Abstract
Background
Vascular endothelial growth factor (VEGF) is a naturally occurring glycoprotein in the body that acts as a growth factor for endothelial cells. It regulates angiogenesis, enhances vascular permeability, and plays a major role in wet age-related macular degeneration. The consistent association between choroidal neovascularization and increased VEGF expression provides a strong reason for exploring the therapeutic potential of anti-VEGF agents in the treatment of this disorder. Blockade of VEGF activity is currently the most effective strategy for arresting choroidal angiogenesis and reducing vascular permeability, which is frequently the main cause of visual acuity deterioration. In recent years, a number of other molecules have been developed to increase the efficacy and to prolong the durability of the anti-VEGF effect. Aflibercept (EYLEA®; Regeneron Pharmaceutical Inc and Bayer), also named VEGF Trap-eye, is the most recent member of the anti-VEGF armamentarium that was approved by the US Food and Drug Administration in November 2011. Because of its high binding affinity and long duration of action, this drug is considered to be a promising clinically proven anti-VEGF agent for the treatment of wet maculopathy.
Objective
This article reviews the current literature and clinical trial data regarding the efficacy and the pharmacological properties of VEGF-Trap eye and describes the possible advantages of its use over the currently used “older” anti-VEGF drugs.
Methods
For this review, a search of PubMed from January 1989 to May 2013 was performed using the following terms (or combination of terms): vascular endothelial growth factors, VEGF, age-related macular degeneration, VEGF-Trap eye in wet AMD, VEGF-Trap eye in diabetic retinopathy, VEGF-Trap eye in retinal vein occlusions, aflibercept. Studies were limited to those published in English.
Results and conclusion
Two Phase III clinical trials, VEGF Trap-eye Investigation of Efficacy and Safety in Wet AMD (VIEW) 1 and 2, comparing VEGF Trap-eye to ranibizumab demonstrated the noninferiority of this novel compound. The clinical equivalence of this compound against ranibizumab is maintained even when the injections are administered at 8-week intervals, which indicates the potential to reduce the risk of monthly intravitreal injections and the burden of monthly monitoring.
Introduction
The neovascular form of age-related macular degeneration (AMD), also known as wet AMD, is characterized by the formation of subretinal choroidal neovascularization (CNV) and is the cause of most cases of blindness in the elderly. Wet AMD is the major cause of severe vision loss in developed nations and is estimated to affect >2.5 million people worldwide.Citation1,Citation2 The patients affected by exudative AMD often experience rapid loss of fine resolution central vision over several months, and early visual stabilization is a key issue in preserving visual acuity.Citation3
Vascular endothelial growth factor (VEGF) is a naturally occurring glycoprotein in the body that acts as a growth factor selective for endothelial cells. It regulates angiogenesis, enhances vascular permeability, and plays a leading role in wet AMD. The consistent association between CNV and increased VEGF expression provides a strong reason for exploring the therapeutic potential of anti-VEGF agents for the treatment of this disorder.Citation4 Blockade of VEGF actions is currently the most effective strategy in arresting choroidal angiogenesis and reducing vascular permeability, which is frequently the main cause of visual acuity deterioration.Citation5
Although pegaptanib (Macugen®; Eyetech Pharmaceuticals Inc, FL, USA and Pfizer Inc, New York, NY, USA) was the first VEGF inhibitor approved by the US Food and Drug Administration (FDA). Important advances in the on-label treatment of CNV in AMD have been achieved with the introduction of ranibizumab (Lucentis; Genentech USA, Inc, San Francisco, CA, USA) in 2006. The off-label use of bevacizumab (Avastin; Genentech USA, Inc) has also shown efficacy for treating wet AMD and other exudative retinal diseases and despite the lack of clinical trials to support its safety or efficacy, anecdotal evidence led to its widespread popularity prior to the approval of ranibizumab.
Aflibercept (EYLEA®; Regeneron Pharmaceutical Inc, Tarrytown, NY, USA and Bayer, Basel, Switzerland), also named VEGF Trap-eye, is the most recent member of the anti-VEGF family. This drug has been recently developed to afford a more potent and prolonged anti-VEGF effect and was approved by the FDA in November 2011.Citation6 This article reviews the efficacy and summarizes the pharmacological properties of VEGF Trap-eye and describes the possible advantages of its use over the currently used “older” anti-VEGF drugs.
Overview of VEGF and its pathological effects in neovascular AMD
VEGF-A (usually simply referred to as VEGF) is a growth factor encoded by a gene family that also includes placental growth factor (PIGF), VEGF-B, VEGF-C, VEGF-D, and the orf virus encoded VEGF-E.Citation7 Differences in exon splicing result in the generation of four main VEGF isoforms: VEGF121, VEGF165, VEGF189, and VEGF206, which have 121, 165, 189, and 206 amino acids after cleavage of the signal sequence, respectively.Citation8
VEGF stimulates the growth of vascular endothelial cells derived from arteries, veins, and the lymphatic system.Citation9 It also induces the formation of thin-walled endothelium-lined structures (ie, angiogenesis) in a variety of in vivo models,Citation10 and induces rapid elevations in microvascular permeability.Citation11 VEGF acts also as a survival factor for endothelial cells, both in vitro and in vivo.Citation12,Citation13 Although endothelial cells represent the primary target of VEGF, several studies have demonstrated that VEGF has mitogenic effects on nonendothelial cell typesCitation14 and promotion effects on monocyte migration.Citation15 VEGF protects neurons from insults such as hypoxia and glutamate toxicityCitation16 and it stimulates neurogenesis in vitro and in vivo.Citation17
VEGF contributes mainly at the initiation stage of CNV by promoting both angiogenesis and vasculogenesis. It acts as an endothelial cell specific mitogen as part of the angiogenesis pathway, and also as a chemoattractant for endothelial cell precursors, inducing their mobilization and differentiation in the vasculogenesis pathway.Citation16 In addition to these activities, VEGF affects vascular permeability by inducing formation of pores in vascular endothelial cellsCitation17,Citation18 and by disrupting the intercellular junction between these cells.Citation19 In turn, this leads to extravasation of fluid, proteins, and circulating cells which disrupts the retinal anatomy and separates the retina from underlying structures, potentially causing severe vision loss.
Although other growth factors can induce the development of blood vessels (ie, transforming growth factor-β, interleukins, insulin-like growth factor-1, and epidermal growth factor), only VEGF appears to be both sufficient and essential for physiologic and pathologic angiogenesis. For this reason, the biochemical pathways involving VEGF are the most studied targets for new potential drugs against neovascular pathologies. Anti-VEGF therapy can arrest choroidal angiogenesis and reduce vascular permeability, which is frequently the main cause of visual acuity deterioration. Pegaptanib and ranibizumab have been approved by the FDA for the treatment of wet AMD, and the off-label use of a third agent, bevacizumab, has shown efficacy for treating wet AMD and other exudative retinal diseases. Pegaptanib was the first anti-VEGF drug FDA approved in December 2004.Citation20–Citation22 However, because it was proven to be less efficacious than other anti-VEGF drugs, possibly owing to its selective binding of VEGF165, it is no longer widely used in most countries. Ranibizumab and bevacizumab, which are nonselective anti-VEGF drugs, are currently the most extensively used drugs worldwide for wet AMD as well as for many other ocular diseases in which VEGF is overexpressed.Citation23
The development of new agents for wet AMD has focused on both improving efficacy and extending the duration of action in comparison with the commonly used anti-VEGF drugs ranibizumab and bevacizumab, which are considered the standard drugs. Ranibizumab is a monoclonal humanized antibody fragment and bevacizumab is a whole monoclonal antibody, and both show a high binding affinity for all isoforms of VEGF. These agents appear to have similar efficacy profiles and mechanisms of action, ie, they block the extracellular availability of VEGF which can arrest choroidal angiogenesis and reduce vascular permeability for a limited period of time.Citation24–Citation27
Bevacizumab has a lower binding affinity for VEGF than ranibizumab.Citation28 However, bevacizumab is approximately three times larger than ranibizumab (149 kDa versus 48 kDa), and its substantially higher molecular weight results in an intra-vitreal half-life that is 36% higher than that of ranibizumab. Accumulating clinical evidence has demonstrated that the effects of a single intravitreal dose of either bevacizumab or ranibizumab effectively reduces the effect of VEGF on CNV for 4–6 weeks in most eyes.Citation29,Citation30
Ranibizumab, which is the only widely used drug that is currently approved by the FDA for the treatment of neovascular AMD, is most extensively studied. Several ranibizumab Phase III clinical trials that have studied different treatment schedules, doses, and populations have obtained good results with monthly injections, ie, a mean number of 25 intravitreal injections over 2 years.Citation31,Citation32
Despite the off-label status of bevacizumab, however, it is preferred over ranibizumab by nearly 60% of physiciansCitation33 because of its significantly lower price (ranibizumab, US $1,950 versus bevacizumab, US $50) and similar efficacy. The FDA originally approved bevacizumab in 2004 for the treatment of metastatic colorectal cancer.Citation34 To deliver an intra-vitreal injection, the physician or pharmacist makes numerous unit doses from a vial of bevacizumab, dramatically lowering the cost of the drug. Moreover, many reports and a 2-year multicenter, randomized clinical trial (the Comparisons of Age-Related Macular Degeneration Treatment Trial [CATT]) demonstrated its near equivalency to ranibizumab with monthly dosing (+7.8 letters versus +8.8 letters) and insignificant poorer outcomes with as-needed dosing (+5.0 versus +6.7 letters).Citation24,Citation25 Moreover, while the systemic half-life of the unbound product of bevacizumab (20 days) was longer than that of ranibizumab (6 hours), severe systemic adverse events occurred at similar frequencies in patients receiving bevacizumab and ranibizumab in the CATT trial.Citation26,Citation35,Citation36
The main problem with the current anti-VEGF therapy is that monthly intravitreal injections are required for maintaining vision. This necessitates an excessive time commitment from patients and institutions, and increases the physical and psychological discomfort and financial burdens for the patients. On the other hand, evidence from the SAILOR (Safety Assessment of Intravitreous Lucentis fOR AMD),Citation37 PIER (A Phase IIIb, Multicenter, Randomized, Double-Masked, Sham Injection-Controlled Study of the Efficacy and Safety of Ranibizumab in Subjects with Subfoveal Choroidal Neovascularization [CNV] with or without Classic CNV Secondary to Age-Related Macular Degeneration),Citation38,Citation39 and EXCITE (Efficacy and Safety of Ranibizumab in Patients With Subfoveal Choroidal Neovascularization [CNV] Secondary to Age-Related Macular Degeneration)Citation40 studies indicates that the efficacy decreases if treatment frequency is reduced. After the loading dose of monthly injections for 3 months of ranibizumab, vision decreases or returns to baseline in most patients if the frequency is reduced to one injection every 2, 3, or 4 months.
Although monthly injections of anti-VEGF represent the best way to preserve vision, most retina surgeons use individualized treatment protocols with monthly assessments after the first three intravitreal injections of anti-VEGF, and further injections are given only if signs of disease activity persist as observed on optical coherence tomography (OCT). This strategy is also abbreviated as “PRN dosing” from the Latin phrase Pro Re Nata, which means “as circumstances arise.” The PrONTO (Prospective Optical Coherence Tomography [OCT] Imaging of Patients With Neovascular AMD Treated With Intra-Ocular Ranibizumab) study used this strategy and obtained visual outcomes similar to those achieved with monthly injections while reducing the number of injections from 25 to 10 over 2 years.Citation41 However, even with this dosing regimen, patients are still required to make monthly visits to the office and undergo frequent and expensive testing because of the constant risk of CNV recurrence.
A treatment approach that aims to reduce the number of injections and the number of visits is the “treat and extend” method. It consists of 3 monthly injections and a follow-up examination after 6 weeks. If the follow-up examination shows evidence of exudation, the patient is treated and told to undergo a follow-up examination in 4 weeks, otherwise the patient is still treated but the follow-up period is extended to 8 weeks. A similar evaluation is performed at the next follow-up visit. However, there is not much evidence in favor of this treatment method. Thus, research on new compounds is focused on inhibiting the VEGF signaling pathway for a more prolonged period.Citation1
Aflibercept (EYLEA®; Regeneron Pharmaceutical Inc and Bayer), or VEGF Trap-eye, is a novel compound derived from the native VEGF receptor (VEGFR) that binds to all VEGF and VEGF-B isoforms as well as to PlGF.Citation42 VEGF Trap-eye promises to decrease the injection frequency in conjunction with the “treat and extend” or “PRN” strategies and appears to serve as an effective alternative drug for patients who are less responsive to the previously approved anti-VEGF drugs.
Structure and mechanism of action
The FDA approved VEGF Trap-eye (EYLEA®, Regeneron Pharmaceutical Inc, and Bayer) for the treatment of subfoveal CNV caused by wet AMD on November 18, 2011.Citation43 VEGF Trap-eye is an intraocular formulation of aflibercept, a product used in oncology (Zaltrap; Regeneron Pharmaceutical Inc), that has been specifically purified and buffered to minimize the risk of eye toxicity when injected intravitreally.Citation44 It is a fully human, recombinant fusion protein that has the property to “trap,” that is to catch, hold, and block certain molecules. Aflibercept was constructed from portions of the human VEGFR fused to the FC portion of a human IgG1.Citation45
Circulating VEGF initiates a biochemical cascade by activating three membrane spanning tyrosine kinase receptors: VEGFR-1, VEGFR-2, and VEGFR-3.Citation46,Citation47 VEGFR-1 (fms-like tyrosine kinase-1, Flt-1) was the first VEGF receptor identified more than a decade ago.Citation48 VEGFR-1 releases tissue specific growth factors, recruits endothelial progenitors, and induces matrix metalloproteinases. It is thought to modulate VEGFR-2 signaling and to act as a dummy/decoy receptor by sequestering VEGF and preventing it from binding to VEGFR-2.Citation7 VEGFR-2 (kinase insert domain-containing receptor or KDR) is considered the major mediator of the mitogenic, angiogenic, permeability enhancing, and anti-apoptotic effects of VEGF.Citation7
Both VEGFR-1 and VEGFR-2 have seven Ig-like binding sequences for VEGF (two of which are incorporated in VEGF Trap-eye) in the extracellular region, a single transmembrane region, and a consensus tyrosine kinase sequence that is interrupted by a kinase insert domain.Citation49–Citation51 The third member of the same family of receptor tyrosine kinases is VEGFR-3.Citation52 This protein is not a receptor for VEGF, but binds VEGF-C and VEGF-D.Citation53 Because VEGFR-1 possesses a higher affinity for VEGF than VEGFR-2, drug developers have used its binding sequences for VEGF Trap-eye.
Structurally, aflibercept is a soluble decoy receptor of 115 kDa that is made by the second binding domain of VEGFR-1 and the third binding domain of VEGFR-2, which then are fused to the FC region of a human IgG1 (). The intermediate size of aflibercept (115 kDa compared to 48 kDa for ranibizumab and 148 kDa for bevacizumab) results in an estimated intravitreal half-life of 7.1 days and a duration of clinical action possibly as long as 2.5 months, which exceeds the 1-month intravitreal binding activity of ranibizumab.Citation54,Citation55 The molecular configuration of aflibercept allows it to bind to all of the VEGF isoforms more tightly than their native receptors (the dissociation constant [Kd] of aflibercept for VEGF165 = 0.49 pmol/L).Citation42 Thus, this compound effectively prevents VEGF from binding and activating its cognate receptors (the Kd of VEGFR-1 and VEGFR-2 for VEGF165 are 9.33 and 88.8 pmol/L, respectively) ().Citation56 Moreover, the binding affinity of aflibercept (Kd = 0.49 pmol/L) is almost 100 times higher than that of ranibizumab (Kd = 46 pmol/L) and bevacizumab (Kd = 58 pmol/L).Citation54,Citation55 This was primarily attributed to the association rate constant for aflibercept binding to human VEGF165, which is almost 80 times faster than the corresponding association rate constant values for ranibizumab and bevacizumab.
Figure 1 Diagram showing the structure of the vascular endothelial growth factor receptor-1 and -2 and the structure of aflibercept (VEGF Trap-eye).
Abbreviation: FC, fragment crystallizable region.
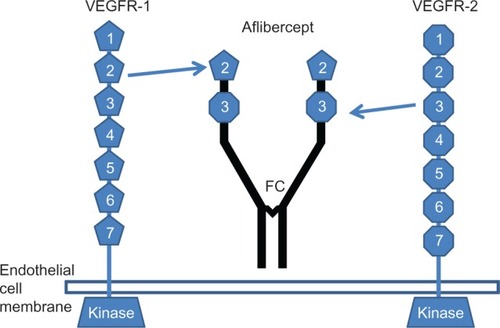
Figure 2 Vascular endothelial growth factor binds to two vascular endothelial growth factor receptors which induces the angiogenic response by activating the tyrosine kinase.
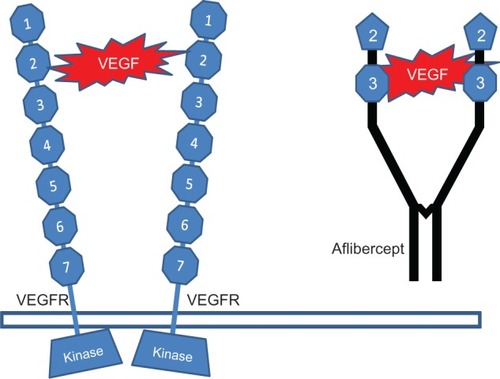
Because of these characteristics, the ability of aflibercept to block VEGF induced activation of VEGFR-1 and -2 in vitro is much stronger than that of ranibizumab and bevacizumab. Additionally, it blocks both PlGF-1 and PlGF-2 mediated activation of VEGFR-1, whereas ranibizumab and bevacizumab do not show such activity. A presumably important functional difference between aflibercept and the other anti-VEGF drugs currently in use is that it can bind and inhibit VEGF as well as PlGF-1 and -2 and VEGF-B, which have also been implicated in pathological vascular remodeling. Experimental evidence shows that targeting VEGF-B and PlGF inhibits CNV and suggests that PlGF synergizes with VEGF in promoting vascular pathology in wet AMD.Citation57
Pharmacodynamics, pharmacokinetics, and metabolism
Aflibercept forms a stable, inert 1:1 complex with either VEGF, VEGF-B, or the PlGF ligand preventing the activation of their receptors, VEGFR-1 and -2.Citation56 The highest intravitreal dose used in pivotal trials for aflibercept is 2 mg, which is 100-fold lower than the dose allowed in oncology (4–6 mg/kg).Citation44,Citation60 Following intravitreal injection of 2 mg of aflibercept, the drug can be detected in plasma as a free drug (a minor quantity) or in a complex bound with VEGF. The drug is rapidly cleared from circulation via pinocytotic proteolysis and glomerular filtration after forming a complex with VEGF via the same pathways that metabolize antibodies.
Following intravitreal injection of 2 mg of aflibercept, the mean maximal plasma concentration of unbound VEGF Trap-eye is attained in 1–3 days, and was estimated to be 200-fold lower than the concentration required for maximal systemic VEGF binding. The systemic half-life of unbound aflibercept is 1.5 days, which is inferior to that of bevacizumab (20 days) and closer to the systemic half-life of ranibizumab (6 hours).Citation59 Free aflibercept has never been detected in plasma at 2 weeks after intravitreal injection and cannot accumulate in plasma in the loading phase.Citation44 Thus, an intravitreal aflibercept dose of 2 mg would be predicted to cause negligible systemic activity and have a systemic safety profile similar to that of ranibizumab.
Therapeutic efficacy
The first surveys regarding the use of aflibercept in treatment of wet AMD emerged from a preclinical study conducted on animal models. This study, published in 2003, showed the first evidence that VEGF Trap-eye is capable of suppressing CNV and VEGF mediated breakdown of the blood–retinal barrier in transgenic mice with laser induced CNV, which was treated with subcutaneous or intravitreal administration.Citation58 The initial use of aflibercept for wet AMD consisted of intravenous injections with doses between 0.3 mg/kg and 3 mg/kg (the usual oncologic dose is 4 mg/kg) and administered every 2 weeks to 25 patients.Citation60 Macular thickness decreased by an average of 66% and vision improved in many patients. Patients receiving the higher dose (3 mg/kg) experienced more systemic hypertension and proteinuria than those treated with the lower dose (1 mg/kg). However, the promising effects obtained intravenously encouraged researchers to transition the trial to intravitreal injections.
The Phase I Clinical Evaluation of Anti-angiogenesis in the Retina Intravitreal Trial (CLEAR-IT 1)Citation60 investigation was a small trial (21 patients) designed to determine the maximum tolerated dose, the bioactivity, and the safety and tolerability of intravitreally administered aflibercept in patients with wet AMD. This study confirmed that aflibercept doses between 0.05 mg and 4 mg were well tolerated. At 6 weeks after a single injection, most patients experienced an improvement in visual acuity (mean visual gain, 4.4 letters) and showed a decrease in macular thickness (−105 μm). Almost 50% of the patients followed for 12 weeks did not show retinal leakage and maintained vision gain.Citation61,Citation62 On the basis of the results of CLEAR-IT 1, the developers hoped to show that an intravitreal formulation of aflibercept could be administered less frequently than once a month.
In a Phase II dose and interval ranging trial, 159 patients with wet AMD (CLEAR-IT 2) were randomized into five treatment groups: the first two groups received 3 monthly aflibercept injections of 0.5 mg or 2 mg and the other three groups received only one aflibercept injection of 0.5 mg, 2 mg, or 4 mg.Citation64 Final global evaluations were performed at 12 weeks. Although visual improvement at week 8 was similar in patients receiving a single dose or two doses (5.7 letters), the average vision in all groups improved more in patients treated monthly (mean gain of ≥8 letters) at 12 weeks. After 12 weeks, the reduction in macular thickness experienced by the patients receiving three monthly injections exceeded that of patients treated only once.Citation63 For this reason, a second part of the CLEAR-IT 2 study was designed in which aflibercept treatment was provided as needed (PRN) from week 12 to 52 and monthly OCT and fluorescein angiography (FAG) examinations were performed starting with a reinjection of all patients at week 12.Citation64 A decision to perform reinjection was made if any of the following conditions were observed: central retinal thickness increase of ≥ 100 μm, loss of at least five lines in the visual acuity chart approved by the Early Treatment Diabetic Retinopathy Study (ETDRS),Citation65 persistent fluid on OCT, new onset of classic neovascularization, persistent leakage on FAG, or the presence of a new hemorrhage on clinical examination. An average of two injections was required with a mean time to the first injection of 129 days. After 1 year (week 52), the average improvement in vision was +5.3 letters. Patients initially treated with 2 mg every 4 weeks had the best visual improvement (mean gain of 9 letters).
The CLEAR-IT 2 study provided the first indication that aflibercept may be dosed as needed with excellent gains in vision.Citation66 Additionally, patients receiving a monthly “loading” dose for 3 months achieved superior visual results than those receiving single injections. Many patients required only two injections after the loading phase and at the last visit after 1 year. Thus, three different dosing regimens were identified for the Phase III studies:Citation67 0.5 mg monthly, 2 mg monthly, or 2 mg every 2 months after the loading phase of three initial monthly doses.
In Phase III, two equivalent pivotal clinical trials of VEGF Trap-eye, VEGF Trap-eye Investigation of Efficacy and Safety in Wet AMD (VIEW) 1 and 2, were conducted to determine if VEGF Trap-eye was noninferior and clinically equivalent to ranibizumab, the drug considered to be the standard against which all subsequent drugs should be compared.Citation66,Citation67 The VIEW 1 study enrolled 1,217 patients in the US and Canada, and the VIEW 2 study enrolled 1,240 patients in Europe, Asia, Japan, and Latin America. Each trial randomized patients among three treatment regimens: 0.5 mg of aflibercept given monthly, 2 mg given monthly, and 2 mg given every two months after 3 monthly loading doses for 3 months. Both studies evaluated the noninferiority efficacy in comparison with a fourth arm of the study in which patients received 0.5 mg of ranibizumab monthly. The first noninferiority endpoint was the percentage of patients who maintained their visual acuity (decrease in vision less than −15 letters); the second noninferiority endpoint was the percentage of patients who gained vision.
After the first year, both the VIEW 1 and 2 studies were continued for a second year (52–96 weeks) in which a modified PRN strategy was adopted. Patients were assessed monthly and were treated only if necessary (with the same drug and dose as in the first year), but the injection was repeated at least every three months in all cases. At week 52, the proportion of patients who maintained their vision (lost <15 ETDRS letters) was approximately 95% when using 2 mg of aflibercept (either monthly or every 2 months after the loading phase). The same results were obtained with 0.5 mg of ranibizumab given monthly. The gains in vision were comparable among the drugs administered monthly: a mean gain of +10.9 letters and +7.6 letters in the aflibercept group and a mean gain of +8.1 letters and +9.4 letters in those receiving ranibizumab, in VIEW 1 and VIEW 2,Citation67 respectively.
In VIEW 1, patients receiving 2 mg of aflibercept every 4 weeks gained more vision than those receiving ranibizumab (+10.9 letters versus +8.1 letters; P = 0.0054).Citation67 Improvements in macular thickness were not statistically different among any of the treatment groups. VIEW 2 patients receiving 2 mg of aflibercept every 8 weeks showed bimonthly fluctuations in macular thickness without corresponding fluctuations in visual acuity.Citation67 The safety of aflibercept was excellent and was comparable with that of ranibizumab in both the VIEW 1 and VIEW 2 studies. Severe extraocular adverse events such as stroke and myocardial infarction occurred with similar frequencies in patients receiving aflibercept (0.7% and 2.6%, respectively) and in patients receiving ranibizumab (1.6% and 2.6%, respectively) in both VIEW trials.
In VIEW 1, the mean vision gain from the baseline (best corrected visual acuity) BCVA at week 52 was greater in the 2 mg aflibercept every month group when compared with the ranibizumab group (mean gain of +10.9 versus +8.1 ETDRS letters).Citation67 Conversely, a statistically significant difference was not found in vision gain in comparison to ranibizumab (mean gain of +7.6 letters versus +9.4 letters) in VIEW 2.Citation67 The reason for this difference in vision results is unknown. However, it is likely that racial and ethnic differences existed between the two trials. Several reports have suggested that the incidence of polypoidal choroidal vasculopathy, which has been suggested to be a variant of neovascular AMD, is markedly high in African-American people, relatively high in the Asian population, and low in white people with AMD.Citation68,Citation69 Polypoidal CNV does not respond well to anti-VEGF therapy alone and should be treated with a combination of photodynamic therapy and anti-VEGF therapy for better results. Thus, a limitation of the two trials was the inclusion of all CNV types by using FAG but not indocyanine green angiography. A comparative subanalysis of the data will be required to address this difference.
However, both VIEW studies showed that 2 mg injections of VEGF Trap-eye every two months delivered a comparable gain in visual acuity to monthly ranibizumab (+7.9 versus +8.1 letters in VIEW 1; +8.9 versus +9.4 letters in VIEW 2).Citation67 Additional efficacy was not demonstrated when VEGF Trap-eye was administered every 4 weeks compared with every 8 weeks, thus suggesting that patients would not require monthly examinations. In the two trials, approximately one third of patients receiving 2 mg of aflibercept every second month experienced a clinical improvement in visual acuity (ranging from +7 to +10 letters). Based on the 1-year efficacy (maintenance of vision) and safety results of the VIEW trials, the FDA approved a regimen of 2 mg of VEGF Trap-eye every 8 weeks for the treatment of wet AMD.Citation70 The recommended treatment regimen includes three loading injections at 4-week intervals, followed by injections every 8 weeks. During the second year (52–96 weeks), patients were assessed monthly and, if necessary, were treated via a modified PRN protocol with a new injection performed not less frequently than once every three months. Between weeks 52 and 96, patients initially receiving 2 mg of aflibercept every 8 weeks and those initially receiving ranibizumab every 4 weeks maintained previous gains in vision.
In an integrated analysis of the VIEW 1 and VIEW 2 studies,Citation70 the visual acuity gain from baseline in the aflibercept group that received 2 mg every 8 weeks was +7.6 letters at week 96 compared to +8.4 letters at week 52, with an average of 11.2 injections over 2 years and 4.2 injections during the second year. The visual acuity gain from baseline in the monthly ranibizumab group was +7.9 letters at week 96 compared to +8.7 letters at week 52, with an average of 16.5 injections over 2 years and 4.7 injections during the second year.Citation70
Only 16% of the patients received six or more injections during the second year.Citation70 In comparison, patients receiving ranibizumab monthly during the first year and PRN the second year received an average of 16.5 injections: 12 during the first year and an average of 4.7 injections over the second year. Approximately 26.5% of the patients required six or more injections during the second year. During year 2 of the VIEW trials,Citation70 48% of the patients receiving 2 mg of aflibercept and 40% of the patients receiving ranibizumab received the minimum number (three) of injections.
In both studies,Citation67 the ocular adverse events experienced across the four treatment groups were those commonly associated with intravitreal injections:Citation35,Citation36 conjunctival hemorrhages, eye pain, and vitreous floaters. Systemic adverse events, such as falls, pneumonia, cancer, and cardiovascular disease were also balanced across the groups and were those commonly found in elderly AMD patients. No evidence of an increased risk of thromboembolic events such as stroke or myocardial infarction was found.Citation71
VEGF Trap-eye: other clinical uses in retinal disease
The VEGF cytokine also plays an important role in the pathogenesis of vascular retinal diseases like diabetic retinopathy, central retinal vein occlusion (CRVO), and branch retinal vein occlusion. It causes an increase in retinal capillary permeability and leakage of fluid into the retina and macula, leading to significant loss of central vision.Citation72 VEGF expression, which is upregulated by hypoxia, was found to be elevated in the ocular fluids of patients with diabetic macular edema (DME) and CRVO.Citation73 Anti-VEGF compounds have been successfully used as the first line of treatment for diabetic retinopathyCitation74 and macular edema due to CRVO, and have replaced laser photocoagulation in some cases.Citation75
Several anti-VEGF agents have been evaluated in numerous clinical trials from 2008 to the present day. Most notably, these include prospective clinical trials regarding intravitreal ranibizumab for the treatment of DME in RD (READ2 [Ranibizumab for Edema of the mAcula in Diabetes], RESOLVE [Safety and Efficacy of Ranibizumab in Diabetic Macular Edema With Center Involvement], RESTORE [A 12 Month Core Study to Assess the Efficacy and Safety of Ranibizumab (Intravitreal Injections) in Patients With Visual Impairment Due to Diabetic Macular Edema and a 24 Month Open-label Extension Study], RISE [A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema (ME) With Center Involvement Secondary to Diabetes Mellitus (RISE)], RIDE [A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema (ME) With Center Involvement Secondary to Diabetes Mellitus (RIDE)]),Citation76 which demonstrated the superiority of this anti-VEGF compound over both sham injection and focal grid laser.Citation77–Citation82 Aflibercept was evaluated in a double-masked, prospective, randomized, multicenter Phase II trial, entitled DME And VEGF Trap-eye: INvestigation of Clinical Impact (DA VINCI),Citation83,Citation84 in which 221 patients with clinically significant DME with central macular involvement were randomized and 219 patients were treated with a balanced distribution over five groups. These groups included monthly doses of 0.5 or 2 mg of VEGF Trap-eye, monthly doses of 2 mg of VEGF Trap-eye for 3 months and then every 8 weeks, monthly doses of 2 mg of VEGF Trap-eye for 3 months and then PRN, and macular laser therapy.Citation83,Citation84 The mean improvements in BVCA at 52 weeks in the VEGF Trap-eye groups were +11.0, +13.1, +9.7, and +12.0 letters, respectively, versus −1.3 letters in the laser group. It is interesting to note these similar results with longer dosing intervals of treatment.
The DA VINCI studyCitation83,Citation84 showed that in addition to the benefits related to the reduction of central macular edema, aflibercept provides secondary benefits related to the nonprogression of retinopathy with the prevents the development of vascular neoproliferation.Citation84 Aflibercept has turned out to be a promising option in DME therapy because of its high binding affinity and extended duration of action. The latter quality is very important in view of the fact that diabetic retinopathy is a chronic disease and that a large percentage of affected patients are of working age.
Presently, no published randomized clinical trials have directly compared any of the anti-VEGF drugs for the treatment of diabetic retinopathy. However, two Phase III clinical studies, the VIVID (VEGF Trap-Eye In Vision Impairment Due to DME)Citation85 and the VISTA (Study of Intravitreal Administration of VEGF Trap-Eye in Patients With Diabetic Macular Edema)Citation75 studies, have been initiated and are evaluating the efficacy and safety of VEGF Trap-eye in comparison with laser treatment over a period of 1 and 2 years, respectively. Finally, a three arm study comparing ranibizumab versus bevacizumab versus aflibercept – the DRCR protocol T – is now in the enrollment phase.Citation75
In September 2012, the FDA approved aflibercept injection for the treatment of macular edema following CRVO.Citation86 This approval was based on data from the Phase III COPERNICUS (Controlled Phase 3 Evaluation of Repeated intravitreal administration of VEGF Trap-Eye In Central retinal vein occlusion: Utility and Safety)Citation87,Citation88 and GALILEO (General Assessment Limiting Infiltration of Exudates in central retinal vein Occlusion with EYLEA) studies.Citation89 In both studies, the results regarding the quality of vision and anatomical outcomes were superior in the aflibercept treated group than in the sham control group. The initial 6-month phase was similar among these studies, during which patients were randomized to receive either an intravitreal injection of 2 mg of aflibercept or a sham injection every month, but the second 6-month phase was different between the two studies. In the GALILEO study,Citation89 patients in the treatment group were treated on a PRN basis with aflibercept, while patients in the placebo group continued to receive treatment with sham injections; in the COPERNICUS study,Citation88 all patients were treated with aflibercept on a PRN basis.
In both the COPERNICUS and GALILEO studies, aflibercept injection resulted in an improvement in visual acuity of >15 letters in 56.1% and 60.2% of patients, respectively, at week 24 compared with those receiving sham injections (12.3% and 22.1%, respectively).Citation87,Citation89 At week 52 in the COPERNICUS study,Citation88 the improvement in visual acuity was 55.3% in the aflibercept/aflibercept PRN patients compared with 30.1% in the sham/aflibercept PRN patients. In the GALILEO study, in which control patients did not receive any aflibercept injections, the improvement was 60.2% and 32.4%, respectively.Citation88,Citation90 The results of these studies showed that it is possible to maintain an excellent visual outcome and to extend the range of administration while using the PRN strategy. These data indicate that aflibercept provides benefits to patients with CRVO and using this drug as needed may become a first line approach that will reduce the burden of monthly injections.
Conclusion
In conclusion, aflibercept, or VEGF Trap-eye, may be considered an attractive alternative to other anti-VEGF agents because it appears to offer visual outcomes similar to ranibizumab and bevacizumab with a longer duration of action. For the first time, an anti-VEGF drug can be given at 2-month intervals with results comparable to ranibizumab given every 4 weeks.Citation91
Aflibercept was shown to be generally well tolerated in the VIEW I and II studies, and the ocular adverse events and adverse events were similar to those of ranibizumab. Patients receiving 2 mg of aflibercept every 8 weeks achieved visual acuity gains similar to those receiving ranibizumab with five fewer injections, on average, over 2 years. Patients who required the most intense therapy received, on average, 1.4 fewer injections in the group receiving 2 mg of aflibercept every 8 weeks when compared to the ranibizumab group in the second year.
Although the future direction of the development of therapeutic management techniques should be driven by improving results, reducing the burden and the cost of treatment should also be considered. In particular, the cost of AMD treatments with the approved anti-VEGF agents is much higher by any metric compared to any previous AMD and retinal treatment. Economic consideration is an important influencing factor in the selection of drugs for individual patients, and the comparable safety and reduced injection burden of aflibercept in comparison with ranibizumab enhances its cost effectiveness. For those clinicians using ranibizumab, the transition to aflibercept (which costs $100 less than ranibizumab) will be easy because the total cost of aflibercept treatment will be even lower than the presumed per vial cost after accounting for the fact that the cost will be lowered further by the greater time interval between injections. However, the transition to aflibercept from off-label bevacizumab (which costs $1,800 less than aflibercept) will be slower for cost conscious physicians. In this case, the relative merits of the more expensive, but less frequently dosed, aflibercept compared to the more frequently dosed, lower cost alternative of off-label bevacizumab must also be considered.
Moreover, aflibercept can be used in shifting patients treated with bevacizumab to aflibercept, as this monthly injection was the only regimen shown to be equivalent to ranibizumab in the comparison of AMD treatment trials.Citation25 Yet another strategy woven into combination therapy stems from the observation that most visual improvements with anti-VEGF agents occur in the first three months, raising the possibility of an initial (albeit high cost) loading treatment with a subsequent (lower cost) maintenance treatment. The addition of new drugs to these combination strategies may diminish both maintenance and loading therapies, achieving better results.
Furthermore, a major concern with chronic therapies is the reduction of the biological effect, which can limit longterm efficacy. This phenomenon has been called tachyphylaxis and has been described as a progressive decrease in the therapeutic response after repetitive administration of anti-VEGF drugs.Citation92 A retrospective review from the National Eye Institute found that between five and ten injections of bevacizumab were required before tachyphylaxis occurred.Citation93 Nonresponder patients, or patients who experience tachyphylaxis, will need alternative treatment strategies to break the cycle of monthly injections with the same stagnant results. A possible solution would be to combine drugs with different mechanisms of action or different pharmacokinetics, for example, switching the treatment to different VEGF blockers. Several reports have shown that administration of aflibercept to eyes that had persistent fluid despite prolonged bevacizumab or ranibizumab therapy resulted in rapid resolution of the subretinal fluid and the flattening of pigment epithelial detachments.Citation6 This indicates that aflibercept can be used with success in patients who show resistance to conventional anti-VEGF drugs and suggest that aflibercept works remarkably well as a “salvage” therapy.
In light of the above analysis based on the literature, the personal opinion of the authors on the therapy for maculopathy is that the best approach for wet AMD is an “attack on several fronts.” In this sense, the first line drugs are anti-VEGF agents that can be used in combination with drugs that inhibit the actions of molecules involved in angiogenesis, including integrins, complements, and PIGF, and with compounds that are able to maintain and preserve the integrity of the retinal photoreceptors and of the choriocapillaris. However, because an effective combination therapy is still several years away, aflibercept promises to become the leading medication in the treatment of wet AMD in the coming years because of its ability to inhibit angiogenesis.
Disclosure
The authors report no conflicts of interest in this work. This review received no specific grant from any funding agency in the public, commercial, or not for profit sector.
References
- Chappelow AV Kaiser PK Neovascular age-related macular degeneration: potential therapies Drugs 2008 68 8 1029 1036 18484796
- La Cour M Kiilgaard JF Nissen MH Age-related macular degeneration: epidemiology and optimal treatment Drugs Aging 2002 19 2 101 133 11950377
- Bloch SB Larsen M Munch IC Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000–2010 Am J Ophthalmol 2012 153 2 209 213 22264944
- Campa C Costagliola C Incorvaia C Inflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implications Mediators Inflamm 2010 2010
- Stewart MW The expanding role of vascular endothelial growth factor inhibitors in ophthalmology Mayo Clin Proc 2012 87 1 77 88 22212972
- Stewart MW Clinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on aflibercept Clin Ophthalmol 2012 6 1175 1186 22973088
- Ferrara N Vascular endothelial growth factor: basic science and clinical progress Endocr Rev 8 2004 25 4 581 611 15294883
- Alitalo K Tammela T Petrova TV Lymphangiogenesis in development and human disease Nature 2005 438 946 953 16355212
- Lee S Jilani SM Nikolova GV Carpizo D Iruela-Arispe ML Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors J Cell Biol 2005 169 681 691 15911882
- Leung DW Cachianes G Kuang WJ Goeddel DV Ferrara N Vascular endothelial growth factor is a secreted angiogenic mitogen Science 1989 246 1306 1309 2479986
- Alon T Hemo I Itin A Stone J Keshet E Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity Nat Med 1995 1 1024 1028 7489357
- Gerber HP Dixit V Ferrara N Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells J Biol Chem 1998 273 13313 13316 9582377
- Carmeliet P Angiogenesis in life, disease and medicine Nature 2005 438 932 936 16355210
- Rosenstein JM Krum JM New roles for VEGF in nervous tissue – beyond blood vessels Exp Neurol 2004 187 246 253 15144851
- Jin K Zhu Y Sun Y Mao XO Xie L Greenberg DA Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo Proc Natl Acad Sci U S A 2002 99 11946 11950 12181492
- Asahara T Takahashi T Masuda H VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells EMBO J 1999 18 3964 3972 10406801
- Roberts WG Palade GE Neovasculature induced by vascular endothelial growth factor is fenestrated Cancer Res 1997 57 765 772 9044858
- Roberts WG Palade GE Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor J Cell Sci 1995 108 6 2369 2379 7673356
- Monsky WL Fukumura D Gohongi T Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor Cancer Res 1999 59 4129 4135 10463618
- Gragoudas ES Adamis AP Cunningham ETJr Feinsod M Guyer DR VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group for neovascular age-related macular degeneration N Engl J Med 2004 351 2805 2816 15625332
- VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group Chakravarthy U Adamis AP Year 2 efficacy results of 2 randomised controlled clinical trials of pegaptanib for neovascular age-related macular degeneration Ophthalmology 2006 113 1508. e1 e25 16828500
- Singerman LJ Masonson H Patel M Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial Br J Ophthalmol 2008 92 1606 1611 18614570
- Frampton JE Ranibizumab: a review of its use in the treatment of neovascular age-related macular degeneration Drugs Aging 2013 30 5 331 358 23539234
- Martin DF Maguire MG Ying GS Grunwald JE Fine SL Jaffe GJ CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration N Engl J Med 2011 364 20 1897 1908 21526923
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group Martin DF Maguire MG Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results Ophthalmology 2012 119 7 1388 1398 22555112
- Ahfat FG Zaidi FH Bevacizumab vs ranibizumab-an appraisal of the evidence from CATT and IVAN Eye (Lond) 3 2013 27 3 289 290 23485959
- Costagliola C Romano M Corte MD Intravitreal bevacizumab for treatment-naive patients with subfoveal occult choroidal neovascularization secondary to age-related macular degeneration: a 12-month follow-up study. Retina Oct 2009 29 9 1227 1234
- Steinbrook R The price of sight-ranibizumab, bevacizumab, and the treatment of macular degeneration N Eng J Med 2006 355 1409 1412
- Meyer CH Krohne TU Holz FG Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans Retina 2011 31 9 1877 1884 21738089
- Conrad PW Zacks DN Johnson MW Intravitreal bevacizumab has initial clinical benefit lasting eight weeks in eyes with neovascular age-related macular degeneration Clin Ophthalmol 2008 2 4 727 733 19668423
- Rosenfeld PJ Brown DM Heier JS MARINA Study Group Ranibizumab for neovascular age-related macular degeneration N Engl J Med 2006 355 1419 1431 17021318
- Brown DM Kaiser PK Michels M ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration N Engl J Med 2006 355 1432 1444 17021319
- Brechner RJ Rosenfeld PJ Babish JD Caplan S Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file Am J Ophthalmol 2011 151 5 887 895. e1 21310390
- Bevacizumab prescribing information Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0168lbl.pdf Accessed July 24, 2013
- Semeraro F Morescalchi F Parmeggiani F Arcidiacono B Costagliola C Systemic adverse drug reactions secondary to anti-VEGF intravitreal injection in patients with neovascular age-related macular degeneration Curr Vasc Pharmacol 2011 9 5 629 646 21470108
- Costagliola C Agnifili L Arcidiacono B Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration Expert Opin Biol Ther 2012 12 10 1299 1313 22866908
- Boyer DS Heier JS Brown DM A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration Ophthalmology 2009 116 9 1731 1739 19643495
- Regillo CD Brown DM Abraham P Randomised, doublemasked, sham-controlled trial of ranibizumab for neovascular agerelated macular degeneration: PIER Study year 1 Am J Ophthalmol 2008 145 239 248 18222192
- Michels M Francom S Wilson L Systemic safety and risk factors associated with intravitreal ranibizumab in patients with choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD) 26th Annual Meeting of the American Society of Retina Specialists October 11–15, 2008 Maui, HI, USA
- Schmidt-Erfurth U Eldem B Guymer R EXCITE Study Group Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study Ophthalmology 2011 118 5 831 839 21146229
- Lalwani GA Rosenfeld PJ Fung AE A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol Jul 2009 148 1 43 58
- Papadopoulos N Martin J Ruan Q Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab Angiogenesis 2012 15 2 171 185 22302382
- US Food and Drug Administration FDA approves Eylea for eye disorder in older people 11 18 2011 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm280601.htm Accessed December 4, 2011
- Dixon JA Oliver SC Olson JL Mandava N VEGF Trap-Eye for the treatment of neovascular age-related macular degeneration Expert Opin Investig Drugs 2009 18 10 1573 1580
- Aflibercept: AVE 0005, AVE 005, AVE0005, VEGF Trap – Regeneron, VEGF Trap (R1R2), VEGF Trap-Eye Drugs R D 2008 9 4 261 269 18588357
- Vaisman N Gospodarowicz D Neufeld G Characterization of the receptors for vascular endothelial growth factor J Biol Chem 1990 265 19461 19466 2246236
- Jakeman LB Armanini M Philips HS Ferrara N Developmental expression of binding sites and mRNA for vascular endothelial growth factor suggests a role for this protein in vasculogenesis and angiogenesis Endocrinology 1993 133 848 859 7688292
- De Vries C Escobedo JA Ueno H Houck K Ferrara N Williams LT The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor Science 1992 255 989 991 1312256
- Shibuya M Yamaguchi S Yamane A Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase (flt) closely related to the fms family Oncogene 1990 5 4 519 524 2158038
- Matthews W Jordan CT Gavin M Jenkins NA Copeland NG Lemischka IR A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit Proc Natl Acad Sci U S A 1991 88 9026 9030 1717995
- Terman BI Carrion ME Kovacs E Rasmussen BA Eddy RL Shows TB Identification of a new endothelial cell growth factor receptor tyrosine kinase Oncogene 1991 6 1677 1683 1656371
- Pajusola K Aprelikova O Korhonen J FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines Cancer Res 1992 52 5738 5743 1327515
- Karkkainen MJ Makinen T Alitalo K Lymphatic endothelium: a new frontier of metastasis research Nat Cell Biol 2002 4 E2 E5 11780131
- Stewart MW Rosenfeld PJ Predicted biological activity of intravitreal VEGF Trap Br J Ophthalmol 2008 92 5 667 668 18356264
- Stewart MW What are the half-lives of ranibizumab and aflibercept (VEGF Trap-eye) in human eyes? Calculations with a mathematical model Eye Reports 2011 1 e5
- Holash J Davis S Papadopoulos N VEGF-Trap: a VEGF blocker with potent antitumor effects PNAS 2002 99 17 11392 11398
- Rakic JM Lambert V Munaut C Mice without uPA, tPA, or plasminogen genes are resistant to experimental choroidal neovascularization Invest Ophthalmol Vis Sci 2003 44 4 1732 1739 12657615
- Saishin Y Saishin Y Takahashi K VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol May 2003 195 2 241 248
- EYLEA™ (aflibercept) injection: US prescribing information Tarrytown, NY, USA Regeneron Pharmaceuticals, Inc Available from: http://www.regeneron.com/Eylea/eylea-fpi.pdf Accessed April 2, 2012
- Nguyen QD Shah SM Hafiz G CLEAR-AMD 1 Study Group A phase I trial of an IV-administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age-related macular degeneration Ophthalmology 2006 113 1522. e1 1532. e14 16876249
- Do DV Nguyen QD Browning DJ Results of a phase I study of intravitreal VEGF Trap in subjects with diabetic macular edema: the CLEAR-IT DME Study IOVS 2007 48 ARVO E-abstract 1430/B486
- Nguyen QD Shah SM Browning DJ A phase I study of intravitreal vascular endothelial growth factor trap-eye in patients with neovascular age-related macular degeneration Ophthalmology 2009 116 2141 2148 19700196
- Brown DM Heier JS Ciulla T CLEAR-IT 2 Investigators Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration Ophthalmology 2011 118 6 1089 1097 21640257
- Heier JS Boyer D Nguyen QD CLEAR-IT 2 Investigators The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fxed dosing Ophthalmology 2011 118 1098 1106 21640258
- Cantrill HL The diabetic retinopathy study and the early treatment diabetic retinopathy study International Ophthalmology Clinics 1984 24 4 13 29 6389409
- Stewart MW Rosenfeld PJ Penha FM Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor trap-eye) Retina 2012 32 3 434 457 22374154
- Heier JS Brown DM Chong V VIEW 1 and VIEW 2 Study Groups Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration Ophthalmology 2012 119 12 2537 2548 23084240
- Sho K Takahashi K Yamada H Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics Arch Ophthalmol 2003 121 10 1392 1396 14557174
- Squirrell DM Bacon JF Brand CS To investigate the prevalence of polypoidal choroidal vasculopathy in presumed age-related peripapillary subretinal neovascular membranes Clin Experiment Ophthalmol 2009 37 4 368 372 19594563
- Regeneron Pharmaceuticals, Inc Two Year Results of Phase 3 Studies with EYLEA™ (aflibercept) injection in wet AMD show sustained improvement in visual acuity [press release] Tarrytown, NY Regeneron Pharmaceuticals, Inc 12 5 2011 Available from: http://investor.regeneron.com/releasedetail.cfm?releaseid=629800 Accessed June 27, 2013
- Regeneron Pharmaceuticals, Inc Bayer and Regeneron report positive top-line results of two phase 3 studies with VEGF Trap-Eye in wet age-related macular degeneration [press release] Tarrytown, NY Regeneron Pharmaceuticals, Inc 11 22 2010 Available from: http://newsroom.regeneron.com/releasedetail.cfm?releaseid=532099 Accessed June 27, 2013
- Antonetti DA Barber AJ Hollinger LA Wolpert EB Gardner TW Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonulaoccluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors J Biol Chem 1999 274 23463 23476 10438525
- Pierce EA Avery RL Foley ED Aiello LP Smith LE Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization Proc Natl Acad Sci U S A 1995 92 905 909 7846076
- Rinaldi M Chiosi F Dell’Omo R Intravitreal pegaptanib sodium (Macugen®) for treatment of diabetic macular oedema: a morphologic and functional study Br J Clin Pharmacol 2012 74 6 940 946 22486651
- Bethke W For DME, one size does not fit all [webpage on the Internet] Review of Opthalmology 8 8 2011 Available from: http://www.revophth.com/content/i/1599/c/29625/ Accessed April 05, 2013
- Clinical Trials Available from: http://www.clinicaltrials.gov/ Accessed July 24, 2013
- Nguyen QD Shah SM Heier JS READ-2 Study Group Primary endpoint (six-months) results of the Ranibizumab for Edema of the macula in Diabetes (READ-2) study Ophthalmology 2009 116 2175 2181 19700194
- Nguyen QD Shah SM Khwaja AA READ-2 Study Group Two-year outcomes of the Ranibizumab for Edema of the macula in Diabetes (READ-2) study Ophthalmology 2010 117 2146 2151 20855114
- Massin P Bandello F Garweg JG Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study Diabetes Care 2010 33 2399 2405 20980427
- Mitchell P Bandello F Schmidt-Erfurth U RESTORE Study Group The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema Ophthalmology 2011 118 615 625 21459215
- Nguyen QD Brown DM Marcus DM et al; RISE RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE Ophthalmology 2012 119 789 801 22330964
- Boyer DS Rundle AC Zhang J Hopkins JJ Ehrlich JS Long-term efficacy and safety of ranibizumab in diabetic macular edema (DME): 36-month results from RISE and RIDE, two phase III clinical trials 45th Annual Scientific Meeting of the Retina Society October 5, 2012 Washington DC, USA
- Do DV Schmidt-Erfurth U Gonzalez VH The DAVINCI study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema Ophthalmology 2011 118 1819 1826 21546089
- Do DV Nguyen QD Boyer D DAVINCI Study Group One-year outcomes of the DAVINCI study of VEGF Trap-Eye in eyes with diabetic macular edema Ophthalmology 2012 119 1658 1665 22537617
- Regeneron Pharmaceuticals Inc Regeneron and Bayer Initiate Phase 3 Trial of EYLEA®(aflibercept) Injection for the Treatment of Diabetic Macular Edema in Asia and Russia Available from: http://investor.regeneron.com/releasedetail.cfm?ReleaseID=741127 Accessed July 24, 2013
- Regeneron Announces FDA Approval of EYLEA®(aflibercept) Injection For Macular Edema Following Central Retinal Vein Occlusion Available from http://investor.regeneron.com/releasedetail.cfm?releaseid=708835 Accessed July 24, 2013
- Boyer D Heier J Brown DM Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study Ophthalmology 2012 119 1024 1032 22440275
- Brown DM Heier JS Clark LW Intravitreal aflibercept injection for macular edema secondary to central vein occlusion: 1-year results from the phase 3 COPERNICUS study Am J Ophthalmol 2013 155 429 437 23218699
- Holz FG Roider J Ogura Y VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study Br J Ophthalmol 2013 97 278 284 23298885
- Holz FG Ogura Y Roider J Intravitreal aflibercept injection for macular edema in central retinal vein occlusion: 1-year results of the phase 3 GALILEO Study [Poster 6929; online] Available from: https://www.abstractsonline.com
- US Food and Drug Administration Eylea US Food and Drug Administration Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125387s004lbl.pdf Accessed June 27, 2013
- Schaal S Kaplan HJ Tezel TH Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology 2008 115 12 2199 2205 18930553
- Forooghian F Cukras C Meyerle CB Chew EY Wong WT Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration Retina 2009 29 6 723 731 19516114