Abstract
Chronic hepatitis C virus (HCV) infection is responsible for the development of liver cirrhosis and hepatocellular carcinoma. It has been a tremendous burden on global health care systems. With the advent of a number of new direct-acting and host-targeting antiviral agents, current interferon-α- and ribavirin-based HCV therapy has started to move towards an interferon-sparing or even interferon-free strategy. In this regard, a recently identified NS5A inhibitor, daclatasvir, showed a great promise in clinical trials as another new class of direct-acting anti-HCV therapeutics, with a distinct mechanism of action. In this review, a variety of preclinical as well as clinical proof-of-concept studies of daclatasvir, including the studies of its discovery, mechanism of action, viral resistance, and host polymorphism profiles are reviewed. In addition, a role of daclatasvir in the future therapy for HCV patients is discussed briefly.
Introduction
The global estimation of hepatitis C virus (HCV)-infected patients is around 170 million, with 4 million new infections reported annually.Citation1,Citation2 There has been a solid cause-and-effect relationship between persistent HCV infection and the development of chronic and often deadly liver diseases, including chronic hepatitis, cirrhosis, and hepatocellular carcinoma.Citation3,Citation4 HCV infection is accountable for more than two-thirds of all cases of liver cancers and transplantations performed in the US.Citation5 In spite of the introduction of two recently approved direct-acting antiviral agents (DAAs), boceprevir and telaprevir, specifically targeting an NS3 viral protease,Citation6 the current standard of care for HCV patients still relies on the combined treatment of a weekly injection of pegylated (PEG) interferon-α (IFNα) and daily administration of the nucleoside analog, ribavirin (RBV). However, this IFNα-based combination therapy has been associated with undesirable side effects, including flu-like symptom, hemolytic anemia, depression, and suicidal thoughts.Citation7 Pulmonary and metabolic complications, including pneumonitis and diabetic ketoacidosis, were also found as IFNα-induced side effects of high severity.Citation8–Citation10 In addition, its unsatisfactory efficacy, which is less than 50% for genotype (GT) 1 and 4 patients has been severe clinical problems.Citation11,Citation12 This emphasizes an urgent need to develop an IFNα-sparing or even an IFNα-free anti-HCV regimen. In this regard, one of the NS5A inhibitors, daclatasvir (DCV), recently developed by Bristol-Myers Squibb and currently progressing through the last stage of clinical trials, has ignited a great excitement as a new and promising component of combination therapy. This review discusses the relevant preclinical as well as clinical data regarding DCV, in order to predict its potential role in HCV treatment in the near future.
Classification and organization of HCV
HCV belongs to the Flaviviridae family of viruses with a single-stranded ribonucleic acid (RNA) of a positive polarity as its viral genome. Following entry into a host liver cell, HCV delivers its RNA genome inside the target cell. Then, internal ribosome entry site (IRES)-assisted translation of its RNA genome results in the production of a polyprotein composed of around 3000 amino acids. This polyprotein subsequently cleaves into ten different viral proteins by virtue of host and virally-encoded proteases.Citation13,Citation14 The first three viral proteins freed from the original polyprotein are called structural proteins. They include core capsid protein and two envelope glycoproteins, E1 and E2.Citation15 They serve as the structural components of a mature virus particle. The remaining seven viral nonstructural (NS) proteins, including p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B, represent the components of a functional replication complex in charge of viral RNA genome amplification on endoplasmic reticulum membranes.Citation16–Citation19 DCV has been shown to abrogate HCV replication by specifically targeting the critical functions of an NS5A protein in the replication complex.Citation20
Role of NS5A in the HCV life cycle
NS5A is a 447 amino acid–long phosphoprotein with an RNA-binding activity.Citation21 In spite of lacking an enzymatic activity, NS5A has been shown to play a major role in HCV RNA replication as well as in infectious particle assembly.Citation22 It consists of an amino terminal amphipathic helix plus three structurally distinct domains. An amino terminal amphipathic helix was demonstrated to be required for the endoplasmic reticulum membrane association, to support HCV RNA replication.Citation23 Domain I was shown to form a dimer, to accommodate a single-strand RNA molecule.Citation24 Replication-enhancing adaptive mutations were found around domain II, suggesting its possible role in HCV RNA replication.Citation25 In addition, the essential role of domain III in HCV infectious particle assembly was also characterized.Citation26 Based on results of these domain mapping studies, NS5A was proposed to play a role in fine-tuning both viral replication as well as particle production in overall HCV life cycle.Citation22
Discovery of DCV and its preclinical studies
DCV was initially discovered by means of a chemical genetic strategy.Citation27,Citation28 In this strategy, a large number of chemically diverse compounds are first screened based on their effect on HCV replication, without any knowledge of their mechanism of action. Then, an error-prone HCV RNA-dependent RNA polymerase (RDRP) generates mutant HCV genomes, which are resistant to identified compounds. Analysis of these resistant mutant genomes leads to the identification of their potential viral targets. Unlike the traditional bottom-up screening approach, which requires the definition of their molecular targets in the first place, this top-down strategy enjoys a freedom of targets selection as long as they are key elements of HCV replication. The discovery of DCV as an NS5A inhibitor proves the usefulness and applicability of this bias-free approach.
By taking advantage of this strategy, Lemm et al conducted a cell-based high-throughput HCV replicon screening.Citation29 In this screening, HCV replication was measured indirectly by monitoring an NS3 protease activity, coupled with fluorescence resonance energy transfer (FRET). Following successful screening, a number of compounds with a thiazolidinone core were identified as inhibitors of HCV replication. Among them, BMS-824 demonstrated a half-maximum effective concentration (EC50) of 5 nM and a half-maximum cytotoxic concentration of more than 50 μM, with a therapeutic index of more than 10,000 in the GT 1b replicon.Citation29 However, BMS-824 showed a relatively poor activity against the GT 1a replicon (> 10 μM). After realizing symmetry as an important contributor to their antiviral activity in GT 1a, BMS-790052 (a previous name of DCV), with a symmetric structure and greater potency against both the GT 1a and 1b replicons, was identified and developed as a candidate for advancement into clinical trials ().Citation27 DCV has been the most potent HCV replication inhibitor reported so far, with a picomolar EC50 towards the GT 1 replicons (50 pM against GT 1a, 9 pM against GT 1b), which represent the majority of HCV patients.Citation27 In addition, DCV also turned out to be an inhibitor of the GT 2a JFH1 replicon and cell culture infectious J6/JFH1 virus, with EC50 values of 46.8 pM and 16.1 pM, respectivelyCitation20 A study of hybrid HCV replicons harboring either GT 3a or 4 NS5A genes also confirmed their susceptibilities to DCV with EC50 values ranging from 120–870 pM for GT 3a and from 7–13 pM for GT 4.Citation30,Citation31 In addition to its potent antiviral activity alone, the additive or synergistic effects of combining NS3 protease or NS5B RNA polymerase inhibitors with DCV on replicon inhibition and clearance were also demonstrated in an in vitro replicon studyCitation32 In addition, GT 1a replicon elimination was shown to be markedly enhanced by the combination of PEG-IFNα and DCVCitation33 These data strongly suggested DCV as a prime candidate for a new component of an IFN-sparing or IFN-free anti-HCV regimen in the future.Citation32
Mechanism of action for DCV
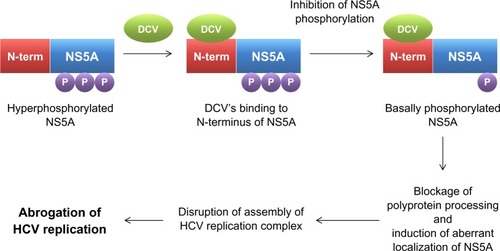
In order to gain an insight into its potential mechanism of action, the HCV replicon of GT 1b was exposed to DCV to generate mutant HCV replicon variants resistant to DCV. After successful sequencing of these variants, a DCV sensitivity domain was found to map to the N terminus of NS5A.Citation29 Identification of NS5A as a possible target of DCV was more or less unexpected, given the ability of DCV to achieve such an extraordinary antiviral potency by targeting a viral protein without any known enzymatic activity. Specifically, DCV was shown to be able to target a specific function of NS5A, which involves downregulation of the hyperphosphorylation of NS5A ().Citation29 By exploring this unique phosphorylation-regulating property of DCV, two distinct functions of NS5A in HCV RNA replication were also discovered. These include a cis-acting function of basally phosphorylated NS5A in maintaining HCV replication complex and a trans-acting function of hyperphosphorylated NS5A in modulating HCV assembly and particle formation. DCV seems to target both the cis- and trans-acting functions of NS5A ().Citation20 When the effect of DCV on polyprotein processing was further examined, DCV was shown to promote the accumulation of an HCV polyprotein intermediate.Citation34 In line with this evidence, the disruption of polyprotein processing by an NS3 protease inhibitor also led to downregulation of the hyperphosphorylation of NS5A, further emphasizing an intimate relationship between polyprotein processing and the phosphorylation status of NS5A.Citation29 In addition, hyperphosphorylated NS5A proteins were demonstrated to separate into different membrane fractions during discontinuous sucrose gradient centrifugation, upon DCV treatment.Citation34 A localization pattern of only hyperphosphorylated NS5A proteins was shown to be disrupted specifically by DCV.Citation34 This finding was further supported by the demonstration of altered biochemical fractionation and the subcellular localization of specific populations of NS5A proteins by DCV.Citation35,Citation36 Based on these observations, DCV seems to perturb the function of new HCV replication complexes, rather than acting on preformed complexes, by modulating the NS5A phosphorylation status ().Citation36 In addition, one modeling studyCitation37 even suggested the possibility of the existence of two modes of action for DCV, due to the multiple roles of NS5A in HCV replication and particle production. These involved the DCV-dependent blockage of two distinct stages of the viral life cycle – viral RNA synthesis and virion assembly/secretion. This study also yielded a more precise estimate of the serum HCV half-life, which was calculated to be around 45 minutes.
In vitro and in vivo HCV resistance to DCV
The analysis of the resistance profiles of HCV variants against DCV is critical in order to predict the potential synergistic efficacy of combination therapy with other anti-HCV agents. Nonoverlapping resistance profiles among different anti-HCV drugs usually lead to a more favorable combination treatment outcome. A majority of DCV-resistant variants turned out to include the L31V, P32L, Q54L, and Y93H mutations of NS5A in the GT 1b replicon, and the M28T, Q30H, Q30R, L31M, L31V, P32L, and Y93C mutations of NS5A in the GT 1a replicon.Citation27,Citation29,Citation38 Specifically, the Y93H mutation displayed the maximum fold resistance phenotype, in spite of its negative impact on fitness of the virus.Citation20 Nevertheless, DCV was reported to be able to maintain subnanomolar potency against all the variants from GT 1b with single amino acid substitutions.Citation38 This suggests a requirement of multiple mutations for the development of significant in vivo resistance in this genetic background.Citation38 Fortunately, most of the DCV-resistant variants remained fully sensitive to IFNα and the small-molecule inhibitors of HCV protease and polymerase.Citation33,Citation38 Most importantly, the resistant variants observed in this in vitro study were very similar to those observed in a multiple ascending dose monotherapy clinical trial of DCV.Citation27,Citation39 These data further validate an in vitro HCV replicon study as an invaluable model to predict clinical efficacy and resistance profile.Citation33
In the following paragraphs, each DCV-related clinical trial data is discussed in depth. In order to conduct a systemic analysis of each DCV-related clinical trial data, all the treatment regimens used in the DCV clinical studies were classified based on the presence or absence of IFN, as studies of DCV alone, studies of IFN-containing regimens, and studies of IFN-free regimens. The characteristics of each clinical study are summarized in .
Table 1 Summary of the clinical trial results of daclatasvir
DCV-alone regimens
Phase I clinical study by Gao et alCitation27
In order to test the clinical efficacy of DCV against HCV infection, a randomized, double-blind, placebo-controlled, single ascending dose study was initiated.Citation27 First, six subjects with GT 1 chronic HCV were treated with doses of 1, 10, and 100 mg of DCV as an oral solution. The mean plasma elimination half-life of DCV was reported to range from 10 to 14 hours by a pharmacokinetic study. After a single oral dose of 10–100 mg of DCV, the 24-hour plasma concentration turned out to be above tenfold the protein-binding-adjusted 90% median effective concentration values (EC90) for HCV GT 1a and 1b. In regards to its antiviral efficacy, the administration of a single 100 mg dose of DCV resulted in a 3.3 log10 reduction in the mean viral load measured 24 hours postdose. This reduction was further sustained for an additional 120 hours in two patients infected with GT 1b virus. Interestingly, a greater and more sustained decline in HCV RNA was detected for subjects infected with GT 1b than for subjects infected with GT 1a.Citation27 The genotypic analysis of clinical samples showed that the major observed HCV variants had substitutions at amino acid positions identified using the in vitro replicon system (M28T, Q30H/R, and L31M mutations for GT 1a and Y93H mutation for GT 1b).Citation27 Headache was the most frequent adverse event, as reported by four subjects after the administration of DCV. This study was the first clinical validation of an the vivo efficacy of the HCV NS5A inhibitor DCV.
Phase I clinical study by Nettles et alCitation39
In the Phase I clinical trial by Nettles et al, a double-blind, placebo-controlled, sequential panel, a multiple ascending dose study was conducted in thirty patients with chronic HCV GT 1 infection.Citation39 The patients were treated with DCV (1, 10, 30, 60, or 100 mg once daily or 30 mg twice daily) or placebo for 14 days. The mean maximum decline from baseline in HCV RNA was observed to be around 2.8–4.1 log10 IU/mL. Inconsistent with a previous result, those who were infected with HCV GT 1b generally demonstrated greater antiviral responses than those who were infected with HCV GT 1a. Specifically, undetectable HCV RNA levels were detected at day 14 in four of the seven patients with GT 1b infection versus none of the 17 patients with GT 1a infection. Viral rebound was also observed on or before day 7 of treatment. Once-daily dosing yielded median peak plasma concentrations at 1–2 hours postdose and a mean terminal half-life of 12–15 hours. A steady state was also achieved following 3–4 days of daily dosing. The identified resistant substitutions included M28, Q30, L31, and Y93 for GT 1a and L31 and Y93 for GT 1b. After 14 days of dosing of 1–100 mg DCV, all patients were able to achieve maximal plasma concentration values above the replicon (EC90) of 0.283 and 0.0362 ng/mL for GT 1a and GT 1b, respectively. This was a first proof-of-concept clinical study with multiple doses of an HCV NS5A inhibitor, DCV.
In vitro and in vivo correlation study by Fridell et alCitation40
An in vitro and in vivo correlation study was conducted for the genotypic and phenotypic analysis of variants resistant to DCV in humans.Citation40 In this study, a sequence analysis was performed on viral complementary DNA isolated from serum specimens, collected at baseline and days 1, 2, 4, 7, and 14 postdosing after a single ascending dose study and a 14-day multiple ascending dose monotherapy study. HCV RNA was reported to remain detectable in all GT 1a–infected patients, and viral breakthrough (VBT) was observed during the course of treatment in the majority of these patients. Due to no cross-resistance observed with other DAAs, DCV was regarded as an excellent candidate for combination therapy. The resistant mutation analysis also found the linkage of L31V/M with Y93H mutations in GT 1b. DCV appeared to have a low genetic barrier for resistance since the plasma minimum concentrations of DCV monotherapy at the dose range used for the multiple ascending dose study were not sufficient to prevent all VBTs because of the emergence of resistant variants.
Single-dose pharmacokinetic study of DCV by Bifano et alCitation41
DCV was shown to be eliminated by hepatic metabolism and direct biliary excretion.Citation41 DCV was also known for its high protein binding. Consistent with its daily dosing, DCV demonstrated a mean plasma half-life of 12 to 15 hours in patients with chronic HCV infection.Citation39 Due to the varying degrees of hepatic impairment, the DCV target population exhibited different levels of drug exposure. In order to examine the influence of hepatic impairment on the singledose pharmacokinetics of DCV, in this open-label, parallel-group study, subjects without active HCV who had mild, moderate, or severe hepatic impairment and controls with normal hepatic function received a single oral dose of 30 mg DCV, and a plasma analysis was performed predose and postdose for 72 hours. DCV turned out to be well tolerated in hepatically impaired patients. The time to reach maximal plasma concentration in the hepatically impaired subjects was similar to that in the controls, and the plasma half-life across the study groups was consistent with the observations in HCV-infected patients.Citation39 In addition, exposure to active and unbound DCV was found to be comparable between the controls and subjects with moderate or severe hepatic impairment. Therefore, DCV dose adjustments in subjects with hepatic impairment would not be recommended.
IFN-containing regimens
Phase IIa clinical study by Pol et alCitation28
In this Phase IIa trial, a randomized parallel-group, double-blind placebo-controlled dose-finding study was conducted for those patients with previously untreated chronic HCV GT 1 infection without cirrhosis.Citation28 The patients were randomly assigned to receive PEG-IFNα (180 μg per week) and RBV (1000–1200 mg daily) plus placebo or 3 mg, 10 mg, or 60 mg of DCV taken once daily, for 48 weeks. The primary efficacy end point was undetectable HCV RNA at 4 weeks and 12 weeks after the start of treatment (extended rapid virologic response [eRVR]). A total of 48 patients were randomly assigned to make 12 per group. Although 15 patients discontinued treatment before week 48, five of 12 patients (42%) who received 3 mg DCV ten of 12 (83%) who received 10 mg DCV nine of 12 (75%) who received 60 mg DCV and one of 12 (8%) who received placebo achieved eRVR. Eleven virologic failures were found including three cases of VBT, four cases of virologic relapse, and four cases of detectable HCV RNA at the end of 48 weeks of therapy. This study suggested a further development of regimens containing 60 mg of DCV for the treatment of chronic GT 1 HCV infection.
Relevant case report by Fontana et alCitation42
In this study, there was a one case report of successful PEG-IFNα, RBV and DCV combination therapy for recurrent cholestatic HCV after liver retransplantation.Citation42 Three months after the second transplant, DCV (20 mg/day), PEG-IFNα (180 μg/week), and RBV (800 mg/day) were prescribed for an early recurrent cholestatic HCV patient. Serum HCV RNA became undetectable at week 3 of treatment and remained undetectable during 24 weeks of triple therapy and during the posttreatment follow up. DCV was well tolerated and the minimum drug concentrations were within the targeted range throughout the treatment.
IFN-free regimens
Phase IIa clinical study by Lok et alCitation43
This was a preliminary study of two antiviral agents, including the NS5 inhibitor DCV and the NS3 protease inhibitor asunaprevir (ASV), in HCV GT 1 patients.Citation43 This open-label, Phase IIa study recruited 21 patients with chronic HCV GT 1 infection who had not had a response to previous treatment with PEG-IFNα and RBV (null responders). Eleven patients (group A) were treated with the NS5A inhibitor DCV (60 mg once daily) and the NS3 protease inhibitor ASV (600 mg twice daily) alone. The other ten patients (group B) were treated with DCV and ASV in combination with PEG-IFNα and RBV for 24 weeks. A sustained virologic response (SVR) 12 weeks after the end of the treatment period was achieved in four patients in group A (36%; two of nine with HCV GT 1a and two of two with GT 1b). Six patients (all with HCV GT 1a) had VBT while receiving therapy, and resistance mutations to both antiviral agents were found in all cases. One patient had a viral response at the end of treatment but had a relapse after the treatment period. On the other hand, all ten patients in group B had an SVR at 12 weeks after treatment; nine patients in group B had an SVR at 24 weeks after treatment. Diarrhea was the most common adverse event in both groups. Six patients had transient elevations of alanine aminotransferase levels to more than three times the upper limit of the normal range. This clinical study further supported a proof of concept that SVR can be achieved by two DAAs without PEG-IFNα and RBV therapy. Nonoverlapping resistance profiles were also found in DCV- and ASV-treated patients. This clinical trial presented proof that the use of DAAs could provide a clinical cure for HCV in patients who are refractory to the current standard of care.
Phase IIa clinical study by Sulkowski et alCitation44,Citation45
This was a randomized, open-label, two-stage, parallel-group, Phase IIa study aiming to evaluate the efficacy and safety of all-oral and once-daily combination of DCV and sofosbuvir (SOF), a nucleotide analog NS5B polymerase inhibitor, with or without RBV, for 24 weeks in treatment-naïve patients infected with HCV GT 1, 2, and 3.Citation44 A total of 126 GT 1 and 44 GT 2/3 patients were randomly assigned to receive a once-daily dose of 60 mg of DCV plus 400 mg of SOF, with or without a prior once-daily dose of 400 mg of SOF for 7 days, or with or without RBV. The primary efficacy end point was undetectable HCV RNA at 24 weeks after the end of treatment (SVR24). It was seen that cotreatment of DCV and SOF with or without RBV achieved SVR24 in more than 93% of patients with HCV GT 1, 2, or 3. The virologic response did not vary according to IL28B GT, viral subtype, or the administration of RBV. The DCV plus SOF regimen was well tolerated, and a low hemoglobin was observed only in patients taking RBV.
In order to extend the potential of the all-oral and once-daily combination of DCV plus SOF regimen in patients who previously failed prior treatment with telaprevir or boceprevir plus PEG IFNα/RBV, another similarly designed clinical study was performed. In this randomized, open-label, parallel-group, Phase IIa clinical study, those 41 difficult-to-treat GT 1 patients, including those with prior nonresponse, relapse, or viral breakthrough, were randomly assigned to receive a once-daily dose of 60 mg of DCV plus 400 mg of SOF with or without RBV.Citation45 The primary efficacy end point was undetectable HCV RNA at 12 weeks after the end of treatment (SVR12). Surprisingly, the all-oral, once-daily combination of DCV and SOF with or without RBV achieved SVR12 in all 41 HCV GT1–infected patients who failed prior treatment. This clinical trial also provided evidence that the use of DCV with other DAA with a high genetic barrier, such as SOF, could result in a clinical elimination of HCV in patients who previously failed current standard of care.
Phase IIa clinical study by Chayama et alCitation46
This was a dual open-label, Phase IIa study with the NS5A inhibitor, DCV, and the NS3 protease inhibitor, ASV, initiated in HCV virus GT 1b–infected null responders, including ten patients with chronic HCV GT 1b infection and previous null response to PEG-IFNα and RBV.Citation46 The patients received dual DAA treatment for 24 weeks with DCV (60 mg once-daily) and ASV (initially 600 mg twice-daily, then subsequently reduced to 200 mg twice daily). The primary efficacy end point was SVR12. Nine patients completed the full course of treatment. Surprisingly, all nine patients achieved SVR12 and SVR24. The HCV RNA also remained undetectable posttreatment, even in the patient who discontinued after 2 weeks. No VBT was detected. Diarrhea and mild headache were the most common adverse events. Transaminase elevations were reported in three patients but did not result in discontinuation. This study clearly showed that a dual therapy with DCV and ASV, without PEG-IFNα and RBV, can achieve high SVR rates in difficult-to-treat patients with HCV GT 1b infection and previous null response to PEG-IFNα and RBV.
Phase IIa clinical study by Suzuki et alCitation47
In this open-label, Phase IIa study, a dual oral therapy with DCV and ASV for 24 weeks was conducted for Japanese patients with HCV GT 1b infection and limited treatment options.Citation47 Among the patients were 21 null responders (<2log10 HCV RNA reduction after 12 weeks of PEG-IFNα/RBV) and 22 patients who were intolerant to or medically ineligible for PEG-IFNα with or without RBV therapy. The patients were treated with a once-daily dose of 60 mg of DCV and a twice-daily dose of 200 mg of ASV for 24 weeks. The primary efficacy end point was SVR12. In all, 36 of the 43 enrolled patients completed 24 weeks of therapy. Serum HCV RNA levels declined rapidly, becoming undetectable in all patients on therapy by week 8, and 33 patients (76.7%) achieved SVR12 and SVR24, 19 (90.5%) of the null responders and 14 (63.6%) of the ineligible/intolerant patients. No virologic failures were observed among the null responders. Three ineligible/intolerant patients experienced VBT, and four relapsed posttreatment. Diarrhea, nasopharyngitis, headache, and mild increases of alanine/aspartate aminotransferases were the most common adverse events. This study indicated that a dual therapy with DCV and ASV, without PEG-IFNα/RBV, was well tolerated and achieved high SVR rates in two groups of difficult-to-treat patients with HCV virus GT 1b infection.
In vitro and in vivo correlation study by Karino et alCitation48
This study was a characterization of virologic escape in HCV GT 1b patients treated with DAAs, DCV, and ASV, including previously described three patients with VBT and four patients with relapse.Citation48 Baseline NS3 polymorphisms (T54S, Q80L, and V170M) at amino acid positions previously associated with low-level resistance (< ninefold) to select NS3 protease inhibitors were detected in four null responders and three ineligibles, but they were not associated with virologic failure. Baseline NS5A polymorphisms (L28M, L31M, and Y93H) associated with DCV resistance (<25-fold) were detected in five null responders and six ineligibles. All three of the patients with VBT and two of the four patients with relapse carried a baseline NS5A-Y93H polymorphism. NS3 and NS5A resistance–associated variants were detected together (NS3-D168A/V and NS5A-L31M/V-Y93H) after virologic failure. While the DCV-resistant substitutions persisted through 48 weeks posttreatment, the ASV-resistant substitutions were no longer detectable. In addition, five out of ten patients with a baseline NS5A-Y93H polymorphism experienced virologic failure, whereas five out of ten achieved SVR.
Polymorphism and HCV clearance
A baseline polymorphism in patients is an important indicator to determine the potential clinical outcome of HCV therapy with a specific DAA. When the impact of a baseline polymorphism on the emergence of resistance to DCV was examined, a Q30R variant was observed at VBT in one of the GT 1a–infected subjects.Citation49 However, another baseline polymorphism (E62D) did not contribute resistance to DCV.Citation49 The linked variant, Q30R-E62D, conferred high-level resistance in vitro and is likely responsible for VBT in vivo.Citation49 A baseline polymorphism with a minimal effect on the anti-HCV effect of DCV can affect the emergence of resistance and significantly affect clinical outcome.Citation49 NS5A sequence heterogeneity present in GT 1a and GT 1b baseline specimens was found to have a minimal effect on the potency of DCV.Citation49 Low rates of naturally occurring resistant variants to DCV were also detected in HCV 1 null responders.Citation50 Due to the low rates of naturally occurring resistant variants in the NS5A region found in HCV 1 null responders to PEG-IFNα plus RBV, routine direct sequencing of HCV before starting DCV was not recommended.Citation50 The prevalence of HCV variants resistant to NS3 protease inhibitors or DCV was also examined in hepatitis patients with GT 1b.Citation51 In this study, drug-resistant mutations were determined in 362 hepatitis patients infected with HCV GT-1b who had not received DAAs before.Citation51 Amino acid substitutions resistant to NS3 inhibitors (V36A, T54S, Q80H, and D168E) were detected in 15 of the 307 (4.9%) patients who had been examined for NS3 substitutions, and in these patients, T54S substitutions (3.3%) predominated over V36A (0.3%), Q80R (0.7%), and D168E (0.7%).Citation51 Amino acid substitutions resistant to DCV (L31M and/or Y93H) were also detected in 33 of the 294 (11.2%) patients who were examined for NS5 substitutions, and the Y93H substitutions (8.2%) were found to predominate over the L31M substitutions (2.7%).Citation51 One of the 239 (0.4%) patients who had been examined for amino acid substitutions in both NS3 and NS5A regions was found to possess HCV GT 1b variants resistant to NS3 inhibitors (T54S) and DCV (L31M).Citation51
The prevalence of natural polymorphisms at the HCV NS5A gene associated with resistance to DCC was also studied in all IFNα treatment-naïve as well as in HIV-HCV-coinfected patients.Citation52 Specifically, changes reported to be associated with DCV resistance in the in vitro replication system for the HCV GT subtypes 1a and 1b (M28T, Q30H/R, L31F/M/V, P32L, and Y93C/H/N) were examined.Citation52 When the samples from 78 HIV-HCV-coinfected individuals, deposited at Los Alamos HCV database, were analyzed, none of the NS5A sequences from HCV GT 1a or HCV GT 3 showed changes associated with DCV resistance, although all NS5A sequences from HCV GT 4 harbored L31M.Citation52 The double mutant L31M+Y93H was found in 7% of HCV GT 1b and in 13% of HCV GT 4.Citation52 Primary resistance mutations to DCV were not seen as natural polymorphisms in HCV GT 1a or in HCV GT 3.Citation52 They could be recognized in most HCV GT 1b and HCV GT 4 strains, regardless of HIV coinfection.Citation52
Potential role of DCV in the future HCV therapy
Since the introduction of IFNα- and RBV-based combination therapy, there have been several medically unmet needs in HCV treatment. These include the need for the improvement of antiviral efficacy in HCV GT 1 and 4, the reduction of viral resistance, minimization of side effects, the shortening of duration and frequency of treatment, an all-oral delivery, and greater affordability. In regards to the improvement of antiviral efficacy in GT 1 and 4, DCV displayed a superior pangenotypic potency in both in vitro and in vivo studies. Therefore, DCV could serve as a critical component of combination therapy, especially for GT 1 patients, who comprised the majority of HCV patients. In addition, the analysis of DCV-induced viral resistance profiles proved its nonoverlapping property with other DAAs. On top of this, ability of DCV to maintain subnanomolar potency against all DCV-resistant variants would be another of its merits. This strongly suggests DCV as the best candidate to achieve the most synergistic antiviral effect when combined with other mechanistically distinct DAAs, by suppressing the emergence of all possible multiple resistant variants. Since most of the serious side effects of standard HCV therapy come from the nonspecific actions of IFNα and RBV, the minimization of side effects also could be achieved by an IFNα-free regimen consisting of DCV and other DAAs, including NS3 protease inhibitors and NS5B polymerase inhibitors. The feasibility of these DCV-containing and IFNα-free DAA combination regimens was already proved by several clinical trials.Citation43,Citation46,Citation47 In order to shorten the duration and frequency of current anti-HCV treatment, DCV also would be a valuable combination option, thanks to its extraordinary antiviral potency and desirable pharmacokinetic profile, which make a once-daily dosing possible. Finally, the all-oral delivery of anti-HCV therapeutics could be realized by implementing a DCV-containing, all-DAA regimen in practice, with no need for muscular injections of IFNα. This would provide a great convenience for HCV patients. In regards to affordability, elimination of the recombinant protein-based IFNα would greatly help to bring down overall cost of current (IFNα-based) combination therapy.
In spite of all the strengths of DCV mentioned above, the most important limitation of DCV turned out to be its low genetic barrier. In vitro and clinical studies demonstrated selection of HCV variants resistant to DCV, more easily for GT 1a isolates than for GT 1b isolates.Citation27,Citation38 Single substitutions in the N terminal region of NS5A were found to confer more than 1000-fold resistance in GT 1a isolates. In order to supplement this weakness, DCV needs to be combined with other DAAs with high genetic barriers and different mechanisms of action. In this regard, a recently developed, second generation NS3 protease inhibitor, asunaprevir, and a nucleotide analog NS5B polymerase inhibitor, sofosbuvir, enjoyed relatively high genetic resistances, and their combined administration with DCV achieved a great antiviral response without the development of viral resistance in HCV patients.Citation44,Citation45,Citation53–Citation55 Therefore, these would potentially serve as great partners for DCV in combination therapy, in the near future.
Conclusion
This review summarizes key preclinical and clinical data of an HCV NS5A inhibitor, DCV, describing its discovery, mechanism of action, resistance profile, in vitro and in vitro efficacy and toxicity, and polymorphism. It also discusses the potential role of DCV in future HCV combination therapy, by satisfying several unmet medical needs in the HCV therapy area, and its limitation due to its low barrier to resistance. Given its extraordinary antiviral potency and high safety profile, its novel mechanism of action, nonoverlapping resistance profile, desirable pharmacokinetic characteristic, and superior synergism with other DAAs, DCV would make an invaluable component of the most ideal, IFNα-free, all-oral anti-HCV combination therapy. A careful evaluation of the relevant Phase III clinical trial results will be required to for DCV to fulfill its critical role in HCV treatment in the near future.
Acknowledgments
This work was supported by the GRRC program of Gyeonggi province (Study of control of viral diseases [grant number GRRC-DONGGUK2011-A01]) and by the Bio and Medical Technology Development Program of the National Research Foundation (NRF), funded by the Korean government (MEST) (grant number 2012053532).
Disclosure
The author declares no conflict of interest in this work.
References
- Shepard CW Finelli L Alter MJ Global epidemiology of hepatitis C virus infection Lancet Infect Dis 2005 5 10 558 567 16122679
- Alter MJ Epidemiology of hepatitis C virus infection World J Gastroenterol 2007 13 17 2436 2441 17552026
- Alter MJ Kruszon-Moran D Nainan OV The prevalence of hepatitis C virus infection in the United States, 1988 through 1994 N Engl J Med 1999 341 8 556 562 10451460
- Di Bisceglie AM Natural history of hepatitis C: its impact on clinical management Hepatology 2000 31 4 1014 1018 10733560
- Armstrong GL Wasley A Simard EP McQuillan GM Kuhnert WL Alter MJ The prevalence of hepatitis C virus infection in the United States, 1999 through 2002 Ann Intern Med 2006 144 10 705 714 16702586
- Pawlotsky JM The results of Phase III clinical trials with telaprevir and boceprevir presented at the Liver Meeting 2010: a new standard of care for hepatitis C virus genotype 1 infection, but with issues still pending Gastroenterology 2011 140 3 746 754 21255572
- Gentile I Viola C Reynaud L Hemolytic anemia during pegylated IFN-alpha2b plus ribavirin treatment for chronic hepatitis C: ribavirin is not always the culprit J Interferon Cytokine Res 2005 25 5 283 285 15871666
- Hegade VS Sood R Saralaya D Moreea S Pulmonary complications of treatment with pegylated interferon for hepatitis C infection-two case reports Ann Hepatol 2013 12 4 629 633 23813142
- Slavenburg S Heijdra YF Drenth JP Pneumonitis as a consequence of (peg)interferon-ribavirin combination therapy for hepatitis C: a review of the literature Dig Dis Sci 2010 55 3 579 585 19399621
- Tosone G Borgia G Gentile I A case of pegylated interferon alpha-related diabetic ketoacidosis: can this complication be avoided? Acta Diabetol 2007 44 3 167 169 17721757
- Liang TJ Rehermann B Seeff LB Hoofnagle JH Pathogenesis, natural history, treatment, and prevention of hepatitis C Ann Intern Med 2000 132 4 296 305 10681285
- Zeuzem S Feinman SV Rasenack J Peginterferon alfa-2a in patients with chronic hepatitis C N Engl J Med 2000 343 23 1666 1672 11106715
- Grakoui A McCourt DW Wychowski C Feinstone SM Rice CM A second hepatitis C virus-encoded proteinase Proc Natl Acad Sci U S A 1993 90 22 10583 10587 8248148
- Grakoui A Wychowski C Lin C Feinstone SM Rice CM Expression and identification of hepatitis C virus polyprotein cleavage products J Virol 1993 67 3 1385 1395 7679746
- Lee C Interaction of Hepatitis C Virus Core Protein with Janus Kinase Is Required for Efficient Production of Infectious Viruses Biomol Ther (Seoul) 21 97 106 24009866
- Moradpour D Penin F Rice CM Replication of hepatitis C virus Nat Rev Microbiol 2007 5 6 453 463 17487147
- Blight KJ Kolykhalov AA Rice CM Efficient initiation of HCV RNA replication in cell culture Science 2000 290 5498 1972 1974 11110665
- Lohmann V Körner F Koch J Herian U Theilmann L Bartenschlager R Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line Science 1999 285 5424 110 113 10390360
- Lee C Discovery of hepatitis C virus NS5A inhibitors as a new class of anti-HCV therapy Arch Pharm Res 2011 34 9 1403 1407 21975800
- Fridell RA Qiu D Valera L Wang C Rose RE Gao M Distinct functions of NS5A in hepatitis C virus RNA replication uncovered by studies with the NS5A inhibitor BMS-790052 J Virol 2011 85 14 7312 7320 21593143
- Foster TL Belyaeva T Stonehouse NJ Pearson AR Harris M All three domains of the hepatitis C virus nonstructural NS5A protein contribute to RNA binding J Virol 2010 84 18 9267 9277 20592076
- Scheel TK Rice CM Understanding the hepatitis C virus life cycle paves the way for highly effective therapies Nat Med 2013 19 7 837 849 23836234
- Elazar M Cheong KH Liu P Greenberg HB Rice CM Glenn JS Amphipathic helix-dependent localization of NS5A mediates hepatitis C virus RNA replication J Virol 2003 77 10 6055 6061 12719597
- Tellinghuisen TL Marcotrigiano J Rice CM Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase Nature 2005 435 7040 374 379 15902263
- Lohmann V Hoffmann S Herian U Penin F Bartenschlager R Viral and cellular determinants of hepatitis C virus RNA replication in cell culture J Virol 2003 77 5 3007 3019 12584326
- Appel N Zayas M Miller S Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly PLoS Pathog 2008 4 3 e1000035 18369481
- Gao M Nettles RE Belema M Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect Nature 2010 465 7294 96 100 20410884
- Pol S Ghalib RH Rustgi VK Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial Lancet Infect Dis 2012 12 9 671 677 22714001
- Lemm JA O’Boyle D Liu M Identification of hepatitis C virus NS5A inhibitors J Virol 2010 84 1 482 491 19812153
- Wang C Valera L Jia L Kirk MJ Gao M Fridell RA In vitro activity of daclatasvir on hepatitis C virus genotype 3 NS5A Antimicrob Agents Chemother 2013 57 1 611 613 23089758
- Wang C Jia L Huang H In vitro activity of BMS-790052 on hepatitis C virus genotype 4 NS5A Antimicrob Agents Chemother 2012 56 3 1588 1590 22203595
- Pelosi LA Voss S Liu M Gao M Lemm JA Effect on hepatitis C virus replication of combinations of direct-acting antivirals, including NS5A inhibitor daclatasvir Antimicrob Agents Chemother 2012 56 10 5230 5239 22850513
- Wang C Huang H Valera L Hepatitis C virus RNA elimination and development of resistance in replicon cells treated with BMS-790052 Antimicrob Agents Chemother 2012 56 3 1350 1358 22214777
- Qiu D Lemm JA O’Boyle DR The effects of NS5A inhibitors on NS5A phosphorylation, polyprotein processing and localization J Gen Virol 2011 92 Pt 11 2502 2511 21795470
- Lee C Ma H Hang JQ The hepatitis C virus NS5A inhibitor (BMS-790052) alters the subcellular localization of the NS5A nonstructural viral protein Virology 2011 414 1 10 18 21513964
- Targett-Adams P Graham EJ Middleton J Small molecules targeting hepatitis C virus-encoded NS5A cause subcellular redistribution of their target: insights into compound modes of action J Virol 2011 85 13 6353 6368 21507963
- Guedj J Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life Proc Natl Acad Sci. USA 2013 110 3991 3996 23431163
- Fridell RA Qiu D Wang C Valera L Gao M Resistance analysis of the hepatitis C virus NS5A inhibitor BMS-790052 in an in vitro replicon system Antimicrob Agents Chemother 2010 54 9 3641 3650 20585111
- Nettles RE Gao M Bifano M Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1 Hepatology 2011 54 6 1956 1965 21837752
- Fridell RA Wang C Sun JH Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations Hepatology 2011 54 6 1924 1935 21809362
- Bifano M Sevinsky H Persson A Single-dose pharmacokinetics of daclatasvir (DCV; BMS-790052) in subjects with hepatic impairment compared with healthy subjects Presented at: 62nd Annual Meeting of the American Association for the Study of Liver Diseases November 6–9, 2011 San Francisco, CA
- Fontana RJ Hughes EA Appelman H Hindes R Dimitrova D Bifano M Case report of successful peginterferon, ribavirin, and daclatasvir therapy for recurrent cholestatic hepatitis C after liver retransplantation Liver Transpl 2012 18 9 1053 1059 22706796
- Lok AS Gardiner DF Lawitz E Preliminary study of two antiviral agents for hepatitis C genotype 1 N Engl J Med 2012 366 3 216 224 22256805
- Sulkowski MS Gardiner DF Rodriguez-Torres M AI444-040 Study Group Sustained virologic response with daclatasvir plus sofosbuvir ± ribavirin (RBV) in chronic HCV genotype (GT) 1-infected patients who previously failed telaprevir (TVR) or boceprevir (BOC) Presented at: 48th Annual Meeting of the European Association for the Study of the Liver April 24–28, 2013 Amsterdam, The Netherlands
- Sulkowski MS Gardiner DF Rodriguez-Torres M AI444040 Study Group High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naive patients chronically infected with HCV GT 1, 2, or 3 Hepatology 2012 56 1516 1517
- Chayama K Takahashi S Toyota J Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders Hepatology 2012 55 3 742 748 21987462
- Suzuki Y Ikeda K Suzuki F Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options J Hepatol 2013 58 4 655 662 23183526
- Karino Y Toyota J Ikeda K Characterization of virologic escape in hepatitis C virus genotype 1b patients treated with the direct-acting antivirals daclatasvir and asunaprevir J Hepatol 2013 58 4 646 654 23178977
- Sun JH O’Boyle Ii DR Zhang Y Impact of a baseline polymorphism on the emergence of resistance to the hepatitis C virus nonstructural protein 5A replication complex inhibitor, BMS-790052 Hepatology 2012 55 6 1692 1699 22234905
- Galmozzi E Aghemo A Colombo M Low rates of naturally occurring resistant variants to the NS5A inhibitor daclatasvir in HCV-1 null responders Hepatology 2013 57 5 2087 22745013
- Suzuki F Sezaki H Akuta N Prevalence of hepatitis C virus variants resistant to NS3 protease inhibitors or the NS5A inhibitor (BMS-790052) in hepatitis patients with genotype 1b J Clin Virol 2012 54 4 352 354 22658798
- Plaza Z Soriano V Vispo E Prevalence of natural polymorphisms at the HCV NS5A gene associated with resistance to daclatasvir, an NS5A inhibitor Antivir Ther (Lond) 2012 17 5 921 926 22436385
- Asselah T Sofosbuvir-based interferon-free therapy for patients with HCV infection J Hepatol Epub July 24 2013
- Chae HB Park SM Youn SJ Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives Scientific World Journal 2013 2013 704912 23844410
- Gentile I Borgia F Buonomo AR Castaldo G Borgia G A novel promising therapeutic option against hepatitis C virus: an oral nucleotide NS5B polymerase inhibitor sofosbuvir Curr Med Chem 2013 20 30 3733 3742 23848533