?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The aim of the current study is to design a new nanocomposite for inducing cytotoxicity of doxorubicin and oxaliplatin toward MDA-MB231, MCF-7, and Caco2 cell lines. A hippuric acid (HA) zinc layered hydroxide (ZLH) nanocomposite was synthesized under an aqueous environment using HA and zinc oxide (ZnO) as the precursors.
Methods
The hippuric acid nanocomposite (HAN) was prepared by the direct reaction of a HA solution with an aqueous suspension of ZnO.
Results
The basal spacing of the nanocomposite was 21.3 Å, which is average of four harmonics at 2θ = 8.32°, 12.50°, 16.68°, and 20.84°. This result indicates that the hippurate anion was successfully intercalated into the interlayer space of ZLH. The combinations of HAN with chemotherapy (drugs) has inhibited the cell growth of the MDA-MB231, MCF-7, and Caco2 cancer cells when compared to drugs alone. An IC50 value for the combination of HAN with doxorubicin toward MCF-7 is 0.19 ± 0.15 μg/mL and toward MDA-MB231 is 0.13 ± 0.10 μg/mL. Similarly, the IC50 for the combination of HAN with oxaliplatin toward Caco2 is 0.24 ± 0.11 μg/mL. In the antiproliferative results, the equal combination of HAN (0.5 μg/mL) with doxorubicin (0.5 μg/mL) has reduced the cell proliferation in MCF-7 and MDA-MB-231 cells into 37.3% and 17.6%, respectively after 24 hours. Similarly, the antiproliferation percentage for equal combination HAN with oxaliplatin (5.00 μg/mL) toward Caco2 is 72.7% after 24 hours.
Conclusion
The resulting combination HAN with drugs has exhibited higher inhibition in cells growth in all cancer cell lines.
Introduction
The structure of layered double hydroxides (LDHs) and zinc layered hydroxide (ZLH) are derived from the structure of brucite, Mg(OH)2. Brucite is the natural mineral form of magnesium hydroxide. The magnesium ions with a (2+) charge are centered to six octahedrally hydroxides with a (1−) charge. Each hydroxide is bonded to three magnesium atoms, resulting in neutral layers that interact with each other through weak hydrogen bonds.Citation1 The chemical structure of LDHs can be represented by the general formula:Citation2
and the layered hydroxide salts can be represented as follows:
where M2+ is a divalent cation, M3+ is a trivalent cation, and An− is an exchangeable anion with a charge (n−).Citation3
The flexibility in composition of LDH and ZLH, and easily replaced the anions located in the interlayer regions leaded to an increased level of interest in these materials. As a result of the relative ease to synthesize, LDHs and LHSs represent inexpensive and versatile compounds that can be used in different advanced applications, such as in a controlled release system for drugs.Citation4
When applying two drugs in a system, the interaction may yield an additive effect, where the total response is the sum of the two drugs individually. In addition, the combination of these two drugs may result in a greater response, which is called a synergistic response. On the other hand, the interaction between the two drugs may also lead to a smaller response, whereby one drug blocks the effects of the other. It is important to note that the synergistic effect of the combination of two drugs against one target has recently been of particular interest.Citation5,Citation6
Hippuric acid (benzoylaminoethanoic acid) can be obtained from the urine of horses and other herbivores. A biological investigation of hippuric acid shows that it has limited antimicrobial activity at acidic pH values,Citation7 and has synergistic potentiating effects on the selective toxicity of a mixture of 13 substances within the circulatory system.Citation8 From our previous work, hippurate nanocompsite HAN has shown synergistic activity with tamoxifen against the HepG2 cell line.Citation9 Therefore the aim of this present work is to study the synergistic properties of HAN when combined with doxorubicin and oxaliplatin against different cell lines (MDA-MB231, MCF-7, and Caco2). It should be known that oxaliplatin has been commonly used for chemotherapy in colon cancer,Citation10 while doxorubicin is the most common treatment for breast cancer.Citation11,Citation12
Materials and methods
Materials
Hippuric acid (98% purity) was purchased from Merck (Darmstadt, Germany) and was used as received. Doxorubicin (98% purity) and oxaliplatin was purchased from Sigma-Aldrich (St Louis, MO). Zinc oxide (ZnO) of American Chemical Society reagent grade was purchased from Fisher Scientific (Waltham, MA), and dimethyl sulfoxide (DDDT) was purchased from Ajax Finechem (Thermo Fisher Scientific, Waltham, MA) and used without further purification. The cell lines MCF-7, MDA-MB231, and Caco2 were purchased from the American Type Culture Collection. Deionized water was used in all the experiments.
Preparation of hippuric acid nanocomposite, HAN
The hippuric acid nanocomposite (HAN) was synthesized by the direct method using ZnO as the starting material as reported previously,Citation9,Citation13,Citation14 with minor modifications. The hippuric acid solution (0.01 Mole) was prepared using 1.8 g of hippuric acid in 20 mL of DDDT, and was adjusted to 50 mL by the addition of deionized water. The ZnO powder (0.2 g) was suspended in 50 mL of water. Hippurate solution was added slowly dropwise to the suspended ZnO, with vigorous stirring until the addition was complete and the solution became clear. The pH was adjusted to 7.9 using an aqueous solution of NaOH (0.5 Mol/L) to get the white precipitate; the resulting precipitate was magnetically stirred for 18 hours at 70°C. The resulting product was centrifuged, thoroughly washed with deionized water, dried in an oven at 60°C overnight, and kept in a sample bottle for further use.
MTT cytotoxicity assay
Breast cancer cell lines (MCF-7 and MDA-MB231) and heterogeneous human epithelial colorectal adenocarcinoma cells (Caco2) were seeded into 96-well plates and kept at 5% CO2 at 37°C for 24 hours, at a cell density of 40–50% confluence. The cells were then treated with doxorubicin and oxaliplatin alone, and with an equivalent cytostatic mixture of either hippuric acid or HAN. After 72 hours of incubation, 20 μL of tetrazolium salts (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT solution (5 mg/mL) was added to each well and incubated for 4 hours. The microplates were turned swiftly to discard the medium, and the formazan precipitate was dissolved in 10% sodium dodecyl sulfate in DDDT containing 0.6% acetic acid. The microplates were then gently shaken in the dark for 30 minutes, and absorbance at 570 nm and 630 nm (background) was measured with a microtiter plate reader. All experiments were carried out in triplicate. The IC50 was generated from the dose-response curves for the cell line.
Antiproliferation assay
MCF-7, MDA-MB231, and Caco2 cells were first seeded in six-well plates. After incubation for 24 hours for cell attachment, exponentially growing cells were exposed to doxorubicin (0.5 μg/mL) alone, or to oxaliplatin (5 μg/mL) and its combination with hippuric acid or HAN. The plates were incubated at 37°C at 5% CO2 for 24, 48, and 72 hours. After incubation, the media was aspirated off, washed with cold phosphate buffered saline to get rid of the dead cells, and replaced with 1 mL of 0.05% (2 mg/mL) trypsin-EDTA. The plates were incubated at 37°C for 10–15 minutes, until the majority of the cells had lifted off. The cells were then harvested. The cell suspension was centrifuged at 1000 rpm for 10 minutes, and the supernatant was discarded. Twenty μL of cell suspension was mixed with 20 μL of 0.4% trypan blue solution. Cells were resuspended and dye-excluding viable cells were microscopically counted using a hemocytometer.
Statistical analysis
All data were expressed as the means ± standard deviation of the values obtained from three replicates. Using analysis of variance, statistical significance was determined. Mean values with probability values of P < 0.05 were taken as statistically significant.
Characterization
Powder X-ray diffraction patterns were recorded with a Shimadzu XRD-6000 instrument (Shimazdu Corporation, Tokyo, Japan) using CuKα radiation (λ = 1.5418 Å) and a dwell time of 4 degrees per minute. Cell count was carried out with a Neubauer hemocytometer (Weber Scientific International Ltd, Middlesex, UK) by way of clear field microscopy (Nikon Corporation, Tokyo, Japan).
Results and discussion
Powder X-ray diffraction
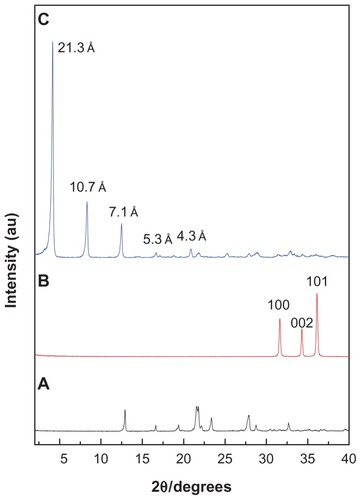
shows the powder X-ray diffraction patterns for hippuric acid, ZnO, and HAN, respectively, which was reported previously in our work.Citation9 The mechanism of formation of hippurate nanocomposite synthesized by direct reaction of ZnO in an aqueous environment can be described as follows. Citation15 Hydrolyzed ZnO in an aqueous environment leads to the formation of thin layers of zinc hydroxide (Zn(OH)2) on the surface of the solid particles. Zn2+ species form of dissociation of Zn(OH)2 in a solution-solid interface. In addition, Zn2+ ions react with hydroxyls, hippurate anions, and water in the solution to form the layered HAN nanocomposite. shows that ZnO exhibited three intense peaks between 30° and 40°, corresponding to diffractions due to the 100, 002, and 101 planes. Complete disappearance of the intense peaks of the ZnO phase and the appearance of a new intense peak at low 2θ with a d value of 21.3 Å indicated that the sample of HAN is pure phase, and that the ZnO was completely converted to ZLH. In addition, shows another four harmonics at 2θ = 8.32°, 12.50°, 16.68°, and 20.84°, with d values of 10.70 Å, 7.10 Å, 5.30 Å, and 4.30 Å, respectively, resulting in an average d value of 21.30 Å.Citation9
Cytotoxicity of the combination of HAN with doxorubicin and oxaliplatin
In this work we used breast cancer cells MCF-7 and MDA-MB231 because they retained several ideal characteristics particular to the mammary epithelium.Citation16,Citation17 Caco2 was used as an in vitro model for colon cancer, which is the second most common cause of cancer in women, and the third most common in men.Citation18 We found that HAN could potentiate the killing of breast and colon cancer cells induced by chemotherapeutic agents. In the absence of hippuric acid or HAN, the doxorubicin suppresses the cells growth of MCF-7 and MDA-MB231 cell lines with IC50 values 0.29 ± 0.09 μg/mL and 0.20 ± 0.06 μg/mL, respectively. In and , the combination of hippuric acid with doxorubicin dose not shown significant reduction in the cell viability (P > 0.05). When closely examining each part in , it is apparent that the combination of doxorubicin with HAN has a higher tumor inhibition efficiency compared to doxorubicin alone, and the IC50 values were 0.19 μg/mL ± 0.15 μg/mL and 0.13 μg/mL ± 0.10 for the MCF-7 and MDA-MB231 cell lines, respectively. There was a significant decrease of MDA-MB231 and Caco2 viability at the combination concentration as low as 0.31 μg/mL till 20 μg/mL (P < 0.05, P = 0.01, respectively). This result indicates that the suppression percentage in cells MCF-7 and MDA-MB231 are 34.4% and 35.0%, respectively.
Table 1 IC50 value of different cell lines treated with free doxorubicin or oxaliplatin, and a combination of free drugs with hippuric acid and HAN
Figure 2 MTT assays of (A) MCF-7, (B) MDA-MB231, and (C) Caco2 cell lines after 72 hours of treatment.
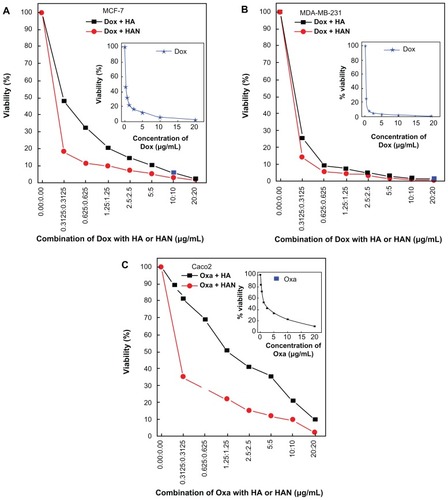
Similarly, the combination of oxaliplatin with HAN against the Caco2 cells shows an suppression efficiency with IC50 of 0.24 μg/mL ± 0.11 compared with 1.48 μg/mL ± 0.10 for oxaliplatin alone. Our findings demonstrated that the co-treatment of Caco2 cell by oxaliplatin and HAN mixture shows maximal efficacy, this may be due to effect of the ZLH nanolayer, which facilitates the entry of the oxaliplatin drug, as compared to doxorubicine.
Antiproliferative effect of free doxorubicin or oxaliplatin and a combination of drugs with hippuric acid and HAN
shows the effect of free doxorubicin and oxaliplatin drugs, and the combination of hippuric acid or HAN with that drugs on the proliferation of MCF-7, MDA-MB231, and Caco2 cell lines at incubation times (24, 48, and 72 hours). However, doxorubicin and oxaliplatin drugs as well as combination of these drugs with HAN suppress the proliferation of MCF-7, MDA-MB231, and Caco2 tumor cells. The combination of these drugs with HAN are more efficient than the drugs alone against the growth of tumor cells. This result indicates that drug delivery to the tumor cell is noticeably enhanced by nanocomposites containing ZLH; however, in the nanocomposites system, drugs can reach the tumor cell membrane without early decomposition, since the drugs are stabilized and protected in the interlayer space of the ZLH layers. These results clearly confirm that the intercalation reaction not only prevents drug denaturation, but it also enhances the permeability of the drug into the target cell without any noticeable side effects.Citation19
Figure 3 Antiproliferative assays of (A) MCF-7, (B) MDA-MB231, and (C) Caco2 cell lines after, 24, 48, and 72 hours of treatment with free doxorubicin or oxaliplatin, and with a combination of doxorubicin or oxaliplatin with hippuric acid and HAN.
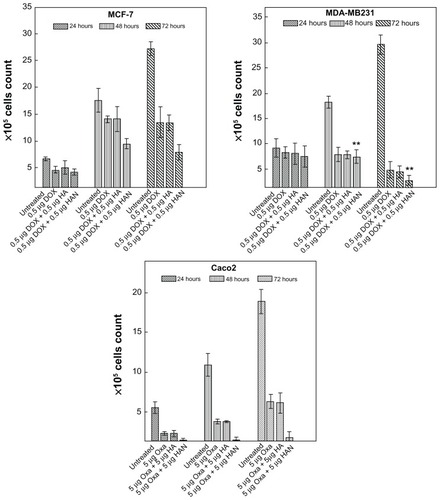
Supporting to MTT assay, trypan blue exclusion results confirmed that the combination of drugs with HAN can inhibit the cell proliferation in a time- and dose-dependent manner. In and after 72 hours exposure, the co-treatment by oxaliplatin with HAN or doxorubicin with HAN results in greater antiproliferative effect of drugs.
Table 2 Antiproliferative assays of the MCF-7, MDA-MB231, and Caco2 cell lines after 24, 48, and 72 hours of treatment with free doxorubicin or oxaliplatin, or with the combination of doxorubicin or oxaliplatin with hippuric acid and HAN
At 48 and 72 hours, there appeared to be maximal and significant (P < 0.05) effects on cell proliferation, when compared to effects noted at 24 hours. This may be due to induce cell death and increasing of the exposure time. From the results of the trypan blue exclusion assay, increasing exposure time to combination of doxorubicin with HAN appeared to have a reduction effect on cell proliferation in MDA-MB231 more than can be seen in MCF-7 line under most circumstances. For MCF-7 cells, antiproliferation decreased at 48 hours (when compared to 24 hours) for both free doxorubicin and for the combination of doxorubicin hippuric acid, and then increases at 72 hours, but this effect was not seen for any other cells/drug treatment. This may be attributable to the high resistance of the estrogen receptor-positive breast cancer MCF-7 cell line to chemotherapy.
Conclusion
The intercalation of hippuric acid into a zinc-layered hydroxide, using a direct method to obtain the HAN nano-composite, was successfully accomplished. The X-ray diffraction results confirmed the intercalation process in which hippuric acid lies between the interlayers. The MTT assay showed that the combination of HAN with doxorubicin or with oxaliplatin induced suppression of cell proliferation for the MCF-7, MDA-MB231, and Caco2 cell lines, as compared to free hippuric acid and free doxorubicin and oxaliplatin, which did not exhibit the same effects. This result can be proved by the antiproliferative study. Finally, the resulting combination of HAN with doxorubicin and oxaliplatin induced cytotoxicity in the MDA-MB231, MCF-7, and Caco2 cell lines.
Acknowledgments
We thank the Ministry of Higher Education of Malaysia for financial support under grant number FRGS/1/11/SG/UPM/01/2 (Vot 5524165) for funding this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- Carlino S The intercalation of carboxylic acids into layered double hydroxides: a critical evaluation and review of the different methods Solid State Ionics 1997 98 1–2 73 84
- Williams GR O’Hare D Towards understanding, control and application of layered double hydroxide chemistry J Mater Chem 2006 16 30 3065 3074
- Rives V Layered Double Hydroxides: Present and Future Hauppage, NY Nova Science Publishers Inc 2001
- Minagawa K Berber MR Hafez IH Mori T Tanaka M Target delivery and controlled release of the chemopreventive drug sulindac by using an advanced layered double hydroxide nanomatrix formulation system J Mater Sci Mater Med 2012 23 4 973 981 22350776
- Cavalieri SJ Biehle JR Sanders WEJr Synergistic activities of clarithromycin and antituberculous drugs against multidrug-resistant Mycobacterium tuberculosis Antimicrob Agents Chemother 1995 39 7 1542 1545 7492101
- Stoppoloni D Canino C Cardillo I Synergistic effect of gefitinib and rofecoxib in mesothelioma cells Mol Cancer 2010 9 27 20122271
- Hamilton-Miller JM Brumfitt W Methenamine and its salts as urinary tract antiseptics: variables affecting the antibacterial activity of formaldehyde, mandelic acid, and hippuric acid in vitro Invest Urol 1977 14 4 287 291 13049
- Kulcsár G Synergistic potentiating effect of D(+)-mannose, orotic, and hippuric acid sodium salt on selective toxicity of a mixture of 13 substances of the circulatory system in culture for various tumor cell lines Cancer Detect Prev 2000 24 5 485 495 11129991
- Hussein Al Ali SH Al-Qubaisi M Hussein MZ Zainal Z Hakim MN Preparation of hippurate-zinc layered hydroxide nanohybrid and its synergistic effect with tamoxifen on HepG2 cell lines Int J Nanomedicine 2011 6 3099 3111 22163163
- Virag P Perde-Schrepler M Fischer-Fodor E Superior cytotoxicity and DNA cross-link induction by oxaliplatin versus cisplatin at lower cellular uptake in colorectal cancer cell lines Anticancer Drugs Epub May 18, 2012 1032 1038
- Zhou Y Gu X Ashayeri E Zhang R Sridhar R Nicotine decreases the cytotoxicity of doxorubicin towards MCF-7 and KB-3.1 human cancer cells in culture J Natl Med Assoc 2007 99 4 319 327 17444420
- Smith L Watson MB O’Kane SL Drew PJ Lind MJ Cawkwell L The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays Mol Cancer Ther 2006 5 8 2115 2120 16928833
- Hussein MZ Al Ali SH Zainal Z Hakim MN Development of antiproliferative nanohybrid compound with controlled release property using ellagic acid as the active agent Int J Nanomedicine 2011 6 1373 1383 21796241
- Hussein Al Ali SH Al-Qubaisi M Controlled-release formulation of antihistamine based on cetirizine zinc-layered hydroxide nanocomposites and its effect on histamine release from basophilic leukemia (RBL-2H3) cells Int J Nanomedicine 2012 7 3351 3363 22848164
- Xingfu Z Zhaolin H Yiqun F Su C Weiping D Nanping X Microspheric organization of multilayered ZnO nanosheets with hierarchically porous structures The Journal of Physical Chemistry C 2008 112 31 11722 11728
- Doyle LA Yang W Abruzzo LV A multidrug resistance transporter from human MCF-7 breast cancer cells Proc Natl Acad Sci U S A 1998 95 26 15665 15670 9861027
- Hiraga T Williams PJ Mundy GR Yoneda T The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases Cancer Res 2001 61 11 4418 4424 11389070
- Darmoul D Lacasa M Baricault L Dipeptidyl peptidase IV (CD 26) gene expression in enterocyte-like colon cancer cell lines HT-29 and Caco-2. Cloning of the complete human coding sequence and changes of dipeptidyl peptidase IV mRNA levels during cell differentiation J Biol Chem 1992 267 7 4824 4833 1347043
- Choy JH Jung JS Oh JM Layered double hydroxide as an efficient drug reservoir for folate derivatives Biomaterials 2004 25 15 3059 3064 14967539