?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pharmaceutical suspension containing oral dosage forms delivering both immediate-release and sustained-release amoxicillin was developed as a new dosage form to eradicate Helicobacter pylori. Amoxicillin-loaded gelatin nanoparticles are able to bind with the mucosal membrane after delivery to the stomach and could escalate the effectiveness of a drug, providing dual release. The objective of this study was to develop amoxicillin nanoparticles using innovative new technology – the Büchi Nano Spray Dryer B-90 – and investigate such features as drug content, particle morphology, yield, in vitro release, flow properties, and stability. The nanoparticles had an average particle size of 571 nm. The drug content and percentage yield was 89.2% ± 0.5% and 93.3% ± 0.6%, respectively. Angle of repose of nanoparticle suspension was 26.3° and bulk density was 0.59 g/cm3. In vitro drug release of formulations was best fitted by first-order and Peppas models with R 2 of 0.9841 and 0.9837 respectively; release profile was 15.9%, while; for the original drug, amoxicillin, under the same conditions, 90% was released in the first 30 minutes. The nanoparticles used in this study enabled sustained release of amoxicillin over an extended period of time, up to 12 hours, and were stable for 12 months under accelerated storage conditions of 25°C ± 2°C and 60% ± 5% relative humidity.
Introduction
β-Lactams are one of the best weapons clinical physicians have against Helicobacter pylori. Amoxicillin is a semisynthetic penicillin that is presently the only β-lactam used to treat H. pylori infection. It is unchanged in gastric acid with a short drug residence time in the stomach. It prevents synthesis of the bacterial cell wall and has good sensitivity to H. pylori in vitro.Citation1 These triple therapies contribute to the usefulness of amoxicillin in therapeutic applications. An effective drug concentration cannot be achieved in the gastric mucosal layer or epithelial cell surface where H. pylori reside.Citation2–Citation4 However, there is some information highlighting that management with amoxicillin cannot completely eliminate H. pylori, signifying that further research is necessary to expand its therapeutic effect.Citation5,Citation6 Therefore, management of large doses of amoxicillin on an everyday basis is essential for eradication of H. pylori. Poor patient compliance due to adverse effects such as nausea, vomiting, and diarrhea are not unusual. Subsequently it is expected that if local delivery of antimicrobial agents from the gastric lumen into the mucus layer can be achieved then the H. pylori eradication rate would be increased.Citation7
Several dosage forms have been recommended to help retain amoxicillin in the stomach, including nanocapsules,Citation8 gellan beads,Citation9 floating microballoons,Citation10 hydrogel nanoparticles,Citation11 and mucoadhesive microparticles,Citation12 which are able to stay in the gastrointestinal tract for an extended time, and can therefore prove more effective for H. pylori eradication. Various methods can be used to prepare polymer nanospheres, such as microemulsion, interfacial polymerization, miniemulsion, salting out, dialysis, super critical fluid technology surfactant-free emulsion, and solvent evaporation.Citation13 Spray drying is a well-known, quick, one-step process frequently used in the pharmaceutical industry to produce a dry powder from a liquid form.Citation14
In light of the above, there is a pressing need to develop a novel formulation that carries amoxicillin into the stomach to bind with the gastric mucosa, to intensify the efficiency of the drug, and to offer a sustained action. To date, to the author’s knowledge, no study has been carried out on the formulation of a suspension containing a dual delivery formulation of amoxicillin. The purpose of this study was to design amoxicillin-loaded gelatin nanoparticles as a new dual delivery suspension containing pure amoxicillin as well as a granulated nanoparticle form of amoxicillin using a Büchi Nano Spray Dryer B-90 (BUCHI Labortechnik AG, Flawil, Switzerland) for H. pylori eradication management.
Materials and methods
Amoxicillin trihydrate was kindly gifted for this research by Karnataka Antibiotics and Pharmaceuticals Ltd (Bangalore, India). Gelatin bloom strength 175, xanthan gum, D-sorbitol powder, citric acid, sodium citrate, sodium lauryl sulfate, and sodium benzoate were obtained from Sigma-Aldrich (St Louis, MO, USA). All other reagents and chemicals used were of analytical grade.
Preparation of nanoparticles by spray drying
Amoxicillin-loaded gelatin nanoparticles (GLAN) were formulated using a spray drying technique.Citation15,Citation16 The experiments were designed according to our previous published work.Citation16 The optimized parameters included the concentration of gelatin (500 mg), inlet temperature (80°C), and flow rate (21 mL/hour). A solution of gelatin (gelatin soaked for 1 hour in 50 mL of water and warmed before spraying) containing a predetermined quantity of amoxicillin was sprayed using spray caps incorporated with mesh of 4 μm hole size. All samples were filtered prior to the spray drying process to avoid blockage of the mesh hole. The piezo-driven spray head generates ultrafine droplets, which are gently dried into solid particles. The dried powder is electrostatically charged and collected at the collecting electrode, and the particles were scraped and stored in a desiccator at 25°C for further investigation.
Morphology of spray-dried particles
The surface morphology of the nanoparticles was observed using scanning electron microscopy (FEI Quanta 200; Quanta Technology, LLC, Raleigh, NC, USA). The nanoparticles were placed in metal stubs and coated with gold using an ion sputter machine (JEOL, Tokyo, Japan) and visualized at 12 kV.Citation17,Citation18
Particle size analysis
A study of amoxicillin nanoparticle size was done using laser light diffraction and an ultrasonic method. The nanoparticles were diluted 5-fold with deionized water using a Malvern Zetasizer Nano-ZS (Malvern Instruments, Malvern, UK).Citation17,Citation19
Determination of amoxicillin drug content in nanoparticles
A known amount of amoxicillin nanoparticles was dissolved in 0.1 M hydrochloric acid. The amoxicillin content was assayed using a spectrophotometer (UV-1601spectrophotometer; Shimadzu, Tokyo, Japan) at 272 nm by constructing a calibration curve. The best-fitting straight line equation of the calibration curve was found to be A = 0.294x + 0.0071.Citation16 The investigations were performed in triplicate. The drug content was considered as the ratio between the actual and theoretical quantity of amoxicillin loaded for an optimized preparation.
Product yield/recovery
The efficiency of the nanoparticle yield was determined as the percentage weight of the nanoparticle achieved compared with the total amount of drug and polymer initially used in the formulation procedure.Citation17
Angle of repose
The angle of repose was assessed to understand the flow ability of the nanoparticle suspension powder by passing the powder suspension through a funnel on a horizontal surface. The height (h) of the heap formed was measured and the radius (r) of the cone base was also determined. The angle of repose (ϕ) was calculated as:Citation20
Bulk density
The spray-dried powder was introduced into a 100 mL graduated cylinder. The volume of the material was noted on the graduated cylinder. The bulk density was determined by following the formula:
where “w” is the weight of the nanoparticle powder and “v” is the volume of the nanoparticle powder.
In vitro drug release and kinetics
In vitro drug release studies were carried out according to a previously published article.Citation16 Briefly both the nanoparticles and the dry powder suspensions were carried out using the paddle method specified in United States Pharmacopeia (USP) XXVII.Citation16 The in vitro release studies were carried out using the USP I basket method (50 rpm, 500 mL, and 37°C ± 1°C). Amoxicillin-loaded nanoparticle suspensions equivalent to 250 mg of drug were positioned in a dialysis cellulose membrane bag (D9402-100FT; Sigma-Aldrich) with a molecular weight cutoff of 12,400 Da, retained and placed into the baskets, and trans ferred to 500 mL of 0.05 M phosphate buffer. Aliquots were withdrawn at preset time intervals and an equal volume of dissolution medium was substituted into the flask to maintain a constant volume. The drawn samples were examined at 272 nm, and the results were used to determine a collective drug release profile for the nanoparticles. Sink conditions were maintained throughout. Examination of the suspension comprising of only amoxicillin was carried out in a similar manner.Citation21,Citation22
Formulation of dry syrup for reconstitution
Oral suspensions for reconstitution were prepared using the formulae shown in . To formulate a dry blend for reconstitution, the powder constituents were decreased to nearly the same particle size. Xanthan gum was incorporated as the efficient suspending agent in the suspensions. D-Sorbitol powder was added to impart a slightly sweet taste. Citric acid and sodium citrate were used to vary the pH of the study preparations. Components present in small quantities (sodium lauryl sulfate [0.05%], sodium benzoate [0.2%], and buffer components) were mixed equally. The same procedure was followed for xanthan gum (0.6%) and amoxicillin-loaded nanoparticles (comprising the equivalent of 500 mg amoxicillin). The ingredients were blended with a portion of D-Sorbitol (20%) according to the geometric dilution procedure. To prepare the reconstituted suspension, approximately 10 mL of water was added to the bottle containing dry suspension powder and stirred with a spoon until a homogeneous product was obtained.Citation23
Table 1 Formulation of dry suspension of amoxicillin-loaded nanoparticles for reconstitution
Stability of dry suspension
The stability model was planned based on the International Conference On Harmonization (ICH) Q2B guidelines.Citation17,Citation24,Citation25 The nanoparticles were stored at 25°C ± 2°C and 60% ± 5% relative humidity for a period of 12 months. This is the long-term storage temperature for products that are intended to be refrigerated when considering the thermosensitivity and thermolability of the polymer.Citation17,Citation24,Citation25 Gelatin is a biopolymer produced by denaturing collagen, which is the major protein present in animal connective tissue; it is sensitive to degradation as long as no gel has formed. The stored samples were confirmed for their physical change, particle size distribution, and drug content. The drug content was analyzed by a UV spectrophotometric method. The testing was carried out at 0, 2, 4, 6, and 12 months (as per the FDA’s guideline for submitting papers on stability of human drugs and biologics, DHHA, February 1987) for long-term storage conditions and at 3-month intervals for a period of 12 months for long-term storage conditions as per ICH guidelines (ICH Q6A) for stability studies.Citation16
Results and discussion
Spray-dried particle morphology
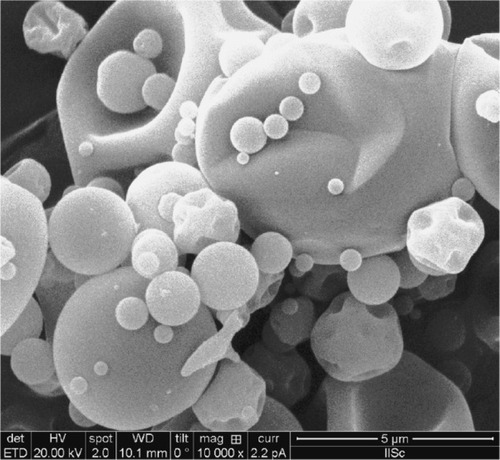
The amoxicillin nanoparticles designed under optimized experimental conditions were shriveled sphere structures and were a free-flowing powder (). Other pharmaceutical scientists have also stated that spray-dried particles developed a shriveled shape and led to high surface area, which is advantageous for dissolution enhancement, and that shrinking may also be due to quick drying in the spraying compartment.Citation26,Citation27 A shriveled shape might also be due to the lack of a plasticizer. We did not use a plasticizer in our experiments due to possible irritation in the gastrointestinal tract as the dosage form was intended to stay in the body for a longer time.Citation28
Drug content, yield, angle of repose, and bulk density
The drug content and percentage yield of the formulation were found to be 89.2% ± 0.5% and 93.3% ± 0.6%, respectively. This is possible in practice only with the use of the Büchi Nano Spray Dryer B-90, which can create much higher yields compared to some traditional preparations.Citation29,Citation30 The minimal losses may be due to particle collection with the manual particle scraper from the collecting electrode. The angle of repose of the nanoparticle suspension powder was 26.3°C, indicating that the formulation was free-flowing in nature and that the bulk density was freely settled having a value of 0.59 g/cm3.
Analysis of nanoparticle size
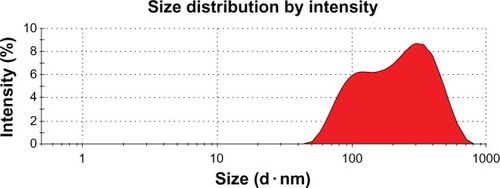
Spray drying has recently been gaining greater consideration as it is a gentle, continuous, and scalable drying method by which to transform liquids to dry powders. It also benefits from marginal loss of high-value powder due to exceptionally high yields, and is an efficient and fast process.Citation16 shows the formation of amoxicillin nanoparticles with heterogeneous particle size distribution. This is important because, when the particles are of uniform size, the spacing is the greatest, but when a range of sizes is used, the void spaces are filled in the target region (stomach). Similar results have been reported elsewhere of nanoparticles formulated by a spray-drying process.Citation31 The average diameter of a nanoparticle was 571 nm.
Release behavior of amoxicillin
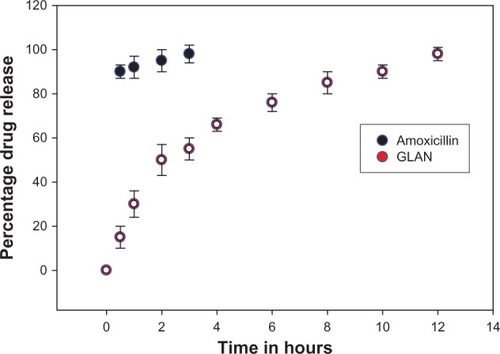
In the drug-manufacturing industry, drug-dissolution testing is routinely done and it is extremely important to know the release pattern and release kinetics of the formulation. illustrates the in vitro drug release from the amoxicillin suspension and the gelatin nanoparticles containing amoxicillin. The formulation shows a close to biphasic release profile. Around 15.9% of the overall amount of amoxicillin in the nanoparticles was released in the first hour. This initial burst of release may be attributed to the drug molecules positioned within the shell or at the interface between the core and the shell. This system can produce a rapid rise in the plasma concentration for some drugs that are required to have a timely therapeutic effect. However, complete drug release from the amoxicillin nanoparticles was prolonged up to 12 hours. During this period, the amount released was 98.0%. In comparison with the amoxicillin nanoparticles, the amoxicillin suspension showed faster amoxicillin release, with 90% of the amoxicillin released in 30 minutes.Citation16 This result specifies that nanoparticles have potential in a controlled-release formulation of amoxicillin. Further studies in animal models are necessary to confirm the in vivo activity of amoxicillin nanoparticles with respect to the eradication of H. pylori infection.
Analysis of release kinetics
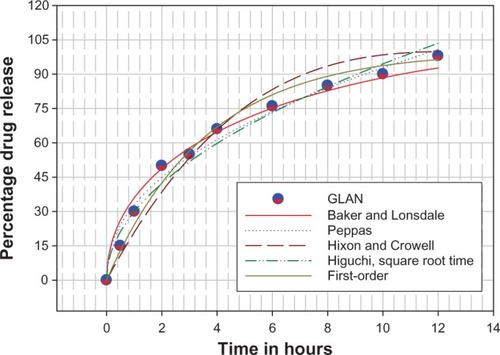
In order to understand the release kinetics of amoxicillin from GLAN nanoparticles, the in vitro release data were fitted to all well-known kinetic equations ().Citation16,Citation32,Citation33 The release mechanism was calculated from the slope of the appropriate plots and the regression coefficient (R2) was determined by Sigma Plot software (v 9.01; Systat Software, Inc, San Jose, CA, USA). It was found that the in vitro drug release of GLAN formulations was best explained by two models (). A novel observation has been made in this study that the drug release agreed well with the first-order and Peppas models. The values of the correlation coefficient (R 2) of the first-order and Peppas models for the obtained release data were approximately 0.9841 and 0.9837, respectively. The first-order kinetics equation describes the dissolution of amoxicillin that is not successfully bound in the gelatin matrix and which is ready to dissolve from the nanoparticle surface. This rapid progression occurs immediately after dipping a sample into release medium and is called “the burst effect.” The Peppas kinetic model defines the drug-release mechanism depending on its data; the release process can be governed by Fickian diffusion (n = 0.5), by gelatin polymeric matrix erosion, or by the combination of both mechanisms. The release pattern was more controlled and was sustained for a longer period of time, up to 12 hours.
Table 2 In vitro release models with correlation coefficient value (R 2)
Stability studies of GLAN
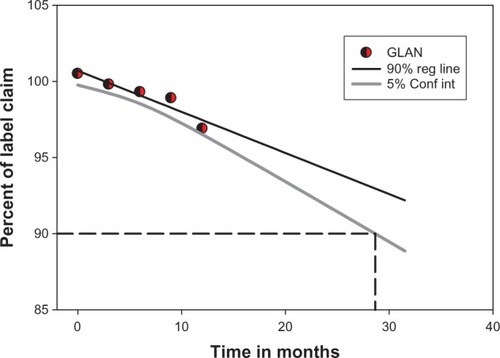
The amoxicillin content under long-term storage conditions did not vary to a large extent in the nanoparticle formulations; the maximum variation of 1.7% from the initial concentration was seen in GLAN 24 months from the date of production. The preparation was stable for 24 months under the long-term storage conditions of 25°C ± 2°C and 60% ± 5% relative humidity. A maximum decrease of 2.6% from the original concentration was observed (). The average particle size was 571 nm and did not vary appreciably. It was more or less shriveled in nature and no physical changes, such as liquefaction, formation of bulges, or bruising, were observed in the nanoparticle preparations throughout the 24-month study period. Nevertheless, the observed changes were found to be insignificant and had no influence on the quality of the preparation.Citation25 shows the fall of drug content of GLAN in long-term storage environments.
Table 3 Interpretations of stability test readings under long-term storage conditions (25°C ± 2°C and 60% ± 5% relative humidity)
Conclusion
In the present study, a pharmaceutical suspension containing oral dosage forms for delivering both immediate-release and sustained-release amoxicillin was developed as a new dosage form with which to eradicate H. pylori. Our results can also be used to develop an effective outline for targeted stomach-specific drug delivery, and can be explored further in vivo with other drugs, using a slight alteration in the methods used here for formulation of the nanoparticles.
The amoxicillin nanoparticle suspension established for this investigation was found to have suitable physical properties or chemical properties and to have a satisfactory particle size. The nanoparticles were found to release the amoxicillin to a maximum extent in vitro. This unusual dualrelease formulation supplements the already existing domain of site-specific drug delivery systems and holds promise as a substitute to conventional drug delivery, allowing that bacteria, such as H. pylori, are extremely challenging to treat and to manage efficiently.
Acknowledgments
The authors wishes to thank Deanship of Scientific Research, King Faisal University, Saudi Arabia for financial support. Research Project number: 140131.
Disclosure
The author reports no conflicts of interest in this work.
References
- Romano A Cuomo A Eradication of Helicobacter pylori: a clinical update Med Gen Med 2004 6 19
- Axon AT The role of acid inhibition in the treatment of Helicobacter pylori infection Scand J Gastroenterol Suppl 1994 201 16 23 8047818
- Fontana G Licciardi M Mansueto S Schillaci D Giammona G Amoxicillin-loaded polyethylcyanoacrylate nanoparticles. influence of PEG coating on the particle size, drug release rate and phagocytic uptake Biomaterials 2001 22 2857 2865 11561891
- Patel JK Chavda JR Formulation and evaluation of stomach-specific amoxicillin-loaded carbopol-934P mucoadhesive microspheres for anti-Helicobacter pylori therapy J Microencap 2009 26 365 376
- Lin CK Hsu PI Lai KH One-week quadruple therapy is an effective salvage regimen for Helicobacter pylori infection in patients after failure of standard triple therapy J Clin Gastroenterol 2002 34 547 551 11960067
- Liu Z Lu W Qian L Zhang X Zeng P Pan J In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin J Control Release 2005 102 135 144 15653140
- Nagahara N Akiyama Y Nakao M Tada M Kitano M Ogawa Y Mucoadhesive microspheres containing amoxicillin for clearance of Helicobacter pylori Antimicrob Agents Chemother 1998 42 2492 2494 9756746
- Jain P Jain S Prasad KN Jain SK Vyas SP Polyelectrolyte coated multilayered liposomes (nanocapsules) for the treatment of Helicobacter pylori infection Mol Pharm 2009 6 593 603 19718807
- Narkar M Sher P Pawar A Stomach-specific controlled release gellan beads of acid-soluble drug prepared by ionotropic gelation method AAPS Pharm Sci Tech 2010 11 267 277
- Awasthi R Kulkarni GT Pawar VK Garg G Optimization studies on gastroretentive floating system using response surface methodology AAPS Pharm Sci Tech 2012 13 85 93
- Moogooee M Ramezanzadeh H Jasoori S Omidi Y Davaran S Synthesis and in vitro studies of cross-linked hydrogel nanoparticles containing amoxicillin J Pharm Sci 2011 100 1057 1066 21280053
- Sambathkumar R Venkateswaramurthy N Vijayabaskaran M Perumal P Formulation of clarithromycin loaded mucoadhesive microspheres by emulsification-internal gelation technique for anti-Helicobacter pylori therapy Int J Pharm and Pharm Sci 2011 3 173 177
- Sham JO Zhang Y Finlay WH Roa WH Löobenberg R Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung Int J Pharm 2004 269 457 467 14706257
- Pilcer G Vanderbist F Amighi K Preparation and characterization of spray-dried tobramycin powders containing nanoparticles for pulmonary delivery Int J Pharm 2009 365 162 169 18782609
- Li X Anton N Arpagaus C Belleteix F Vandamme TF Nanoparticles by spray drying using innovative new technology: the Buchi nano spray dryer B-90 J Control Release 2010 147 304 310 20659510
- Harsha S Dual drug delivery system for targeting H. pylori in the stomach: preparation and in vitro characterization of amoxicillin-loaded Carbopol® nanospheres Int J Nanomedicine 2012 7 4787 4796 22969298
- Harsha NS Rani RH Drug targeting to lungs by way of microspheres Arch Pharm Res 2006 29 598 604 16903082
- Nowacek AS Balkundi S McMillan J Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages J Control Release 2011 150 204 211 21108978
- Lu B Zhang JQ Yang H Lung-targeting microspheres of carboplatin Int J Pharm 2003 265 1 11 14522113
- Gaba P Singh S Gaba M Gupta GD Galactomannan gum coated mucoadhesive microspheres of glipizide for treatment of type 2 diabetes mellitus: in vitro and in vivo evaluation Saudi Pharmaceutical Journal 2011 19 143 152 23960752
- Singh SK Chidrawar VR Ushir YV Vadalia KR Sheth NR Singh S Pharmaceutical characterization of amoxicillin trihydrate as mucoadhesive microspheres in management of H Pylori Int J Pharm Tech Res 2010 6 348 358
- Nayak AK Malakar J Formulation and in vitro evaluation of hydrodynamically balanced system for theophylline delivery J Basic Clin Pharm 2011 2 133 137
- Raula J Eerikäinen H Kauppinen EI Influence of the solvent composition on the aerosol synthesis of pharmaceutical polymer nanoparticles Int J Pharm 2004 284 13 21 15454292
- Lacoulonche F Gamisans F Chauvet A Garcí a ML Espina M Egea MA Stability and in vitro drug release of flrbiprofen-loaded poly-epsilon-caprolactone nanospheres Drug Dev Ind Pharm 1999 25 983 993 10518238
- Harsha S R C Rani S Ofloxacin targeting to lungs by way of microspheres Int J Pharm 2009 380 127 132 19646516
- Miller DA Gil M Spray-drying technology Williams ROIII Watts AB Miller DA Formulating Poorly Water Soluble Drugs New York Springer 2012 363 442
- De Jaeghere F Allémann E Cerny R pH-Dependent dissolving nano- and microparticles for improved peroral delivery of a highly lipophilic compound in dogs AAPS Pharm Sci 2001 3 E8
- Coowanitwong I Arya V Kulvanich P Hochhaus G Slow release formulations of inhaled rifampin AAPS J 2008 10 342 348 18584334
- Sansone F Rossi A Del Gaudio P De Simone F Aquino RP Lauro MR Hesperidin gastroresistant microparticles by spray-drying: preparation, characterization, and dissolution profiles AAPS Pharm Sci Tech 2009 10 391 401
- Atuah KN Walter E Merkle HP Alpar HO Encapsulation of plasmid DNA in PLGA-stearylamine microspheres: a comparison of solvent evaporation and spray-drying methods J Microencapsul 2003 20 387 399 12881118
- Maas SG Schaldach G Littringer EM The impact of spray drying outlet temperature on the particle morphology of mannitol Powder Technology 2011 213 27 35
- Rosario-Meléndez R Ouimet MA Uhrich KE Formulation of salicy-late-based poly(anhydride-ester) microspheres for short- and long-term salicylic acid delivery Polymer Bulletin 2013 70 343 351 23420391
- Nochos A Douroumis D Bouropoulos N In vitro release of bovine serum albumin from alginate/HPMC hydrogel beads Carbohydrate Polymers 2008 74 451 457