Abstract
Purpose
7-Difluoromethyl–5, 4′-dimethoxygenistein (DFMG), prepared by the difluoromethylation and alkylation of Genistein, is an active new chemical entity. Its anti-atherosclerosis effect was found in a series of studies in vitro. In this article, we explored and evaluated the anti-atherosclerosis effect via its protection of endothelial function in ApoE−/− mice that were fed a high-fat diet.
Methods
Five C57BL/6J mice were selected as a control group and were fed a 1% high-fat diet (control group, n = 5). Five ApoE−/− mice that were fed a high-fat diet for 16 weeks were selected as the atherosclerosis model group (model group, n = 5). In the phase I study, 25 ApoE−/− mice were provided a prophylactic treatment with different drugs at the beginning of the 16 week high-fat diet: 5 mg/gk genistein (genistein 1 group, n = 5), 5 mg/kg lovastatin (lovastatin1 group, n = 5), 2.5 mg/kg DFMG (DFMG L1 group, n = 5), 5 mg/kg DFMG (DFMG M1 group, n = 5), and 10 mg/kg DFMG (DFMG H1 group, n = 5). In the phase II study, 25 atherosclerosis model, ApoE−/− mice were treated with different drugs and fed a high-fat diet for 16 weeks: 5 mg/gk genistein (genistein 2 group, n = 5), 5 mg/kg lovastatin (lovastatin 2 group, n = 5), 2.5 mg/kg DFMG (DFMG L2 group, n = 5), 5 mg/kg DFMG (DFMG M2 group, n = 5), and 10 mg/kg DFMG (DFMG H2 group, n = 5). The plasma levels of lipids, von Willebrand factor (vWF), and nitrite were compared between phases I and II. Endothelium-dependent relaxation (EDR), aortic lesion development, and quantification in thoracic aortas were measured during these two phase studies.
Results
Compared to the model group, the lipid and vWF plasma levels were significantly lower, the plasma nitrite levels were significantly higher, the fatty streaks of aortic lesions were significantly lower, and the endothelium dependent relaxation was significantly higher after both phase studies (P < 0.05). The DFMG supplementation led to significant plasma nitrite increment in all groups after both phase studies (P < 0.05). There were significantly decreased fatty streaks of aortic lesions in DFMG-prevented and DFMG-treated mice (P < 0.05). There was a significant increase in EDR in all prophylactic treatment groups and treatment groups (P < 0.05). We further demonstrated that the preventative effect was more obvious than the therapeutic effect.
Conclusion
Our results suggest that DFMG could work in prophylactic and therapeutic treatments for atherosclerosis development.
Introduction
Atherosclerosis (AS) is a chronic and degenerative disease of the large artery walls. It is a leading cause of mortality and morbidityCitation1 and is the single most important cause of cardiovascular disease (CVD), a predominant health problem worldwide.Citation2 Clinical manifestations of atherosclerosis include myocardial infarction, heart failure, stroke, and peripheral artery disease, all of which resulting in irreversible organ damage.Citation3 The incidence rate of atherosclerotic disease has been on the rise due to dietary habits that originated in the West, insufficient exercise brought about by persistent motorization, increased mental stress from social downturn, and the rapid progression of aging in society.Citation4 As atherosclerosis has a long asymptomatic phase and the first manifestation of the disease may be sudden cardiac death, it is essential to find effective strategies to prevent it.Citation5,Citation6 Current guidelines for the prevention of atherosclerotic diseases focus on treatment of established cardiovascular risk factors to attenuate the subsequent endothelial dysfunction and damage.Citation6 Endothelial dysfunction (ED) is an early event in atherosclerosis and plays a pivotal role in the atherogenesis process.Citation7
The endothelium is the main regulator of vascular wall homeostasis. In response to a variety of noxious stimuli, the endothelium undergoes phenotypical modulation to a non-adaptive state (known as “endothelial dysfunction”), which is characterized by the loss or dysregulation of the homeostatic mechanisms that operate in healthy endothelial cells. This pathophysiological condition is associated with the increased expression of adhesion molecules, the increased synthesis of proinflammatory and pro-thrombotic factors, increased oxidative stress, and the abnormal modulation of vascular tone, all of which may lead to functional manifestations, including impaired endothelium-dependent vasodilatation.Citation8 Current evidence suggests that endothelial dysfunction occurs early in the process of atherogenesis and contributes to the formation, progression, and complications of atherosclerotic plaque. A number of studies have shown that endothelial dysfunction affects patients with cardiovascular risk factors (but who are still without any clinical signs of atherosclerosis), as indicated by their impaired response to endothelial vasodilators, such as acetylcholine and bradykinin.Citation9–Citation11 In addition to the endothelium’s pathophysiological role, these observations strongly suggest that endothelial dysfunction is a common mechanistic link between atherosclerotic risk factors and the development of atherosclerosis. Furthermore, various studiesCitation7,Citation9 have shown that endothelial dysfunction is an independent predictor of future cardiovascular events in patients with atherosclerotic risk factors, stable ischemic heart disease, or acute coronary syndromes. Therefore, endothelial dysfunction seems to be a systemic vascular process that not only mediates the development of atherosclerotic plaque but also modulates the clinical course of atherosclerosis.
Genistein (GEN), 4′, 5, 7-trihydroxyisoflavone, is an isoflavonoid compound derived from soy products. GEN could have a role similar to estrogen because its chemical structure is similar to estradiol.Citation12,Citation13 The protective effects of soy isoflavones in AS include a series of mechanisms, such as the prevention of low-density lipoprotein (LDL) oxidation, the improvement of vascular reactivity, the inhibition of proinflammatory cytokines or cell adhesion proteins, and the reduction of platelet aggregation.Citation14 Recent studies have demonstrated that GEN inhibits the hyperpermeability of cultured vascular endothelial cells (ECs)Citation15 and acts as antiinflammatory and antiatherogenic agents in human ECs.Citation16 However, the low absorption of GEN in the intestine and the rapid metabolic elimination of GEN, due to the hydroxyls at the C-5, C-7, and C-40 positions (allowing GEN to bind to glucuronic acid and sulfuric acid), reduce its bioavailability and bioactivity in vivoCitation17 and restrict its clinical application. To overcome this problem, more effective GEN analogues were designed still using GEN as the leading compound.
We previously synthesized a series of novel GEN derivatives and demonstrated that 7-difluoromethyl–5, 4′-dimethoxygenistein (DFMG) had the strongest inhibitory effect on hydrogen peroxide-induced impairment in human umbilical vein endothelial (HUVE-12) cells in vitro.Citation18 Our previous study showed that DFMG could decrease the release of the cell adhesion molecule and inflammatory factor by down-regulating the expression of NF-κB and decreasing the adhesion of circulating monocytes to endothelial cells, a critical step in the early stages of atherosclerosis.Citation19 In the present study, using lovastatin as an active control, we further investigated the preventive effect of DFMG on atherosclerosis in vivo in ApoE−/− mice, a mouse model for atherosclerosis.
Materials and methods
Reagents
DFMG (98% pure) was synthesized in the Pharmacy Department of Hunan Normal University (People’s Republic of China) and identified in the Analysis Center of Hunan University.Citation18 The molecular formula of DFMG is C18H14O5F2, and its molecular weight is 348. It has the characteristics of a pallide-flavens crystal powder, and the detailed spectroscopic analysis is as follows: infrared radiation (IR) õmax (cm−1, KBr): 1430, 1469, 1512, 1580, 1612, and 1637 (C=O) and 1H nuclear magnetic resonance (NMR) (300 MHz, CDCl3): 3.834 (3H, s), 3.970 (3H, s), 6.54 (1H, d, J = 2.1 Hz), 6.642 (1H, t, J = 72.3 Hz), 6.724 (1H, d, J = 2.1 Hz), 6.940 (1H, d, J = 8.7 Hz), 7.472 (1H, d, J = 8.7 Hz), and 7.811 (1H, s). GEN and dimethyl sulfoxide were purchased from Sigma-Aldrich (St Louis, MO, USA). Lovastatin (20 mg/tablet) was purchased from the Yangtze River Pharmaceutical Group (Guangzhou, People’s Republic of China).
Animal model and treatment
The Institutional Animal Care and Use Committee of Hunan Normal University approved this study, and the study conformed to the procedures described in the Guide for the Care and Use of Laboratory Animals of the Hunan Normal University. Male C57BL/6J ApoE knockout and C57BL/6J mice (6–8 weeks old), weighing 20–25 g, were purchased from the Shanghai Slac Laboratory Animal Co, Ltd (Shanghai, People’s Republic of China). The animals were housed in microisolator cages in a room at 20°C ± 2°C, with a relative humidity of 55% ± 15%, and a 12/12 hour light/dark cycle. Care of the animals and all experimental procedures were conducted in accordance with the institutional guidelines for animal research. After a 10-day acclimation period and an overnight fasting, blood samples were taken from the mice for pre-experiment sampling. Collected blood samples were centrifuged (1000 g), and the resulting plasma was stored at −70°C until measurements were performed. Five C57BL/6J mice (6–8 weeks old) were selected as the control group (wild type group) and were fed a high-fat diet (78.8% standard diet, 10.0% yolk powder, 10.0% lard 1.0% cholesterol, and 0.2% sodium taurocholate) (control group, n = 5). The experimental diet was purchased from the Shanghai Slac Laboratory Animal Co, Ltd (Shanghai, People’s Republic of China). Five ApoE−/− mice that were fed a high-fat diet for 16 weeks were selected as the atherosclerosis model group (model group, n = 5). The animal model of atherosclerosis was built as previously described by Plump.Citation20 This study was divided into two phases: phase I, the study of prevention, and phase II, the study of treatment.
For phase I, the study of prophylactic treatment, against AS 25 ApoE−/− mice (6–8 weeks old) were provided prophylactic treatment with different drugs at the beginning of a 16-week, high-fat diet. The drug groups included: 5 mg/gk GEN (genistein 1 group, n = 5), 5 mg/kg lovastatin (lovastatin 1 group, n = 5), 2.5 mg/kg DFMG (DFMG L1 group, n = 5), 5 mg/kg DFMG (DFMG M1 group, n = 5), and 10 mg/kg DFMG (DFMG H1 group, n = 5).
For phase II, the study of treatment, 25 atherosclerosis model, ApoE−/− mice were treated with different drugs after they were fed the high fat diet for 16 weeks. The drug groups included: 5 mg/gk GEN (genistein 2 group, n = 5), 5 mg/ kg lovastatin (lovastatin 2 group, n = 5), 2.5 mg/kg DFMG (DFMG L2 group, n = 5), 5 mg/kg DFMG (DFMG M2 group, n = 5), and 10 mg/kg DFMG (DFMG H2 group, n = 5).
Measurement of serum lipid levels
Blood samples were collected via sinus orbital bleeding of the mouse model at three points: before feeding of the high-fat diet, after feeding of the high-fat diet for 16 weeks, and after treatment for 16 weeks. Plasma was separated and stored at −70°C until further analysis was needed. Concentrations of plasma Total cholesterol, triglyceride, high-density lipoprotein (HDL), and LDL cholesterol were determined using an automatic analyzer (Roche P800; Roche Diagnostics, Indianapolis, IN, USA).
Plasma vWF and nitrite measurement
Von Willebrand factor (vWF) is a well-established in vivo marker of endothelial cell activation and injuryCitation21 Plasma vWF was measured by sandwich enzyme-linked immunosorbent assay (ELISA), using paired capture and detecting anti-vWF The vWF antibody (Cedarlane Co, Burlington, ON, Canada) was as previously described.Citation21
The plasma level of nitrite (stable NO metabolite) was measured using a colorimetric assay kit (R&D Systems, Minneapolis, MN, USA) that involved the Griess reaction, as previously described.Citation22
Aortic lesion development and quantification in thoracic aortas
Lesion development in ApoE−/− mice was quantified via stains with the neutral lipid-targeting lysochrome Oil Red O (ORO; Sigma, Poole, UK), and then the dye retained by the aorta tissue was solubilized and quantified as previously described.Citation23,Citation24 A 3% solution (w/v) of ORO in 2-propanol was prepared by heating the reagents to 56°C for 1 hour, and then the solution was cooled before filtration through a number 1 filter paper (Whatman, Maidstone, UK). A working solution was prepared by diluting the stock solution 6:4 with deionized water. Before use, the working solution was passed through a 0.2 μM filter. Each aorta was rinsed in distilled water, quickly rinsed in 70% 2-propanol, and stained for 30 minutes in the ORO working solution, then rinsed again for 10 seconds in 70% 2-propanol, and then returned to the distilled water. Aortas were inspected under the microscope, and any small pieces of stained adventitial fat that remained attached were removed carefully without disturbing the plaques. Stained aortic arches and descending thoracic aortas were gently blotted and then transferred into 96-well polypropylene plates (Greiner Bio-One Ltd Stonehouse, UK) containing 100 μL chloroform/methanol (2:1 v/v). The plates were placed on an orbital shaker for 4 minutes, until all the stain was dissolved. Aorta samples were then removed rinsed in phosphate buffered saline (PBS), and homogenized in 200 μL of PBS using a Dounce homogenizer and 1 mm zirconia beads (5.5 g/cm3, BioSpec Products Inc, Bartlesville, OK, USA). The homogenate was centrifuged on a bench-top microcentrifuge at 13,000 rpm for 5 minutes, and protein was measured in the supernatant using the BCA protein assay (Pierce, Rockford IL, USA). ORO was measured using a μQuant plate reader (Bio-Tek Instruments Inc, Winooski, VT, USA) at 520 nm, and the quantity (μmol) of ORO retained and released from the tissue was calculated using a standard curve of ORO (3.12-100 μM) in chloroform/methanol 2:1. Results were expressed as μmol ORO/μg protein.
Preparation of aortic rings and tension recording
Mechanical function studies of isolated thoracic aortas were carried out. Briefly, the thoracic aorta without adventitial tissue was removed and cut into rings (4 mm in length). For isometric tension recording, the rings were mounted in a thermostatic organ bath. After excision, the descending thoracic aorta was immediately immersed in chilled Tyrode solution containing (in mM): NaCl 118.0, CaCl2 2.5, KCl 4.73, MgCl2 1.2, KH2PO4 1.2, NaHCO3 25.0, EDTA 0.026, D(+)glucose 5.5 (pH 7.4), and adventitial tissue was carefully removed. The 4 mm rings were mounted in organ bath chambers and attached to an isometric tension transducer (Chengdu Instruments Factory, Chengdu, People’s Republic of China). The aortic rings were equilibrated at a resting tension of 2 mN, which was maintained throughout the experiment. Following equilibration, aortic rings were precontracted with an α-agonist (R)-(−)-phenylephrine-HCl (10 μM). After a steady state of contraction had been induced drugs were added in increasing concentrations to obtain cumulative concentration-response curves for acetylcholine (representing an endothelium-dependent relaxing agent, 1 nM–1 mM) and glyceryl-trinitrate (as a nitric oxide donor, representing a non-endothelium-dependent relaxing agent, 100 nM–100 mM). The drugs were washed out before adding the next substance. The relaxing effect of acetylcholine was abolished by adding Nω-nitro-L-arginine methyl ester (L-NAME, 1 μM). The presence of functional endothelium was verified by the ability of acetylcholine (10 μM) to induce more than 70% relaxation of rings precontracted with phenylephrine (1 μM).Citation25,Citation26 Aortic rings without any response to acetylcholine (relaxation < 10%) were excluded from statistical analysis due to presumable damage of the endothelium. The contractile activity was digitalized with the BL-420E+ biological and functional experimental system (Chengdu TME Technology Co., Ltd Chengdu, People’s Republic of China). Cumulative concentration-response curves for acetylcholine (1 nM–100 mM) and glyceryl trinitrate (100 nM–100 mM) were obtained (as described above), abolishing the relaxing effect of acetylcholine by adding L-NAME (1 μM).
Statistical analysis
The data are reported as the mean ± SEM. Phosphate buffered saline (PBS). The acetylcholine- or glyceryl trinitrate-induced maximal relaxation (Emax) in the aortic rings was calculated as a percentage of the contraction in response to phenylephrine (10 μM). A statistical software package, SPSS (version 13, SPSS Inc., IBM, Chicago, IL, USA), was used to perform the statistical analysis. The data were tested for normality and homogeneity of variance. ANOVA repeated measures were used to assess changes within a group, and one-way ANOVA (followed by Bonferroni’s t-test) within groups were used to assess the significance of any change between groups. Non-parametric methods (Wilcoxon rank-sum test and Kruskal-Wallis test) were used for the comparison of aortic lesion quantification within and between groups. Statistical significance was accepted at P < 0.05.
Results
DFMG decreased the plasma level of lipids and lipoproteins in ApoE−/− mice
The overproduction of lipoproteins and the impaired plasma clearance secondary to the down-regulation of LDL receptor expression in the liver are responsible for the hyperlipidemia induced by dietary cholesterol in ApoE−/− mice.Citation27 shows the effects of a 16-week, high-fat diet on cholesterol and LDL levels. Basal values of lipids and lipoproteins were similar in all groups. The cholesterol-rich diet induced a significant increase of total cholesterol, LDL- and HDL-cholesterol, and triglyceride (TG) in all atherosclerosis model groups (P < 0.05) through the phase I study. By the end of phase II, plasma cholesterol, LDL, and HDL were reduced significantly, but they did not return to baseline values in all groups (). The plasma TG level was reduced significantly, and there was no difference between pre-experiment and ending values (). The lipid and lipoprotein levels in the control group remained statistically unchanged throughout the study. By the end of phase II, the animals treated with different dosages of DFMG had similar levels of cholesterol, LDL, and TG as the DFMG-prevented groups by the end of phase I (P > 0.05). All of the lipids and lipoproteins in the DFMG-prevented and DFMG-treated groups were significantly higher than the control group (P < 0.05) (). These results showed that DFMG had some lipid-lowering effects in our study.
Table 1 The plasma levels of lipids, lipoproteins, nitrite, and vWF in different groups of the study
DFMG increased the plasma level of nitrite in ApoE−/− mice
During the phase I study, the plasma level of nitrite significantly increased in the DFMG-prevented groups (P < 0.05), and it was also significantly higher than the level in the model group (P < 0.05) (). Compared with the control group, the plasma nitrite in the model group was significantly lower (control, 11.68 ± 0.21; Model, 8.22 ± 0.17; P < 0.05). In the phase I study, the plasma nitrite level in the lovastatin-prevented group, genistein-prevented group, and DFMG-prevented group was significantly ameliorated when compared to the model group, but it was similar to the plasma nitrite level in the normal group. An entirely similar situation existed in the phase II study. Although a slight increase in plasma nitrite was found in DFMG-prevented groups, there was significant difference in plasma nitrite between DFMG-prevented and DFMG-treated groups (P < 0.05). At the same time, there was no significant difference in the plasma nitrite between DFMG groups with a lower dosage (2.5 mg/kg d−1) and lovastatin groups with a higher dosage (5 mg/kg d−1) (P > 0.05) in phase I ().
DFMG decreased the plasma level of vWF in ApoE−/− mice
Contrary to the change in plasma nitrite, the plasma level of vWF significantly decreased in DFMG-prevented groups (P < 0.05) during the phase I study, and it was significantly lower than the plasma vWF in the model group (P < 0.05) (). Compared with the control group, the plasma vWF in the model group was significantly higher (control, 0.57 ± 0.09; Model, 16.41 ± 0.55; P < 0.05). In the phase I study, the plasma vWF in the lovastatin-prevented group, genistein-prevented group, and DFMG-prevented group was significantly decreased when compared to the model group, but it was similar to the plasma vWF in the control group. An entirely similar situation existed in the phase II study. Although a slight decrease in plasma vWF was found in the DFMG-prevented groups, there was significant difference in plasma vWF between DFMG-prevented and DFMG-treated groups. At the same time, there was no significant difference in the plasma vWF between DFMG groups with a lower dosage (2.5 mg/kg•d−1) and lovastatin groups with a slightly higher dosage (5 mg/kg•d−1) (P > 0.05) in phase I ().
The effect of DFMG on aortic lesion development and quantification
shows the extent of atherosclerotic lesions on the thoracic arteries. In the phase I study, the ORO staining of aortas from the model group of ApoE−/− mice revealed early atheromatous lesions (fatty streaks) in the aortic arteries. The staining in aortas from control mice was diffuse, and there was no evidence of plaque formation. Quantification of the ORO stain in the aortic arch of ApoE−/− mice showed a very significant decrease () compared to the atherosclerosis model group, as would be expected from the visually evident staining within the aorta tissue (). The atherosclerotic lesions in the model group averaged 7.70 ± 0.33 μmol/μg. Prevention and treatment with lovastatin, GEN, and different dosages of DFMG decreased this (P < 0.05). Atherosclerotic lesions between the lovastatin and DFMG groups showed no significant difference. The quantification of atherosclerotic lesions in the DFMG-prevented groups was significantly less than the quantification in the DFMG-treated groups (P < 0.05).
The effect of DFMG on the EDR of mice aortas
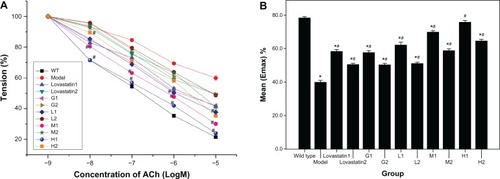
The model group had a significantly reduced (49% at 10 μM acetylcholine; P < 0.05) endothelium-dependent relaxation (EDR) in response to acetylcholine when compared to the control mice ( and 4). The EDR of aortic tissue in response to acetylcholine was improved in the DFMG-prevented and DFMG-treated groups of ApoE−/− mice at all concentrations when compared to the model mice (P < 0.05). At the same time, the Emax (acetylcholine-induced maximal relaxation) in the aortic rings from the DFMG-prevented groups was significantly higher than the Emax from the DFMG-treated groups. These data confirmed that DFMG could significantly ameliorate EDR in the rings of mice aortas and thus prevent endothelium dysfunction.
Discussion
The present study demonstrated that DFMG had some effect on the hyperlipidemia in ApoE−/− mice. Compared with the model group, the plasma levels of lipids in the DFMG groups were significantly in both study phases (early prevention and late treatment) but were still higher than those in the control group. Thus, DFMG could protect the arteries from atherosclerosis through the prevention of endothelium dysfunction and the reduction of arterial plaque. DFMG could also lead to a significant plasma nitrite increase, a fatty streak decrease, and an EDR elevation, especially as evident in the early prevention group in the phase I study when compared to late treatment group in the phase II study.
A new chemical entity, DFMG () was synthesized, as reported.Citation16 Data suggested that DFMG possesses a protective effect against vascular endothelium injury caused by oxidative stress. Our team previously focused on the protective effects and mechanisms of DFMG as applied to the damaged endothelium.Citation18,Citation19,Citation28,Citation29 We had also previously revealed that DFMG has an antiatherosclerosis effect, decreasing hyperlipidemia and possessing antioxidant and plaque stabilization properties in a rabbit model of atherosclerosis.Citation30 Thus, DFMG may be useful for the prevention of atherosclerosis.
Figure 1 Structure of genistein and DFMG (A) the structure of genistein and (B) the structure of 7-Difluoromethyl-5, 4′-dimethoxygenistein (DFMG).
Numerous animal species have been used to study the pathogenesis and potential treatment of atherosclerotic lesions. Shore first described ApoE as a lipoprotein constituent of triglyceride-rich very low-density lipoproteins (VLDLs).Citation31 ApoE is a major component in several classes of plasma lipoproteins and has been implicated in the maintenance of overall plasma cholesterol homeostasis because of it facilitating the hepatic uptake of lipoproteins by binding to their receptors. ApoE also carries out additional functions, including the stimulation of cholesterol efflux from macrophages, the prevention of platelet aggregation, and the inhibition of proliferation of T-lymphocytes and endothelial cells. ApoE−/− mice are severely hypercholesterolemic when compared to the wild type counterpart, and the increase in cholesterol is largely distributed in LDLs due to their impaired clearance.Citation20,Citation32,Citation33 When compared to the rabbit model, the ApoE−/− mouse contains the entire spectrum of lesions observable during atherogenesis, and the ApoE−/− was the first mouse model to develop lesions similar to those of humans. Hence, ApoE−/− mice are one of the most relevant models for atherosclerosis because they are hypercholesterolemic and develop spontaneous arterial lesions.Citation34 Based on these facts, we selected ApoE−/− mice to produce the atherosclerosis model for the present research.
In this study, we administered to the mice upon their being given a high-fat diet for 16 weeks. We did this to examine the preventive effects of DFMG on atherosclerosis and hyperlipemia in phase I and to examine its therapeutic effects in phase II. After 16 weeks of administration, serum total cholesterol and LDL-cholesterol levels in the DFMG groups of phase I and phase II were significantly reduced when compared with the levels in the atherosclerotic mouse model; this effect was similar to that of the lovastatin group in phase I and phase II ().
Lovastatin belongs to the statin family and is commonly used to prevent and treat hyperlipemia and atherosclerosis.Citation35 The atherosclerotic lesions in thoracic aortas of the DFMG-prevented or DFMG-treated groups were markedly smaller than those in the model group (). The quantification of atherosclerotic lesions in thoracic aortas in the DFMG-treated groups was similar to the quantification in the lovastatin-treated group. Moreover, the quantification of atherosclerotic lesions in the DFMG-prevented groups was significantly less than the quantification in the DFMG-treated groups. These data demonstrated that DFMG had obvious preventive and therapeutic effects on atherosclerosis and hyperlipemia in an atherosclerotic animal model.
Figure 2 Aortic lesion development (A) and quantification (B). (A) ORO-stained aortic arches from ApoE−/− mice that were fed a high-fat diet for 16 weeks in different groups and phases. Lesion staining is intensely focused at the aortic arch and at the branch points of the aorta. Compared with the atherosclerosis model group, the aortic lesion developed differently in the prevented and treated groups with different drugs. (B) Quantification of ORO staining in the aortic arch of ApoE−/− mice after consuming a high-fat diet in different groups and phases.
Abbreviations: DFMG-H(I), DFMG-prevented group (10 mg/kg/d, ig); DFMG-H(II), DFMG-treated group (10 mg/kg/d, ig); DFMG-L(I), DFMG-prevented group (2.5 mg/kg/d, ig); DFMG-L(II), DFMG-treated group (2.5 mg/kg/d, ig); DFMG-M(I), DFMG-prevented group (5 mg/kg/d, ig); DFMG-M(II), DFMG-treated group (5 mg/kg/d, ig); Genistein(I), genistein-prevented group (5 mg/kg/d, ig); Genistein(II), genistein-treated group (5 mg/kg/d, ig); Lovastatin(I), lovastatin-prevented group (5 mg/kg/d, ig); Lovastatin(II), lovastatin-treated group (5 mg/kg/d, ig); Model, atherosclerosis model group; ORO, Oil red O; WT, wild type group (C57BL/6J mice).
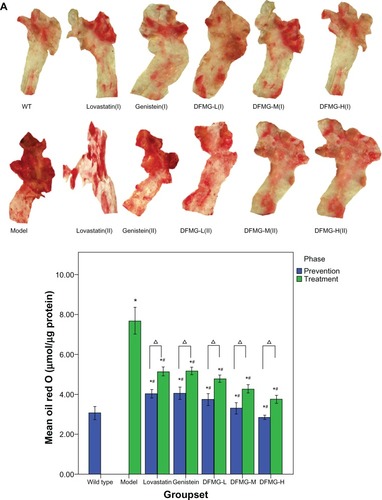
Figure 3 Acetylcholine (ACh)-induced endothelium-dependent relaxation (A) and the Emax (B) of thoracic aortic rings in different groups of mice previously contracted by phenylephrine (PE).
Abbreviations: GI, genistein-prevented group (5 mg/kg/d, ig); G2, genistein-treated group (5 mg/kg/d, ig); HI, DFMG-prevented group (10 mg/kg/d, ig); H2, DFMG-treated group (10 mg/kg/d, ig); LovastatinI, lovastatin-prevented group (5 mg/kg/d, ig); Lovastatin2, lovastatin-treated group (5 mg/kg/d, ig); LI, DFMG-prevented group (2.5 mg/kg/d, ig); L2, DFMG-treated group (2.5 mg/kg/d, ig); Model, atherosclerosis model group; MI, DFMG-prevented group (5 mg/kg/d, ig); M2, DFMG-treated group (5 mg/kg/d, ig); WT, wild type group (C57BL/6J mice).
The endothelium is a major regulator of vascular tone, remodeling, and arterial inflammation and thrombosis; thus, it is the key to the atherosclerotic disease process. Endothelial dysfunction is primarily characterized by impaired NO-induced vasodilation.Citation36–Citation38 Furthermore, endothelium-derived NO promotes endothelium-dependent vasodilation,Citation39 such as during physical exercise, and it also has important anti-inflammatory and antithrombotic effects, thereby actively suppressing leukocyte adhesion and arterial thrombosis. Experimental studies could then confirm that endothelium-derived NO prevents development of atherosclerotic lesions.Citation38,Citation40–Citation42 Consequently, endothelium-derived NO can be considered an important endogenous antiatherogenic system.
Significantly, endothelial dysfunction represents a common pathway for all known cardiovascular risk factors for the development and progression of vascular disease, such as dyslipidemia, smoking, diabetes, hypertension, obesity, and mental stress. Most studies have used the assessment of EDR as an index of “endothelial function.” Endothelial dysfunction represents an early event in the development of atherosclerosis and is induced by all known cardiovascular risk factors.Citation43
As lovastatin could prevent the increase of serum vWF, DFMG could decrease the level of serum vWF in atherosclerosis for the ApoE−/− mouse model. The level of serum nitrite in our DFMG-prevented group was significantly higher than the level in our DFMG-treated group. When compared to the model mice, all of the DFMG-prevented and DFMG-treated groups of our ApoE−/− mice had improved EDR of the aortic tissue in response to acetylcholine. At the same time, the Emax in the aortic rings from the DFMG-prevented groups was significantly higher than the Emax for DFMG-treated groups. These data confirmed that DFMG could significantly ameliorate the EDR in rings of mice aortas and prevent endothelium dysfunction.
GEN has estrogenic activities and can bind to estrogen receptors (ERs). GEN can also activate inducible nitric oxide (iNOS) and up-regulate NO production through the estrogen receptor α (ERα) pathway in RAW264.7 cells.Citation44 As the structure of DFMG is similar to GEN, we speculate that the mechanism of DMFG action was primarily mediated through the ERa pathway.
Conclusion
We have shown that DFMG had some effect on the hyperlipidemia in ApoE−/− mice and could protect arteries from atherosclerosis. DFMG could prevent endothelium dysfunction in the early stage of atherosclerosis and could improve the EDR of aortic arteries in ApoE−/−. The attenuating NO production of DFMG may be responsible for mediating this vascular protective effect. Thus, our research suggests that DFMG may be useful for the development of prophylactic and therapeutic treatments for atherosclerosis.
Acknowledgment
This study was supported by a grant from the Natural Science Foundation of China (No 30971270) and the Science-Technology Foundation of Hunan Province, China (Grant No 2012SK3125).
Disclosure
The authors declare that they had no financial or personal relations with other parties whose interests could have positively or negatively affected the content of this article in any way.
References
- Libby P Ridker PM Hansson GK Progress and challenges in translating the biology of atherosclerosis Nature 2011 473 317 325 21593864
- Murray CJ Lopez AD Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study Lancet 1997 349 1436 1442 9164317
- Werner N Nickenig G Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol 2006 26 257 266 16322535
- Fuster V Kelly BB Vedanthan R Promoting global cardiovascular health: moving forward Circulation 2011 123 1671 1678 21502585
- Napoli C Lerman LO de Nigris F Gossl M Balestrieri ML Lerman A Rethinking primary prevention of atherosclerosis-related diseases Circulation 2006 114 2517 2527 17146003
- Mensah GA Ryan US Hooper WC Vascular endothelium summary statement II: cardiovascular disease prevention and control Vascul Pharmacol 2007 46 318 320 17229595
- Bonetti PO Lerman LO Lerman A Endothelial dysfunction: a marker of atherosclerotic risk Arterioscler Thromb Vasc Biol 2003 23 168 175 12588755
- Tritto I Ambrosio G The multi-faceted behavior of nitric oxide in vascular “inflammation”: catchy terminology or true phenomenon? Cardiovasc Res 2004 63 1 4 15194454
- Zardi EM Afeltra A Endothelial dysfunction and vascular stiffness in systemic lupus erythematosus: are they early markers of subclinical atherosclerosis? Autoimmun Rev 2010 9 684 686 20553974
- Furchgott RF Zawadzki JV The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine Nature 1980 288 373 376 6253831
- Bonetti PO Lerman LO Lerman A Endothelial dysfunction: a marker of atherosclerotic risk Arterioscler Thromb Vasc Biol 2003 23 168 175 12588755
- Huang R Shi F Lei T Song Y Hughes CL Liu G Effect of the isoflavone genistein against galactose-induced cataracts in rats Exp Biol Med (Maywood) 2007 232 118 125 17202592
- Kwon SH Kang MJ Huh JS Comparison of oral bioavailability of genistein and genistin in rats Int J Pharm 2007 337 148 154 17280808
- Wenzel U Fuchs D Daniel H Protective effects of soy-isoflavones in cardiovascular disease. Identification of molecular targets Hamostaseologie 2008 28 85 88 18278168
- Liu D Jiang H Grange RW Genistein activates the 3′, 5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function Endocrinology 2005 146 1312 1320 15591142
- Simoncini T Garibaldi S Fu XD Effects of phytoestrogens derived from red clover on atherogenic adhesion molecules in human endothelial cells Menopause 2008 15 542 550 18467954
- Kroon PA Clifford MN Crozier A How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr 2004 80 15 21 15213022
- Fu XH Wang L Zhao H Xiang HL Cao JG Synthesis of genistein derivatives and determination of their protective effects against vascular endothelial cell damages caused by hydrogen peroxide Bioorg Med Chem Lett 2008 18 513 517 18068980
- Wang L Zheng X Xiang HL Fu XH Cao JG 7-Difluoromethyl–5, 4′-dimethoxygenistein inhibits oxidative stress induced adhesion between endothelial cells and monocytes via NF-κB Eur J Pharmacol 2009 605 31 35 19248247
- Plump AS Smith JD Hayek T Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells Cell 1992 71 343 353 1423598
- Haghjooyjavanmard S Nematbakhsh M Monajemi A Soleimani M Von Willebrand factor, C-reactive protein, nitric oxide, and vascular endothelial growth factor in a dietary reversal model of hypercholesterolemia in rabbit Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2008 152 91 95 18795081
- Haghjooy Javanmard S Nematbakhsh M Monajemi A L-arginine supplementation influenced nitrite but not nitrate and total nitrite in rabbit model of hypercholesterolemia Iran Biomed J 2008 12 179 184 18762822
- Nunnari JJ Zand T Joris I Majno G Quantitation of oil red O staining of the aorta in hypercholesterolemic rats Exp Mol Pathol 1989 51 1 8 2767215
- Beattie JH Duthie SJ Kwun IS Ha TY Gordon MJ Rapid quantification of aortic lesions in apoE−/− mice J Vasc Res 2009 46 347 352 19142014
- Qian LB Wang HP Qiu WL Huang H Bruce IC Xia Q Interleukin-2 protects against endothelial dysfunction induced by high glucose levels in rats Vascul Pharmacol 2006 45 374 382 16837248
- Schlimmer N Kratz M Bohm M Baumhakel M Telmisartan, ramipril and their combination improve endothelial function in different tissues in a murine model of cholesterol-induced atherosclerosis Br J Pharmacol 2011 163 804 814 21323898
- Wang BY Singer AH Tsao PS Drexler H Kosek J Cooke JP Dietary arginine prevents atherogenesis in the coronary artery of the hypercholesterolemic abbit J Am Coll Cardiol 1994 23 452 458 8294700
- Wang L Zheng X Xiang HL Fu XH Cao JG 7-difluoromethyl–5, 4′-dimethoxygenistein, a novel agent protecting against vascular endothelial injury caused by oxidative stress Clin Exp Pharmacol Physiol 2009 36 e90 e95 19793105
- Liu F Cao JG Li C Tan JS Fu XH Protective effects of 7-difluoromethyl–5, 4′-dimethoxygenistein against human aorta endothelial injury caused by lysophosphatidyl choline Mol Cell Biochem 2012 363 147 155 22198288
- Zhao H Li C Cao JG 7-Difluoromethyl-5, 4′-dimethoxygenistein, a novel genistein derivative, has therapeutic effects on atherosclerosis in a rabbit model J Cardiovasc Pharmacol 2009 54 412 420 19730393
- Shore VG Shore B Heterogeneity of human plasma very low density lipoproteins. Separation of species differing in protein components Biochemistry 1973 12 502 507 4345806
- Zhang SH Reddick RL Piedrahita JA Maeda N Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E Science 1992 258 468 471 1411543
- Zhang SH Reddick RL Burkey B Maeda N Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption J Clin Invest 1994 94 937 945 8083379
- Nakashima Y Plump AS Raines EW Breslow JL Ross R ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree Arterioscler Thromb 1994 14 133 140 8274468
- Senaratne MP Thomson AB Kappagoda CT Lovastatin prevents the impairment of endothelium dependent relaxation and inhibits accumulation of cholesterol in the aorta in experimental atherosclerosis in rabbits Cardiovasc Res 1991 25 568 578 1913746
- Ignarro LJ Napoli C Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis Curr Diab Rep 2005 5 17 23 15663912
- Maxwell AJ Mechanisms of dysfunction of the nitric oxide pathway in vascular diseases Nitric Oxide 2002 6 101 124 11890735
- Napoli C de Nigris F Williams-Ignarro S Pignalosa O Sica V Ignarro LJ Nitric oxide and atherosclerosis: an update Nitric Oxide 2006 15 265 279 16684613
- Furchgott RF Zawadzki JV The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine Nature 1980 288 373 376 6253831
- Kuhlencordt PJ Gyurko R Han F Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice Circulation 2001 104 448 454 11468208
- Rassaf T Kleinbongard P Kelm M The L-arginine nitric oxide pathway: avenue for a multiple-level approach to assess vascular function Biol Chem 2006 387 1347 1349 17081105
- Miller MR Megson IL Recent developments in nitric oxide donor drugs Br J Pharmacol 2007 151 305 321 17401442
- Giannotti G Landmesser U Endothelial dysfunction as an early sign of atherosclerosis Herz 2007 32 568 572 17972030
- Nakaya M Tachibana H Yamada K Isoflavone genistein and daidzein up-regulate LPS-induced inducible nitric oxide synthase activity through estrogen receptor pathway in RAW264.7 cells Biochem Pharmacol 2005 71 108 114 16271352