Abstract
Purpose
Patients with glioblastoma (GBM) have poor prognosis and limited therapeutic options, largely because of chemoresistance to temozolomide (TMZ) treatment. Ubiquitin conjugating enzyme E2 T (UBE2T) plays a key role in regulating the malignancy of various tumors, including GBM; however, its role in TMZ resistance of GBM has not been elucidated. The purpose of this study was to clarify the role of UBE2T in mediating TMZ resistance and investigate the specific underlying mechanism.
Methods
Western blotting was used to detect the protein levels of UBE2T and Wnt/β-catenin-related factors. CCK-8, flow cytometry, and colony formation assays were used to examine the effect of UBE2T on TMZ resistance. Wnt/β-catenin signaling pathway activation was inhibited using XAV-939, and a xenograft mouse model was generated to clarify the function of TMZ in vivo.
Results
UBE2T knockdown sensitized GBM cells to TMZ treatment, whereas UBE2T overexpression promoted TMZ resistance. The specific UBE2T inhibitor, M435-1279, increased the sensitivity of GBM cells to TMZ. Mechanistically, our results demonstrated that UBE2T induces β-catenin nuclear translocation and increases the protein levels of downstream molecules, including survivin and c-Myc. Inhibition of Wnt/β-catenin signaling using XAV-939 blocked TMZ resistance due to UBE2T overexpression in GBM cells. In addition, UBE2T was shown to facilitate TMZ resistance by inducing Wnt/β-catenin signaling pathway activation in a mouse xenograft model. Combined treatment with TMZ and UBE2T inhibitor achieved superior tumor growth suppression relative to TMZ treatment alone.
Conclusion
Our data reveal a novel role of UBE2T in mediating TMZ resistance of GBM cells via regulating Wnt/β-catenin signaling. These findings indicate that targeting UBE2T has promising potential to overcome TMZ resistance of GBM.
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor and is associated with higher recurrence and lower survival rates.Citation1,Citation2 Temozolomide (TMZ)-based chemotherapies are the standard treatment options for GBM;Citation3 however, GBM tumors inevitably become resistant to TMZ and patients have poor prognosis, due to the molecular heterogeneity, hyperactivation of pro-survival pathways, rapid growth rate, and immunosuppression of GBM tumors.Citation4 Aberrantly activated genes, such as O6-methylguanine-DNA methyltransferase (MGMT), which can repair DNA alkylation damage caused by TMZ, have key roles in TMZ resistance of GBM.Citation5 Although there have been many attempts to improve the efficacy of therapy for GBM, unfortunately, none have achieved the desired therapeutic effect.Citation6 Therefore, it is of great clinical significance to elucidate the mechanism underlying TMZ resistance and develop new targets for therapeutic intervention.
Ubiquitin conjugating enzyme E2 T (UBE2T, also known as HSPC150) belongs to the ubiquitin E2 family and participates in ubiquitin activation by transfer from the E1 activating enzyme to the substrate.Citation7 UBE2T was initially reported as a negative regulator of the Fanconi anemia (FA) pathway; its deletion leads to impaired DNA repair ability and the formation of abnormal chromosomes, which are a feature of FA.Citation8,Citation9 In addition to its relevance in FA, UBE2T also has vital roles in the development, progression, and recurrence of various tumors.Citation10–14 UBE2T facilitates the occurrence and development of ovarian cancer by regulating epithelial–mesenchymal transition (EMT) through the PI3K-AKT pathway.Citation15 Further, silencing of UBE2T represses lung adenocarcinoma progression by increasing the expression of fibulin 5,Citation16 and UBE2T can also promote pyrimidine metabolism by enhancing Akt K63-linked ubiquitination, thus contributing to hepatocellular carcinoma (HCC) development.Citation17
Recent studies have discovered that UBE2T plays a key role in therapy resistance in various tumors. For example, UBE2T enhances radiation resistance through ubiquitination-mediated FOXO1 degradation and Wnt/β-catenin signaling pathway activation in non-small cell lung cancer.Citation18 Further, UBE2T-mediated mono-ubiquitination of H2AX/γH2AX decreases HCC radiosensitivity by activating cycle checkpoint kinase 1,Citation19 while hypoxia-induced downregulation of UBE2T increases tumor sensitivity to the DNA crosslinking agent, mitomycin C.Citation20 Moreover, UBE2T promotes proliferation and inhibits apoptosis of GBM cells through inducing K48-linked polyubiquitination of ribosomal protein L6 in an E3 ligase-independent manner.Citation21 Another group reported that UBE2T overexpression enhances GBM cell invasion and migration through stabilizing glucose-regulating protein 78 and facilitating EMT.Citation22
Overall, the available evidence indicates that UBE2T may be a valuable therapeutic target in the treatment of GBM. Nevertheless, the function and underlying mechanism of UBE2T in TMZ resistance of GBM remain unclear. In the current study, we aim to clarify the role of UBE2T in mediating TMZ resistance and investigate the specific underlying mechanism.
Materials and Methods
Cell Lines
The U87 MG and HEK293T cell lines were purchased from American Type Culture Collection (ATCC). U251 cells were obtained from Shanghai Cell Bank (Chinese Academy of Science, Shanghai, China). All cell lines were authenticated by Short Tandem Repeat (STR) assay. All cells were cultured in DMEM (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco) in a 5% CO2 atmosphere at 37°C.
Antibodies and Reagents
Antibodies for UBE2T (ab140611, 1:1000), survivin (ab76424, 1:1000), β-catenin (ab32572, 1:1000), and Lamin B1 (ab133741, 1:1000) were purchased from Abcam (Cambridge, UK). C-Myc (10828-1-AP, 1:1000) antibody was obtained from Proteintech (Wuhan, China). Antibodies for β-actin (AC026, 1:50000) and GAPDH (AC002, 1:5000) were purchased from Abclonal (Wuhan, China). XAV-939 (S1180), M435-1279 (S1180), puromycin (S7417), polybrene (E1299), and TMZ (S1237) were acquired from Selleck Chemicals (Houston, TX, USA).
Plasmids and shRNAs
A vector containing the UBE2T coding sequence was purchased from Wzbio (CH858804, Jinan, China). UBE2T was subcloned into pCMV (PS100069, OriGene, Rockville, MD, USA) to generate the pCMV-UBE2T vector. Vectors containing UBE2T-specific shRNA1 (TRCN0000098575) and shRNA2 (TRCN0000098576) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
UBE2T Overexpression and Knockdown
Lentiviruses were produced by transfecting overexpression or shRNA constructs into HEK293T cells with packaging plasmids (psPAX2 and pMD2.G), using Lipofectamine 2000 reagent (11668019, ThermoFisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. Cell supernatants were harvested 48 h after transfection. To obtain stable cell lines, U87 and U251 cells were infected with lentiviral supernatants for 24 h in the presence of polybrene (10 ng/mL) and then selected by culture in medium supplemented with puromycin (2 μg/mL). UBE2T overexpression and knockdown efficiencies were assessed by Western blotting assay.
Cell Survival Rate Assay
Cell survival rate was examined using the Cell Counting Kit-8 (CCK-8, C0037, Beyotime, Shanghai, China). In brief, U251 and U87 cells were seeded into 96-well plates (3000 cells per well) and treated as required. Subsequently, 10 μL of CCK-8 reagent was added to the wells and cells cultured for 2 h at 37°C in 5% CO2. Plates were then analyzed by spectrometry (450 nm) using a ThermoFisher Scientific Multiskan FC instrument.
Western Blotting
Cells were harvested and lysed with RIPA lysis buffer (P0013B, Beyotime) supplemented with 1 mM PMSF. Protein concentrations were determined using a bicinchoninic acid kit (P0012S, Beyotime). Equal amounts of protein samples were separated by SDS-PAGE, transferred onto polyvinylidene fluoride membranes, then probed with primary antibodies at 4°C overnight, and incubated with secondary antibodies at room temperature for 1 h. Western blotting band signals were detected using an enhanced chemiluminescence kit and analyzed using ImageJ software.
Apoptosis Assay
Cells were seeded into 6-cm dishes and treated as required. After treatment, cells were trypsinized, collected, washed with PBS, and gently resuspended in 500 μL binding buffer (556547, BD Bioscience, Franklin Lakes, NJ, USA). Thereafter, cells were stained with 5 μL Annexin V-FITC for 15 min and 5 μL propidium iodide for another 5 min. Finally, the stained cells were harvested using a BD FACSCalibur instrument and analyzed with FlowJo software.
Colony Formation Assay
Cells (800–1200/well) were seeded into 6-well plates and treated as indicated. Next, cells were rinsed with PBS, fixed with methanol, and stained with crystal violet. Colonies with >50 cells were counted using ImageJ software.
Separation of Nuclear and Cytoplasmic Fractions
Separation of cell nuclear and cytoplasmic fractions was carried out using NE-PER Nuclear and Cytoplasmic Extraction Reagents (78833, ThermoFisher Scientific), according to the manufacturer’s protocol. Western blotting was then performed to detect proteins in the nucleus and cytoplasm.
Xenograft Mouse Model
BALB/c nude mice (20–25 g, 4–6 weeks) were purchased from Gempharmatech Co., Ltd (Nanjing, China). U251 cells (5 × 106) transfected with pCMV or pCMV-UBE2T were subcutaneously injected into the left flanks of nude mice. When the tumor size approached 100 mm3, mice were randomly assigned to three groups: pCMV+TMZ, pCMV-UBE2T+TMZ, and pCMV-UBE2T+TMZ+XAV-939. Then, mice were intraperitoneally injected with TMZ (50 mg/kg) and/or XAV-939 (15 mg/kg) once a day. Two weeks later, mice were sacrificed by cervical dislocation and the xenograft tumors were excised, photographed, and tumor volume was calculated using the formula: (length × width2)/2.
Statistical Analysis
Bars and errors represent the mean ± standard error of the mean (SEM) from at least three independent experiments. Student’s t-test and one-way ANOVA were used for group comparisons. GraphPad Prism and Adobe Photoshop were used to generate and process graphs. P < 0.05 was considered statistically significant.
Results
UBE2T Knockdown Increases GBM Cell Sensitivity to TMZ
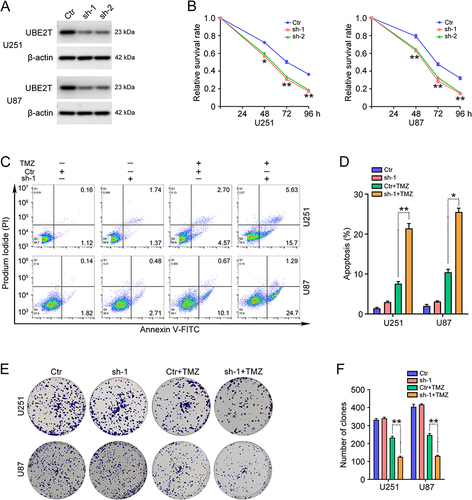
To clarify the function of UBE2T in GBM cell sensitivity to TMZ, UBE2T shRNAs (shRNA1 and shRNA2) were stably transferred into U251 and U87 cells by lentiviral transduction; Western blotting showed that UBE2T protein levels are significantly reduced in GBM cells transduced with UBE2T shRNA (). Next, U251 and U87 cells with UBE2T knocked down were treated with TMZ (100 μM) for different periods of time (48, 72, and 96 h), and CCK-8 assays were performed to examine the cell survival rate. UBE2T deficiency decreased the viability of GBM cells compared with that of the control group (). Flow cytometry analysis was conducted to detect apoptosis of U251 and U87 cells challenged with TMZ (100 μM) for 72 h. Knockdown of UBE2T significantly promoted GBM cell apoptosis ( and ). In addition, colony formation assays demonstrated that UBE2T knockdown decreases the colony forming abilities of U251 and U87 cells ( and ). Thus, our data indicate that UBE2T knockdown sensitizes GBM cells to TMZ treatment.
Figure 1 Knockdown of UBE2T sensitizes GBM cells to TMZ treatment. (A) UBE2T protein levels determined by Western blot in U251 and U87 cells with UBE2T stably knocked down. PLKO.1 (Ctr), UBE2T shRNA-1 (sh-1), UBE2T shRNA-2 (sh-2). (B) U251 and U87 cells with UBE2T knocked down were treated with TMZ (100 μM) for 48, 72, and 96 h, and cell survival rates assessed by CCK-8 assay. (C) U251 and U87 cells with UBE2T knocked down were treated with TMZ (100 μM) for 72 h, and apoptosis detected by flow cytometry analysis. (D) Apoptotic cell ratios in (C). (E) U251 and U87 cells with UBE2T knocked down were treated with TMZ (20 μM) for 3–7 days, and colony formation efficiency determined by colony formation assay. (F) Numbers of clones in (E). n = 3, *P < 0.05, **P < 0.01, compared with the indicated group.
UBE2T Overexpression Promotes TMZ Resistance in GBM Cells
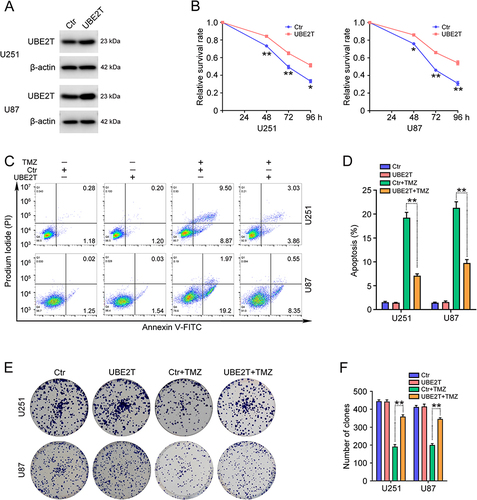
To further scrutinize the role of UBE2T in the TMZ resistance of GBM cells, U251 and U87 cells stably overexpressing UBE2T were constructed. UBE2T protein levels in cells transfected with pCMV-UBE2T (UBE2T) were significantly higher than those in cells transfected with empty vector (pCMV) (). We then treated GBM cells overexpressing UBE2T with TMZ (100 μM) for 48, 72, and 96 h. CCK-8 assays were used to determine cell survival rates and suggested that ectopic expression of UBE2T increases GBM cell viability (). Flow cytometry analysis also illustrated that UBE2T overexpression decreases the apoptosis ratio in GBM cells treated with TMZ (100 μM) ( and ). Consistent with these findings, the colony forming ability in cells overexpressing UBE2T was higher than that in control cells ( and ). Collectively, our results indicate that UBE2T overexpression confers TMZ insensitivity to GBM cells.
Figure 2 Overexpression of UBE2T promotes TMZ resistance in GBM cells. (A) UBE2T protein levels in U251 and U87 cells stably overexpressing UBE2T were determined by Western blot. pCMV (Ctr), pCMV-UBE2T (UBE2T). (B) U251 and U87 cells with UBE2T overexpression were challenged with TMZ (100 μM) for 48, 72, and 96 h and cell survival rates determined by CCK-8 assay. (C) U251 and U87 cells with UBE2T overexpression were challenged with TMZ (100 μM) for 72 h and flow cytometry analysis conducted to detect apoptosis. (D) Apoptotic cell ratios in (C). (E) U251 and U87 cells overexpressing UBE2T were challenged with TMZ (20 μM) for 3–7 days and colony formation efficiency determined by colony formation assay. (F) Numbers of clones in (E). n = 3, *P < 0.05, **P < 0.01, compared with the indicated group.
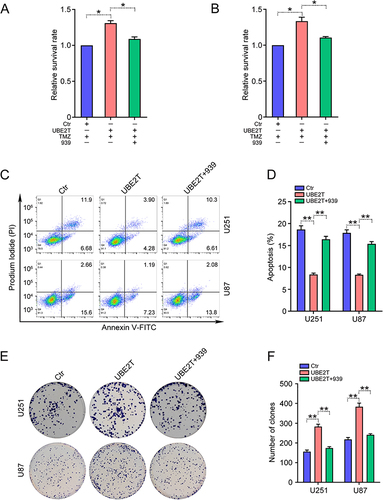
UBE2T Inhibitor Increases the Efficacy of TMZ Treatment of GBM Cells
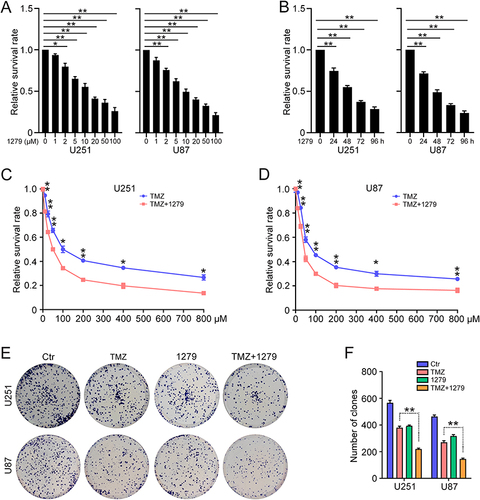
As high UBE2T expression leads to TMZ resistance in GBM cells, we investigate whether TMZ is more effective when used in combination with a UBE2T-specific inhibitor. Consistent with our previous findings, the administration of M435-1279 significantly inhibited U251 and U87 cell proliferation ( and ). Next, U251 and U87 cells were treated with various concentrations of TMZ (0, 10, 25, 50, 100, 200, 400, 800 μM) combined with M435-1279 (5 μM) for 72 h. CCK-8 assays showed that addition of M435-1279 increases the growth suppressive effect of TMZ ( and ). Subsequently, we conducted colony formation assays to investigate the colony forming ability of GBM cells treated with TMZ and/or M435-1279. Compared with treatment with TMZ alone, administration of M435-1279 combined with TMZ led to reduced colony formation of U251 and U87 cells ( and ). Hence, our data suggest that UBE2T inhibition increases the sensitivity of GBM cells to TMZ treatment, and that combination treatment with a UBE2T inhibitor and TMZ may improve the therapeutic effect of TMZ and overcome TMZ resistance.
Figure 3 The UBE2T inhibitor, M435-1279, increases TMZ sensitivity in GBM cells. (A) U251 and U87 cells were treated with the indicated concentrations of M435-1279 (0, 1, 2, 5, 10, 20, 50, 100 μM) for 48 h and cell survival rate determined by CCK-8 assay. (B) U251 and U87 cells were treated with 10 μM M435-1279 for 24, 48, 72, and 96 h and cell survival rate determined by CCK-8 assay. (C) U251 and (D) U87 cells were treated with the indicated concentrations of TMZ (0, 10, 25, 50, 100, 200, 400, 800 μM) in combination with or without M435-1279 (5 μM) for 72 h and cell survival rate determined by CCK-8 assay. (E) U251 and U87 cells were treated with TMZ (20 μM) in combination with or without M435-1279 (2 μM) for 3–7 days. (F) Colony formation efficiency was determined by colony formation assay. n = 3, *P < 0.05, **P < 0.01, compared with the indicated group.
UBE2T Promotes Activation of the Wnt/β-Catenin Signaling Pathway in GBM Cells
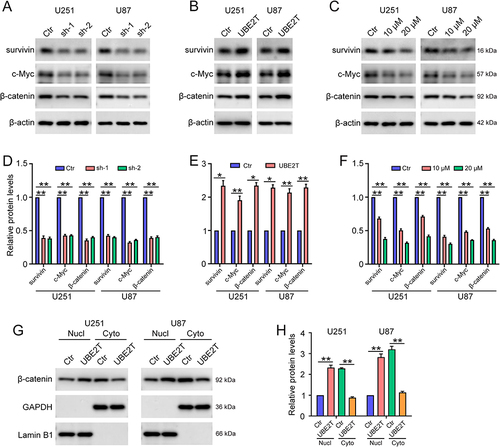
Next, we explored the molecular mechanisms underlying UBE2T mediation of TMZ resistance in GBM cells. As UBE2T regulates the Wnt/β-catenin signaling pathway in various tumors,Citation11,Citation13,Citation14 we next investigated whether UBE2T participates in regulation of Wnt/β-catenin signaling in GBM cells. Western blotting showed that UBE2T knockdown decreases β-catenin expression, as well as that of its downstream molecules, such as survivin and c-Myc ( and ), whereas, UBE2T overexpression increased the expression of these proteins ( and ). Notably, the administration of UBE2T inhibitor strongly reduced the protein levels of β-catenin, survivin, and c-Myc ( and ). In addition, UBE2T overexpression enhanced the nuclear translocation of β-catenin, indicating activation of the Wnt/β-catenin pathway ( and ). Thus, our results suggest that UBE2T positively regulates Wnt/β-catenin signaling in GBM cells, while UBE2T inhibition can suppress Wnt/β-catenin signaling activity.
Figure 4 UBE2T activates the Wnt/β-catenin signaling pathway in GBM cells. Survivin, c-Myc, β-catenin, and β-actin protein levels detected by Western blot in U251 and U87 cells: (A) transfected with pLKO.1 (Ctr), UBE2T shRNA-1 (sh-1), and UBE2T shRNA-2 (sh-2); (B) transfected with pCMV (Ctr) and pCMV-UBE2T (UBE2T); and (C) treated with M435-1279 (10 and 20 μM) for 48 h. (D–F) Quantification of protein levels in (A–C), respectively. (G) β-catenin protein levels detected by Western blot in the cytoplasm (cyto) and nuclear (nucl) fractions of U251 and U87 cells. GAPDH and Lamin B1 were used as markers of cytoplasm and nucleus fractions, respectively. (H) Quantification of UBE2T protein levels in (G). n = 3, *P < 0.05, **P < 0.01, compared with the indicated group.
UBE2T Confers TMZ Resistance to GBM Cells via Wnt/β-Catenin Signaling
Subsequently, we examined whether Wnt/β-catenin signaling is involved in the role of UBE2T in TMZ resistance of GBM cells. A well-known Wnt/β-catenin inhibitor, XAV-939, was applied to block β-catenin activation.Citation23,Citation24 CCK-8 assay results demonstrated that the effects of UBE2T overexpression are greatly reduced in the presence of XAV-939 ( and ). Compared with cells with UBE2T overexpression alone, the apoptosis of cells treated with XAV-939 was dramatically increased ( and ). Correspondingly, colony formation assays also demonstrated that XAV-939 decreases the colony forming abilities of U251 and U87 cells overexpressing UBE2T ( and ). Collectively, these data suggest that UBE2T mediates TMZ resistance by activating Wnt/β-catenin signaling in GBM cells.
Figure 5 UBE2T promotes TMZ resistance via Wnt/β-catenin signaling. GBM cells with or without UBE2T overexpression were treated with TMZ (100 μM) and/or XAV-939 (10 μM) for 72 h, and CCK-8 assays used to detect cell survival rates in (A) U251 and (B) U87 cells. (C) Apoptosis was detected by flow cytometry analysis. (D) Ratio of apoptotic cells in (C). (E) GBM cells with or without UBE2T overexpression were treated with TMZ (20 μM) and/or XAV-939 (2 μM) for 3–7 days. Colony formation efficiency detected by colony formation assay. (F) Numbers of clones in (E). n = 3, *P < 0.05, **P < 0.01, compared with the indicated group.
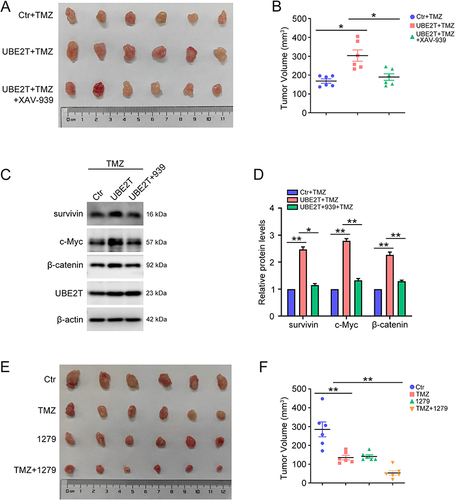
UBE2T Promotes TMZ Resistance in vivo Through Wnt/β-Catenin Signaling
Next, subcutaneous xenografts were used to evaluate the role of UBE2T in a mouse model. As shown in , UBE2T overexpression promoted tumor growth, whereas blocking Wnt/β-catenin using XAV-939 reversed this phenomenon ( and ). Further, UBE2T overexpression also activated Wnt/β-catenin in tumors, but not in mice injected with XAV-939 ( and ). Furthermore, we explored the combined effect of TMZ and UBE2T inhibitor treatment in vivo, and found that the combination of TMZ and UBE2T inhibitor was more effective than TMZ treatment alone ( and ). Together, our results demonstrate that UBE2T promotes TMZ resistance by activating the Wnt/β-catenin signaling pathway, and that UBE2T inhibitor treatment can enhance the growth suppressive effect of TMZ in a mouse model.
Figure 6 Inhibition of UBE2T increases TMZ sensitivity in a mouse xenograft model. (A) U251 cells with or without stable overexpression of UBE2T were implanted subcutaneously into nude mice. When the tumor volumes were approximately 100 mm3, mice were randomly divided into three groups: pCMV+TMZ (Ctr+TMZ), pCMV-UBE2T+TMZ (UBE2T+TMZ), and pCMV-UBE2T+TMZ+XAV-939 (UBE2T+TMZ+XAV-939). Then, mice were intraperitoneally injected with TMZ (50 mg/kg) or/and XAV-939 (15 mg/kg) once a day. After two weeks, ectopic xenograft tumors were excised and photographed. (B) Measurement of tumor volumes in (A). (C) Survivin, c-Myc, β-catenin, UBE2T, and β-actin protein levels in tumors detected by Western blot. (D) Quantification of protein levels in (C). (E) U251 cells were implanted subcutaneously into nude mice. When the tumor volumes were approximately 100 mm3, mice were randomly divided into four groups: unstimulated (Ctr), TMZ, M435-1279 (1279), and TMZ+M435-1279 (TMZ+1279). Then, mice were intraperitoneally injected with TMZ (50 mg/kg) and/or M435-1279 (10 mg/kg) once a day. After two weeks, ectopic xenograft tumors were excised and photographed. (F) Measurement of tumor volumes in (E). n = 6, *P < 0.05, **P < 0.01, compared with the indicated group.
Discussion
GBM is the most aggressive primary brain tumor in adults and accounts for more than 50% of all gliomas.Citation25 Although the treatment of GBM has evolved over the years, most patients with GBM still have a very poor prognosis, with a 5-year relative survival rate of 5%.Citation26 TMZ is a common clinical chemotherapy for GBM, which can cross the blood–brain barrier and trigger tumor cell apoptosis;Citation27 however, resistance to TMZ develops quickly and frequently. In the current study, we found that ectopic expression of UBE2T decreases TMZ-induced GBM cell apoptosis, whereas UBE2T knockdown induces apoptosis. UBE2T inhibition using specific inhibitors also increased GBM cell sensitivity to TMZ both in vitro and in vivo. Our findings suggest that UBE2T is a promising therapeutic target to overcome TMZ resistance in patients with GBM.
Recently, targeted therapies have shown great prospects for application in the treatment of malignant tumors. Yu et al identified a novel UBE2T inhibitor, M435-1279, that can inhibit Wnt/β-catenin signaling hyperactivation. This inhibitor suppresses gastric cancer progression by blocking UBE2T-mediated degradation of receptor for activated C kinase 1 (RACK1), while reducing cytotoxicity.Citation13 UBE2T is also significantly overexpressed in GBM and contributes to GBM malignant progression,Citation21,Citation22 making it an attractive target for GBM-targeted therapy.
In this study, we demonstrated that treatment with UBE2T inhibitors significantly reduces GBM cell proliferation and enhances the growth suppressive effect of TMZ on GBM cells. Further, we showed that M435-1279 combined with TMZ is more effective in inhibiting tumor growth than TMZ alone in a mouse xenograft model. Thus, targeted inhibition of UBE2T has significant prospects for clinical application to overcome TMZ resistance. In our future investigations, we will further explore the clinical effects of UBE2T inhibition.
Many reports have indicated that UBE2T participates in regulation of various intracellular oncogenic signaling pathways. UBE2T was originally shown to bind to FA Complementation Group L and regulate the FA pathway.Citation8 Further, UBE2T overexpression enhances malignant progression by activating the PI3K-AKT signaling pathway in ovarian cancer, lung adenocarcinoma, breast cancer, renal cell carcinoma, and osteosarcoma.Citation10,Citation28–31 UBE2T significantly promotes retinoblastoma tumorigenesis via STAT3 signaling.Citation32 Notably, UBE2T induces Wnt/β-catenin pathway activation through RACK1 ubiquitination and degradation, resulting in promotion of gastric cancer progression.Citation13 Further, UBE2T physically binds to and increases the ubiquitination of Mule, which subsequently mediates β-catenin degradation, thereby regulating liver cancer stem cells.Citation14 UBE2T can also promote β-catenin nuclear translocation through activating MAPK/ERK signaling in hepatocellular carcinoma.Citation11 Wnt/β-catenin signaling is a highly conserved pathway that mediates biological processes, including proliferation, genetic stability, apoptosis, and stem cell renewal.Citation33 Once activated, β-catenin translocates to the nucleus and induces activation of its target genes (encoding c-Myc, survivin, cyclin D1) via TCF/LEF transcription factor activity.Citation34 Our data demonstrated that UBE2T overexpression increases the levels of β-catenin, c-Myc, and survivin, and induces β-catenin nuclear translocation, and that these activities are inhibited by UBE2T knockdown. Moreover, the use of a specific UBE2T inhibitor also suppressed Wnt/β-catenin signaling hyperactivation.
Abnormal activation of the Wnt/β-catenin pathway is strongly implicated in various human tumors, indicating that it is a promising therapeutic target.Citation35,Citation36 Wnt/β-catenin signaling is also highly activated in GBM and active Wnt/β-catenin is associated with reduced survival of patients with GBM;Citation37,Citation38 hence, targeting specific regulatory components of the Wnt/β-catenin signaling pathway has the potential to enhance the survival rate of patients with GBM. Wnt/β-catenin signaling promotes chemoresistance of various tumors by transcriptionally activating oncogenes that promote cell survival, such as those encoding c-Myc, survivin, and cyclin D1.Citation39 Further, the Wnt/β-catenin pathway enhances TMZ resistance by inducing MGMT expression, and Wnt inhibition, thereby augmenting the effects of alkylating drugs.Citation40 Furthermore, Wnt/β-catenin signaling contributes to TMZ resistance through regulating cell stemness and EMT in GBM.Citation41 Here, we showed that inhibition of Wnt/β-catenin abolishes UBE2T overexpression-induced TMZ resistance both in vitro and in vivo. Therefore, UBE2T confers TMZ resistance via activating Wnt/β-catenin signaling in GBM. Inhibition of the Wnt/β-catenin pathway by targeting UBE2T is a potential approach to overcome TMZ resistance.
Conclusion
Overall, our data demonstrated that UBE2T promotes TMZ resistance by inducing Wnt/β-catenin signaling activation in GBM cells and in a mouse xenograft model. Treatment with a UBE2T-specific inhibitor greatly facilitated the growth suppressive effect of TMZ. These findings indicate that targeting UBE2T has the potential to overcome TMZ resistance in the clinic.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
All animal experiments were conducted following the principles approved by the Ethical Committee of Animal Experiments of the First Affiliated Hospital of the University of Science and Technology of China [2020-N(A)-076].
Consent for Publication
All authors have approved the manuscript and given their consent for submission and publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Nguyen HM, Guz-Montgomery K, Lowe DB, Saha D. Pathogenetic features and current management of glioblastoma. Cancers. 2021;13(4):856. doi:10.3390/cancers13040856
- Khan I, Baig MH, Mahfooz S, et al. Nanomedicine for glioblastoma: progress and future prospects. Semin Cancer Biol. 2022;86(Pt 2):172–186. doi:10.1016/j.semcancer.2022.06.007
- Gan T, Wang Y, Xie M, et al. MEX3A impairs DNA mismatch repair signaling and mediates acquired temozolomide resistance in glioblastoma. Cancer Res. 2022;82(22):4234–4246. doi:10.1158/0008-5472.CAN-22-2036
- Goenka A, Tiek D, Song X, Huang T, Hu B, Cheng SY. The many facets of therapy resistance and tumor recurrence in glioblastoma. Cells. 2021;10(3):484. doi:10.3390/cells10030484
- Zhao J, Yang S, Cui X, et al. A novel compound EPIC-0412 reverses temozolomide resistance via inhibiting DNA repair/MGMT in glioblastoma. Neuro Oncol. 2022. doi:10.1093/neuonc/noac242
- Ntafoulis I, Koolen SLW, Leenstra S, Lamfers MLM. Drug repurposing, a fast-track approach to develop effective treatments for glioblastoma. Cancers. 2022;14(15):3705. doi:10.3390/cancers14153705
- Zhu J, Ao H, Liu M, Cao K, Ma J. UBE2T promotes autophagy via the p53/AMPK/mTOR signaling pathway in lung adenocarcinoma. J Transl Med. 2021;19(1):374. doi:10.1186/s12967-021-03056-1
- Machida YJ, Machida Y, Chen Y, et al. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Mol Cell. 2006;23(4):589–596. doi:10.1016/j.molcel.2006.06.024
- Schultz-Rogers L, Lach FP, Rickman KA, et al. A homozygous missense variant in UBE2T is associated with a mild Fanconi anemia phenotype. Haematologica. 2021;106(4):1188–1192. doi:10.3324/haematol.2020.259275
- Huang W, Huang H, Xiao Y, et al. UBE2T is upregulated, predicts poor prognosis, and promotes cell proliferation and invasion by promoting epithelial-mesenchymal transition via inhibiting autophagy in an AKT/mTOR dependent manner in ovarian cancer. Cell Cycle. 2022;21(8):780–791. doi:10.1080/15384101.2022.2031426
- Lioulia E, Mokos P, Panteris E, Dafou D. UBE2T promotes beta-catenin nuclear translocation in hepatocellular carcinoma through MAPK/ERK-dependent activation. Mol Oncol. 2022;16(8):1694–1713. doi:10.1002/1878-0261.13111
- Ren X, Li A, Ying E, Fang J, Li M, Yu J. Upregulation of ubiquitin-conjugating enzyme E2T (UBE2T) predicts poor prognosis and promotes hepatocellular carcinoma progression. Bioengineered. 2021;12(1):1530–1542. doi:10.1080/21655979.2021.1918507
- Yu Z, Jiang X, Qin L, et al. A novel UBE2T inhibitor suppresses Wnt/beta-catenin signaling hyperactivation and gastric cancer progression by blocking RACK1 ubiquitination. Oncogene. 2021;40(5):1027–1042. doi:10.1038/s41388-020-01572-w
- Ho NPY, Leung CON, Wong TL, et al. The interplay of UBE2T and mule in regulating Wnt/beta-catenin activation to promote hepatocellular carcinoma progression. Cell Death Dis. 2021;12(2):148. doi:10.1038/s41419-021-03403-6
- Cui P, Li H, Wang C, et al. UBE2T regulates epithelial-mesenchymal transition through the PI3K-AKT pathway and plays a carcinogenic role in ovarian cancer. J Ovarian Res. 2022;15(1):103. doi:10.1186/s13048-022-01034-9
- Li Y, Yang X, Lu D. Knockdown of ubiquitin-conjugating enzyme E2T (UBE2T) suppresses lung adenocarcinoma progression via targeting fibulin-5 (FBLN5). Bioengineered. 2022;13(5):11867–11880. doi:10.1080/21655979.2022.2060162
- Zhu Z, Cao C, Zhang D, et al. UBE2T-mediated Akt ubiquitination and Akt/beta-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism. Cell Death Dis. 2022;13(2):154. doi:10.1038/s41419-022-04596-0
- Yin H, Wang X, Zhang X, et al. UBE2T promotes radiation resistance in non-small cell lung cancer via inducing epithelial-mesenchymal transition and the ubiquitination-mediated FOXO1 degradation. Cancer Lett. 2020;494:121–131. doi:10.1016/j.canlet.2020.06.005
- Sun J, Zhu Z, Li W, et al. UBE2T-regulated H2AX monoubiquitination induces hepatocellular carcinoma radioresistance by facilitating CHK1 activation. J Exp Clin Cancer Res. 2020;39(1):222. doi:10.1186/s13046-020-01734-4
- Ramaekers CH, van den Beucken T, Meng A, et al. Hypoxia disrupts the Fanconi anemia pathway and sensitizes cells to chemotherapy through regulation of UBE2T. Radiother Oncol. 2011;101(1):190–197. doi:10.1016/j.radonc.2011.05.059
- Tao X, Wu X, Zhou P, et al. UBE2T promotes glioblastoma malignance through ubiquitination-mediated degradation of RPL6. Cancer Sci. 2022;114:521–532. doi:10.1111/cas.15604
- Huang P, Guo Y, Zhao Z, et al. UBE2T promotes glioblastoma invasion and migration via stabilizing GRP78 and regulating EMT. Aging. 2020;12(11):10275–10289. doi:10.18632/aging.103239
- Lee R, Li J, Li J, et al. Synthetic essentiality of tryptophan 2,3-Dioxygenase 2 in APC-mutated colorectal cancer. Cancer Discov. 2022;12(7):1702–1717. doi:10.1158/2159-8290.CD-21-0680
- Lu Y, Zhao X, Liu Q, et al. lncRNA MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance via Wnt/beta-catenin signaling. Nat Med. 2017;23(11):1331–1341. doi:10.1038/nm.4424
- He Z, Cheng M, Hu J, et al. miR-1297 sensitizes glioma cells to temozolomide (TMZ) treatment through targeting adrenomedullin (ADM). J Transl Med. 2022;20(1):443. doi:10.1186/s12967-022-03647-6
- Cantidio FS, Gil GOB, Queiroz IN, Regalin M. Glioblastoma - treatment and obstacles. Rep Pract Oncol Radiother. 2022;27(4):744–753. doi:10.5603/RPOR.a2022.0076
- Choo M, Mai VH, Kim HS, et al. Involvement of cell shape and lipid metabolism in glioblastoma resistance to temozolomide. Acta Pharmacol Sin. 2022;44:670–679. doi:10.1038/s41401-022-00984-6
- Chen Y, Hong H, Wang Q, et al. NEDD4L-induced ubiquitination mediating UBE2T degradation inhibits progression of lung adenocarcinoma via PI3K-AKT signaling. Cancer Cell Int. 2021;21(1):631. doi:10.1186/s12935-021-02341-9
- Qiao L, Dong C, Ma B. UBE2T promotes proliferation, invasion and glycolysis of breast cancer cells by regulating the PI3K/AKT signaling pathway. J Recept Signal Transduct Res. 2022;42(2):151–159. doi:10.1080/10799893.2020.1870495
- Hao P, Kang B, Li Y, Hao W, Ma F. UBE2T promotes proliferation and regulates PI3K/Akt signaling in renal cell carcinoma. Mol Med Rep. 2019;20(2):1212–1220. doi:10.3892/mmr.2019.10322
- Wang Y, Leng H, Chen H, et al. Knockdown of UBE2T inhibits osteosarcoma cell proliferation, migration, and invasion by suppressing the PI3K/Akt signaling pathway. Oncol Res. 2016;24(5):361–369. doi:10.3727/096504016X14685034103310
- Xu N, Cui Y, Shi H, et al. UBE2T/STAT3 signaling promotes the proliferation and tumorigenesis in retinoblastoma. Invest Ophthalmol Vis Sci. 2022;63(9):20. doi:10.1167/iovs.63.9.20
- Liu J, Xiao Q, Xiao J, et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. doi:10.1038/s41392-021-00762-6
- Zhang Y, Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. doi:10.1186/s13045-020-00990-3
- Perugorria MJ, Olaizola P, Labiano I, et al. Wnt-beta-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16(2):121–136. doi:10.1038/s41575-018-0075-9
- Zhao H, Ming T, Tang S, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022;21(1):144. doi:10.1186/s12943-022-01616-7
- Barzegar Behrooz A, Talaie Z, Jusheghani F, Los MJ, Klonisch T, Ghavami S. Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in glioblastoma. Int J Mol Sci. 2022;23(3):1353. doi:10.3390/ijms23031353
- Shahcheraghi SH, Tchokonte-Nana V, Lotfi M, Lotfi M, Ghorbani A, Sadeghnia HR. Wnt/beta-catenin and PI3K/Akt/mTOR signaling pathways in glioblastoma: two main targets for drug design: a review. Curr Pharm Des. 2020;26(15):1729–1741. doi:10.2174/1381612826666200131100630
- Sun J, Ma Q, Li B, et al. RPN2 is targeted by miR-181c and mediates glioma progression and temozolomide sensitivity via the wnt/beta-catenin signaling pathway. Cell Death Dis. 2020;11(10):890. doi:10.1038/s41419-020-03113-5
- Wickstrom M, Dyberg C, Milosevic J, et al. Wnt/beta-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun. 2015;6:8904. doi:10.1038/ncomms9904
- Singh N, Miner A, Hennis L, Mittal S. Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review. Cancer Drug Resist. 2021;4(1):17–43. doi:10.20517/cdr.2020.79
