet 2004 65 5 419 422 15099351
- Naoumova RP Tosi I Patel D Severe hypercholesterolemia in four British families with the D374Y mutation in the PCSK9 gene: long-term follow-up and treatment response Arterioscler Thromb Vasc Biol 2005 25 12 2654 2660 16224054
- McNutt MC Kwon HJ Chen C Chen JR Horton JD Lagace TA Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells J Biol Chem 2009 284 16 10561 10570 19224862
- Shan L Pang L Zhang R Murgolo NJ Lan H Hedrick JA PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide Biochem Biophys Res Commun 2008 375 1 69 73 18675252
- Liu M Wu G Baysarowich J PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain J Lipid Res 2010 51 9 2611 2618 20453200
- Zhang Y Zhou L Kong-Beltran M Calcium-independent inhibition of PCSK9 by affinity-improved variants of the LDL receptor EGF(A) domain J Mol Biol 2012 422 5 685 696 22728257
- O’Keefe JHJr Cordain L Harris WH Moe RM Vogel R Optimal low-density lipoprotein is 50 to 70 mg/dL: lower is better and physiologically normal J Am Coll Cardiol 2004 43 11 2142 2146 15172426
- Baigent C Blackwell L Emberson J Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials Lancet 2010 376 9753 1670 1681 21067804
- Kapur NK Musunuru K Clinical efficacy and safety of statins in managing cardiovascular risk Vasc Health Risk Manag 2008 4 2 341 353 18561510
- Hou R Goldberg AC Lowering low-density lipoprotein cholesterol: statins, ezetimibe, bile acid sequestrants, and combinations: comparative efficacy and safety Endocrinol Metab Clin North Am 2009 38 1 79 97 19217513
- Bruckert E Hayem G Dejager S Yau C Begaud B Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO study Cardiovasc Drugs Ther 2005 19 6 403 414 16453090
- Duff CJ Scott MJ Kirby IT Hutchinson SE Martin SL Hooper NM Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor Biochem J 2009 419 3 577 584 19196236
- Chan JC Piper DE Cao Q A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates Proc Natl Acad Sci U S A 2009 106 24 9820 9825 19443683
- Ni YG Di Marco S Condra JH A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo J Lipid Res 2011 52 1 78 86 20959675
- Condra J Cubbon R Hammond Hinventors Merck assignee 1D05 PCSK9 antagonists United States patent US 20120301461A1 11 12 2012
- Zhang L McCabe T Condra JH An anti-PCSK9 antibody reduces LDL-cholesterol on top of a statin and suppresses hepatocyte SREBP-regulated genes Int J Biol Sci 2012 8 3 310 327 22355267
- Liang H Chaparro-Riggers J Strop P Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates J Pharmacol Exp Ther 2012 340 2 228 236 22019884
- Chaparro-Riggers J Liang H DeVay RM Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9 J Biol Chem 2012 287 14 11090 11097 22294692
- Davies J Darling R Allan BInventors Eli Lilly and Company, assignee Antibodies to PCSK9 and uses thereof US patent US 20130071405A1 3 21 2013
- Stein EA Mellis S Yancopoulos GD Effect of a monoclonal antibody to PCSK9 on LDL cholesterol N Engl J Med 2012 366 12 1108 1118 22435370
- McKenney JM Koren MJ Kereiakes DJ Hanotin C Ferrand AC Stein EA Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy J Am Coll Cardiol 2012 59 25 2344 2353 22463922
- Roth EM McKenney JM Hanotin C Asset G Stein EA Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia N Engl J Med 2012 367 20 1891 1900 23113833
- Dias CS Shaywitz AJ Wasserman SM Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins J Am Coll Cardiol 2012 60 19 1888 1898 23083772
- Sullivan D Olsson AG Scott R Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial JAMA 2012 308 23 2497 2506 23128163
- Raal F Scott R Somaratne R Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial Circulation 2012 126 20 2408 2417 23129602
- Koren MJ Scott R Kim JB Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study Lancet 2012 380 9858 1995 2006 23141812
- Giugliano RP Desai NR Kohli P Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study Lancet 2012 380 9858 2007 2017 23141813
- Pfizer Monthly and twice monthly subcutaneous dosing of PF-04950615 (RN316) in hypercholesterolemic subjects on a statin Available from: http://clinicaltrials.gov/ct2/show/NCT01592240. http://clinicaltrials.gov/ct2/show/NCT01592240. NLM identifier: NCT01592240Accessed September 12, 2013
- Rue S Cohen S Li J Yowe Dinventors IRM LLC, Novartis AG, assignee PCSK9 antagonists United States patent US 20110142849A1 6 16 2011
- Novartis Pharmaceuticals Safety, pharmacokinetics and pharmacodynamics of LGT209 in healthy volunteers with elevated cholesterol and in hypercholesterolemic patients treated with statins Available from: http://www.clinicaltrials.gov/ct2/show/NCT00265317. http://clinicaltrials.gov/ct2/show/NCT01859455. NLM identifier: NCT01859455Accessed September 12, 2013
- Genentech A phase II study of the safety and efficacy of MPSK3169A in patients with coronary heart disease or high risk of coronary heart disease Available from: http://clinicaltrials.gov/ct2/show/NCT01609140. http://clinicaltrials.gov/ct2/show/NCT01609140. NLM identifier: NCT01609140Accessed September 12, 2013
- Poirier S Mayer G Benjannet S The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2 J Biol Chem 2008 283 4 2363 2372 18039658
- Canuel M Sun X Asselin MC Paramithiotis E Prat A Seidah NG Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1) PLoS One 2013 8 5 e64145 23675525
- Jonas MC Costantini C Puglielli L PCSK9 is required for the disposal of non-acetylated intermediates of the nascent membrane protein BACE1 EMBO Reports 2008 9 9 916 922 18660751
- Sharotri V Collier DM Olson DR Zhou R Snyder PM Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9) J Biol Chem 2012 287 23 19266 19274 22493497
- Labonté P Begley S Guévin C PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression Hepatology 2009 50 1 17 24 19489072
- Hofer F Gruenberger M Kowalski H Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus Proc Natl Acad Sci U S A 1994 91 5 1839 1842 8127891
- Finkelshtein D Werman A Novick D Barak S Rubinstein M LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus Proc Natl Acad Sci U S A 2013 110 18 7306 7311 23589850
- Agnello V Abel G Elfahal M Knight GB Zhang QX Hepatitis C virus and other faviviridae viruses enter cells via low density lipoprotein receptor Proc Natl Acad Sci U S A 1999 96 22 12766 12771 10535997
- Pileri P Uematsu Y Campagnoli S Binding of hepatitis C virus to CD81 Science 1998 282 5390 938 941 9794763
- Marlovits TC Abrahamsberg C Blaas D Very-low-density lipoprotein receptor fragment shed from HeLa cells inhibits human rhinovirus infection J Virol 1998 72 12 10246 10250 9811769
- Sanofi Evaluation of cardiovascular outcomes after an acute coronary syndrome during treatment with alirocumab SAR236553 (REGN727) (ODYSSEY outcomes) Available from: http://clinicaltrials.gov/ct2/show/NCT01663402. http://clinicaltrials.gov/ct2/show/NCT01663402. NLM identifier: NCT01663402Accessed September 12, 2013
- Amgen Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER) Available from: http://clinicaltrials.gov/ct2/show/NCT01764633. http://clinicaltrials.gov/ct2/show/NCT01764633. NLM identifier: NCT01764633Accessed September 12, 2013
- Amgen GLobal Assessment of plaque reGression with a PCSK9 antibOdy as measured by intraVascular ultrasound (GLAGOV) Available from: http://clinicaltrials.gov/ct2/show/NCT01813422. http://clinicaltrials.gov/ct2/show/NCT01813422. NLM identifier: NCT01813422Accessed September 12, 2013
- Amgen Trial evaluating PCSK9 antibody in subjects with LDL receptor abnormalities (TESLA) Available from: http://clinicaltrials.gov/ct2/show/NCT01588496. http://clinicaltrials.gov/ct2/show/NCT01588496. NLM identifier: NCT01588496Accessed September 12, 2013
- Cannon CP Braunwald E McCabe CH Intensive versus moderate lipid lowering with statins after acute coronary syndromes N Engl J Med 2004 350 15 1495 1504 15007110
- Law MR Wald NJ Rudnicka AR Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis BMJ 2003 326 7404 1423 12829554
- Mihaylova B Emberson J Blackwell L The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials Lancet 2012 380 9841 581 590 22607822
- Ridker PM Danielson E Fonseca FA Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial Lancet 2009 373 9670 1175 1182 19329177
- Ridker PM Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep 2013 15 1 295 23225175
- Sun XM Eden ER Tosi I Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia Hum Mol Genet 2005 14 9 1161 1169 15772090
- Herbert B Patel D Waddington SN Increased secretion of lipoproteins in transgenic mice expressing human D374YPCSK9 under physiological genetic control Arterioscler Thromb Vasc Biol 2010 30 7 1333 1339 20448210
- Denis M Marcinkiewicz J Zaid A Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice Circulation 2012 125 7 894 901 22261195
- Al-Mashhadi RH Sørensen CB Kragh PM Familial hypercholesterolemia and atherosclerosis in cloned minipigs created by DNA transposition of a human PCSK9 gain-of-function mutant Sci Transl Med 2013 5 166 166ra161
- Rudenko G Henry L Henderson K Structure of the LDL receptor extracellular domain at endosomal pH Science 12 20 2002 298 5602 2353 2358 12459547