Abstract
Background
Diabetic nephropathy (DN), as a chronic inflammatory complication of diabetes, is characterized by hyperglycemia, albuminuria and edema, which ultimately becomes the leading cause of end-stage renal disease (ESRD). Astragalus polysaccharide (APS), extracted from the Astragalus membranaceus, was widely used in the treatment of diabetes mellitus. However, the functional roles of APS ameliorate inflammatory responses in DN, which remain poorly understood. Therefore, the purpose of this study was to explore the molecular mechanism of APS on DN in vivo and vitro models.
Methods
We explored the beneficial effects of APS in streptozotocin (STZ)-induced DN rat model and high glucose (HG)-treated glomerular podocyte model. The fasting blood glucose (FBG) and ratio of kidney weight to body weight were measured after 4 weeks of APS treatment. The renal injury parameters containing serum creatinine (Scr), blood urea nitrogen (BUN) and 24 h urinary protein were evaluated. The renal pathological examination was observed by hematoxylin-eosin (HE) staining. The levels of IL-1β, IL-6 and MCP-1 were evaluated by ELISA assay. The proliferation of podocytes was determined using CCK-8 assay and flow cytometry. qRT-PCR and Western blot analysis were performed to determine the amounts of TLR4/NF-κB-related gene expression.
Results
Our results indicated that APS effectively decreased the levels of FBG, BUN, Scr and renal pathological damage when compared with STZ-induced DN model group. Additionally, APS significantly ameliorated renal injury by reducing inflammatory cytokines IL-1β, IL-6, MCP-1 expression and inhibiting the TLR4/NF-κB pathway activity in DN rats. Consistent with the results in vitro, the HG-induced inflammatory response and proliferation of glomerular podocytes were also alleviated through APS administration.
Conclusion
We found that APS ameliorated DN renal injury, and the mechanisms perhaps related to relieving inflammatory responses and attenuating the TLR4/NF-κB signaling pathway.
Introduction
Diabetes nephropathy (DN) is one of the most important microvascular complications of diabetes patients, with hyperglycemia, proteinuria and decreased glomerular filtration rate in early stage.Citation1,Citation2 In the wake of DN constantly aggravated, the pathological are mainly characterized by accumulation of extracellular matrix, decreased glomerular podocyte, thickened glomerular basement membranes and mesangial hypertrophy, which eventually leads to glomerulosclerosis and renal failure.Citation3–5 Accumulating evidence has demonstrated that its primary initiating mechanism is related to glucose and lipid metabolism disorder, and hemodynamic changes.Citation6,Citation7 However, its development progression are caused by a variety of pathological mechanisms, containing cytokines, inflammatory responses, oxidative stress, fibrosis, etc.Citation8,Citation9 Owing to the pathogenesis of DN is complex and has not been adequately elucidated, which potential mechanism of action is still being explored.Citation10,Citation11 In addition, the current clinical treatments of DN are not absolutely effective and additional in-depth research is still needed.
In general, DN has been considered endocrine and metabolic diseases, and an increasing research has emphasized that inflammatory responses play an important role in the progression of this pathology.Citation12,Citation13 Hyperglycemia aggravates inflammation and stimulates the activation and release of various cytokines, chemokines, growth factors and cell adhesion molecules. Recent evidence has indicated that some inflammatory cytokines such as IL-1β, IL-6, MCP-1, TNF-α, and TGF-β1 played a critical role in hyperglycaemia-induced renal injury.Citation14,Citation15 Therefore, inhibiting inflammatory responses and reducing the expression of cytokines will effectively improve renal injury and enhance renal function in DN. Previous researches have demonstrated that TLR4/NF-κB as crucial pathway had played crucial role in the DN by mediating immune inflammatory response.Citation16,Citation17 TLR4 is a membrane transmembrane protein, which could recognize extracellular antigen or pathogens and transmit it into intracellular.Citation18 Afterwards, further activates NF-κB and up-regulates inflammatory cytokines, including IL-6 and IL-1β, subsequently trigger inflammation and leukocyte aggregation.Citation19 Accordingly, we hypothesise that suppressing the TLR4/NF-κB signaling pathway will contribute to help ameliorate DN renal inflammatory responses.
Astragalus polysaccharide (APS), as one of the polysaccharide bioactive components of Astragalus membranaceus, has been demonstrated to be effective in anti-inflammatory, antioxidant and hypoglycemic biological effects.Citation20,Citation21 Some researchers have demonstrated that APS alleviated T2DM rats by activating the sweet taste receptors pathway and promoted glucose transport and lipogenesis.Citation22 In addition, others have revealed APS has protective effect on acute renal injury in sepsis.Citation23 Even if APS has been widely used in endocrine system diseases, its specific molecular mechanism is still not completely clear. Therefore, this study aims to explore the therapeutic mechanisms of APS on DN by improving inflammatory responses and inhibiting TLR4/NF-κB pathway.
Materials and Methods
Animal and Ethics Statement
Male Sprague Dawley rats (220±20 g) were purchased from Experimental Animal Center of Anhui Medical University (Hefei, China). All animals were housed in the standard laboratory with a constant 12 h light/dark cycle and freely access to food and water. The animal experiments were approved by the Committee of Animal Experiment Center of Anhui University of Chinese Medicine, which complies with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
DN Model Preparation and Treatment Protocols
After a week of adaptive feeding, the rats were randomly divided into six groups (n=10): control group, diabetic nephropathy group (DN), low dosage of APS (200 mg/kg) (purity >90%, Solarbio, Beijing, China), medium dosage of APS (400 mg/kg), high dosage of APS (800 mg/kg) and positive control group (metformin, 180 mg/kg). Model and administration group rats were intraperitoneally injected with 65 mg/kg streptozotocin (dissolved in 0.01 mM citrate buffer, pH 4.5) to replicate DN. Meanwhile, control group rats were injected with equal volume of the citrate buffer. The blood glucose readings above 11.7 mmol/L were taken as the success criterion of the DN model. One week later, the drugs were administered by gavage at a dose of 1mL/100g once a day. The control and model groups were given the same amount of solvent.
Collection of Blood and Urine
The blood glucose and body weight of rats were measured once a week of study. At the end of the experiment, rats were individually housed in metabolic cages and collected 24 h urine samples to assess the level of urinary protein. Before sacrifice, blood samples were collected from abdominal aorta of rats under anesthesia conditions (pentobarbital, 30mg/kg, i.p.), centrifuged to separate serum, and stored at -80°C until the time of assay. Furthermore, the kidneys were collected and calculated kidney weight index.
Pathological Analysis
The kidneys (in 4% paraformaldehyde solution) were embedded in paraffin and cut into 6μm section. The sections were respectively stained with hematoxylin and eosin (HE) to observe histopathological abnormalities. Images were observed by the microscope (Olympus Corp, Japan) to examine glomerular, tubular morphology and collagen deposition.
Inflammatory Cytokines Analysis
To assess renal function, the expression of serum creatinine (Scr) and blood urea nitrogen (BUN) were detected using a full-automatic biochemistry analyzer (Hitachi, Tokyo, Japan). The levels of inflammatory cytokines, including interleukin 1β (IL-1β), interleukin 6 (IL-6) and monocyte chemotactic protein 1 (MCP-1) in the serum were detected by using ELISA kits (Invitrogen, USA) following the instructions of the manufacturer.
Cell Culture and Treatment
A murine podocyte cell line, MPC5, was acquired from Cell Bank of Type Culture Collection (Shanghai, China) and cultured in DMEM supplemented with 5.5 mM glucose, 10% fetal bovine serum, 100 U/mL penicillin/streptomycin and 10 U/mL IFNγ at 33 °C in a 5% CO2 humidified incubator. The cells were pretreated with 30 mM high glucose (HG) for 24 h before exposure to varying APS medicated serum with different concentrations for 48 h or 100 nM of the TLR-4 antagonist resatorvid (TAK-242) 4 h prior to APS treatment.
Cell Proliferation Analysis
The podocytes were seeded into a 96-well plate with a density of 1×105 cells/well. After 24 h incubation, the cells were treated with different concentrations of APS medicated serum for 24 hours, 48 hours, or 72 hours. After that, 10 µL of CCK-8 (Servicebio, Wuhan, China) solution was added to each well. After 1 hour incubation, the absorbance was examined at 450 nm by a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Quantitative Real-Time PCR (qRT-PCR)
The total RNA was extracted from renal tissues and podocytes with TRIzol reagent (Invitrogen, USA). cDNA was synthesized from 1 µg total RNA by reverse transcription using PrimeScript RT Master Mix (TaKaRa, Japan). Quantitative PCR analysis was performed with SYBR Premix Ex Taq II kit (TaKaRa, Japan) and specific primers on an Applied Biosystems 7900 Real-Time PCR system (Applied Biosystems, USA). The relative quantification of mRNA levels was calculated based on the 2−ΔΔCt method and normalized to GAPDH. The primer sequences were shown in .
Table 1 The Primer Sequences
Western Blot Analysis
Kidney tissue homogenates or podocytes were collected and lysed using RIPA buffer supplemented with protease and phosphatase inhibitor cocktail for protein extraction. Equal amount of protein lysates (30 μg) were separated by 10% SDS-PAGE and electrotransferred to nitrocellulose membranes (Millipore, USA).Citation24,Citation25 After being blocked with 5% non-fat dry milk in PBS for 1 h, the membranes were probed with the primary antibodies at 4 °C overnight. Afterwards, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. The protein was visualized with enhanced chemiluminescence, and protein intensity was quantified using ImageJ software. All employed antibodies were obtained from Affinity Biologicals, USA.
Statistical Analysis
All data were displayed as the means ± standard deviation and analyzed by SPSS 17.0 software. The differences among multiple groups were performed using one-way ANOVA. P < 0.05 was considered statistically significant.
Results
APS Attenuated the Fasting Blood Glucose and Body Weight
In order to evaluate whether APS alleviate typical symptoms in diabetes mellitus, we monitored FBG level. Relative to the control group, the fasting blood glucose was remarkable increased in the DN group on week 0, which suggested that STZ effectively induced hyperglycemia in the DN model (). The FBG levels were continuously elevated once every week in DN rats. After APS treatment, the FBG levels have displayed dose-dependently decreased, indicating that APS could effectively ameliorate symptoms of DN.
Figure 1 The physiological outcomes of APS in the treatment of DN rats. (A) Fasting blood glucose levels. (B) Body weight. (C) Ratio of kidney weight to body weight. **P<0.01 vs control, ##P<0.01 vs model.
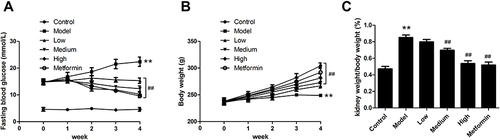
Meanwhile, the body weight of rats was measured once a week. The weights were displayed a continuous increase in control group, while it gradually decreased in the DN group (). In addition, treatment with APS significantly increased the body weight compared with the DN group. Moreover, the ratio of kidney weight to body weight exhibited same significant influence (). Therefore, the results indicated that high dose of APS could effectively ameliorate diabetes symptoms in DN rats.
APS Ameliorates Renal Injury in DN Rats
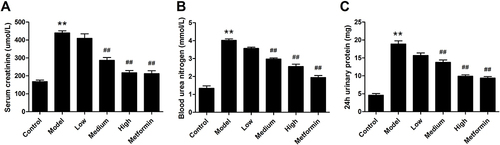
The biochemical indexes associated with kidney function were measured to evaluate the protective effect of APS in DN, including Scr, BUN and 24 h urinary protein. The results revealed that these parameter levels of DN were significantly increased in comparison to control rats (). Administration with APS significantly attenuated these effects compared with the DN rats. Collectively, our results demonstrated that APS treatment could ameliorate renal injury.
Effect of APS on Kidney Pathological Injury
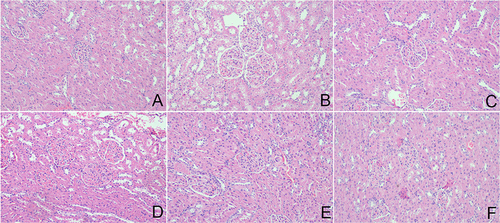
Hematoxylin and eosin (HE) staining was carried out to investigate the glomerular structural alterations in kidney tissue, which could directly reflect the renal histopathology injury. As results showed the sections from the control rats displayed normally sized structures (). Conversely, the DN rats exhibited the diffuse mesangial matrix expansion, thicker glomerular basement membrane and capillary lumen diminution (). However, APS treatment attenuated renal structural injury, which could remarkably ameliorate mesangial cell hyperplasia and thicker glomerular basement membrane (). In addition, metformin has similar therapeutic effects as high-dose APS in improving renal tissue damage ().
APS Decreased HG-Induced Proliferation of Podocytes
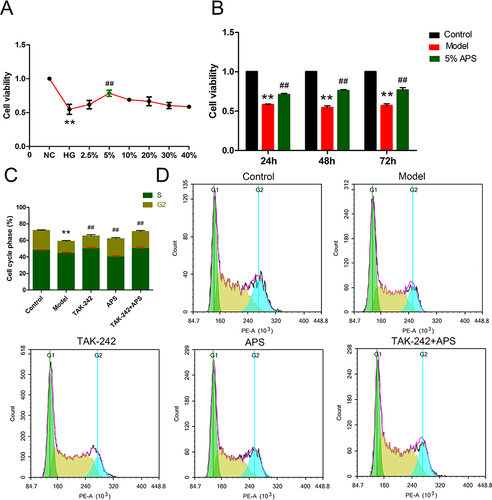
To determine the effect of APS in DN, we established an in vitro model by treating podocytes with different concentrations of glucose medium. When the podocytes were cultured at 30 mM HG for 24 h, the highest OD value was acquired and adopted as model group. TAK-242, a TLR4 inhibitor, was stimulated with 30 mM HG in the presence or absence of 100 nM TAK-242, which was employed to explore the underlying mechanism of APS in DN in podocytes. The podocytes were cultured in100μL 5% APS serum for 48 hours, which has the higher cell viability and used for further experiments ( and ). The results exhibited HG treatment strikingly inhibited podocytes proliferation relative to the control group (). While, after the APS or TAK-242 treatment, the results were conspicuously up-regulated in comparison with the HG-induced model group. It has been demonstrated that APS could notably enhanced HG-induced proliferation of podocytes ().
Figure 4 APS promotes HG-induced podocytes growth and cell cycle progression. (A) APS medicated serum with different concentrations. (B) Cell viability at different culture times. (C and D) Flow cytometric detection of cell cycle distribution and proliferation. **P<0.01 vs control, ##P<0.01 vs model.
APS Attenuates Renal Inflammatory Responses
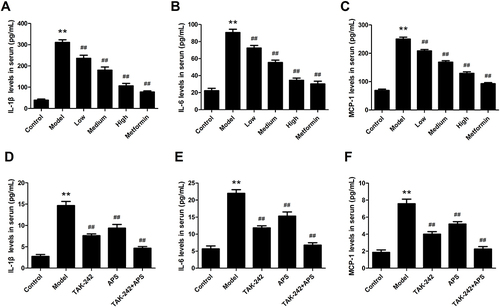
The serum inflammatory factors IL-1β, IL-6 and MCP-1 were determined, which played a vital role in the pathogenesis of DN. The ELISA results showed that compared with control rats, IL-1β (), IL-6 () and MCP-1 () were significantly increased in DN rats. After the APS administration, the levels of inflammatory factors were significantly decreased when compared with DN rats. Consistently with the in vivo results, the inflammatory factor amounts of IL-1β (), IL-6 () and MCP-1 () were remarkably up-regulated in HG-induced podocytes. However, HG-induced inflammatory response was inhibited by APS or TAK-242, especially combination treatment with TAK-242 and APS more effectively suppressed the amounts of these factors. Therefore, these results suggested that APS attenuated the inflammatory response and elevated the renal function.
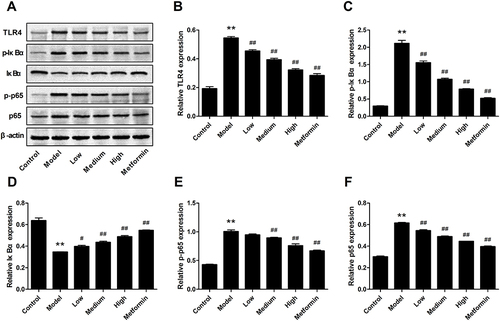
Effect of APS on the mRNA Expression of TLR4, p65 and IκBα
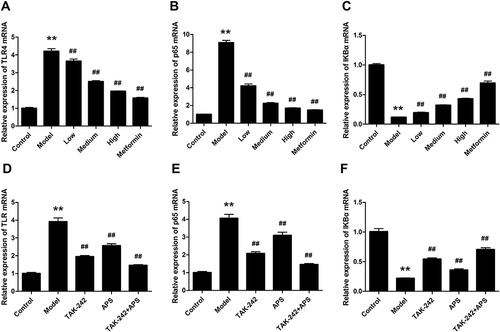
The effect of APS on TLR4, p65 and IκBα mRNA expression in DN rats is shown in . The results revealed that the mRNA expression levels of TLR4 and p65 were significantly increased in the DN rats compared with the control rats ( and ). The expression of IκBα was decreased in DN rats, which could be markedly reversed by APS administration (). Similarly, the expression of TLR4 and p65 in HG-stimulated podocytes was significantly increased, whereas treatment with APS markedly decreased the levels of these effects ( and ). Interestingly, the level of IκBα was significantly enhanced by APS treatment compared to the HG-stimulated podocytes ().
Effect of APS on the TLR4/NF-κB Signaling Pathway
To elucidate the mechanism underlying the effects of APS on hyperglycemia-induced inflammation in DN rats, the TLR4/NF-κB pathway was determined by Western blot (). The levels of TLR4, p-IκBα, p-p65 and p65 were strikingly improved in DN rats compared with control rats, whereas APS dramatically reversed these effects ( and ). In addition, IκBα was significantly decreased in STZ-induced DN rats, while the effects were significantly inverted by APS treatment (). These results suggested that the activation of TLR4/NF-κB signaling pathway in DN rats was markedly suppressed after APS treatment.
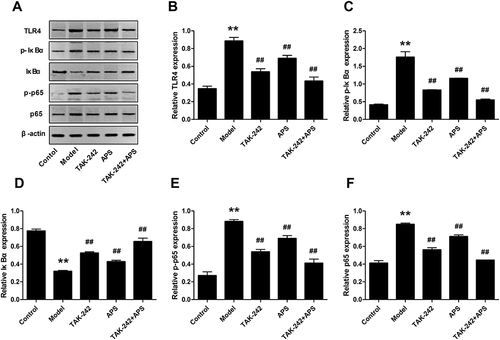
APS Suppressed Inflammatory Response in Podocytes by Inhibiting the TLR4/NF-κB Pathway
Podocytes were stimulated with 30 mM HG in the presence or absence of 100 nM TAK-242, followed by treated with 100 μM 5% APS for 48 h. The Western blot results manifested that TAK-242 administration prominently repressed up-regulation of TLR4, p-IκBα, p65 and p-p65 in HG-induced podocytes ( and ). However, both APS and TAK-242 combination treatment markedly decreased above trends in HG-induced podocytes. Additionally, IκBα was obviously reduced, which has evidently ascended after the intervention of APS or TAK-242 (). Taken together, these findings demonstrated that APS treatment significantly suppressed the inflammatory response in HG-induced podocytes by blocking TLR4/NF-κB pathway.
Discussion
DN is one of the most severe microvascular complications of diabetes mellitus, glucose metabolic disturbance and abnormal hemodynamics, which plays a crucial role in the progression and development. In addition, accumulating evidence supposed that oxidative stress and inflammatory stimulation also promoted the aggravation of symptoms. However, the underlying pathogenesis of DN remains largely unclear. Therefore, it is meaningful to further investigate the intrarenal immunity mechanisms of DN, and seeking drugs available to reduce inflammation and delay the development of DN.Citation26,Citation27
APS, naturally extracted from the plant Astragalus membranaceus, is the most effective bioactive ingredients with a wide range of pharmacological effects. Numerous studies had reported APS played a vital role in inflammatory responses under a variety of pathological conditions.Citation28 Prior experimental pieces of evidence demonstrated APS alleviated LPS-induced inflammation via the NF-κB/MAPK signaling pathway, and decreased the expression of IL-Iβ, IL-6 and TNF-α.Citation21 Meanwhile, APS could protected renal function and affected the TGF-β/Smad pathway in STZ-induced diabetic rats.Citation29 Zhou et al had pointed out that APS performed immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo.Citation30 Additionally, APS treatment significantly decreased blood glucose and blood lipid levels, as well as ameliorated insulin resistance in T2DM mice.Citation31
In our research, the rats appeared to have hyperglycemia and lose weight after STZ injection in 2 weeks, which had expressed the common characteristics of DN. Proteinuria as a key index in the development of DN, it directly reflects renal function through glomerular filtration rate and renal tubular reabsorption.Citation32 Our study unveiled that APS not only effectively attenuated FBG levels but also improved Scr, BUN and 24 h urinary protein. Moreover, the introduction of APS dramatically alleviated the expression of inflammatory cytokines, namely, IL-1β, IL-6 and MCP-1, which was involved in DN development and progression. Our data disclosed APS has an effect on enhancing the anti-inflammatory capacity of the kidneys and alleviating diabetic renal injury.
More recently, there are increasing evidences supporting that inflammation performed critical roles in the onset and advancement of DN.Citation33 Accumulating studies have shown that TLR4/NF-κB as one of the most important proinflammatory pathway, which has been confirmed to be involved in alleviating renal inflammation of DN.Citation15 TLR4, as a family of Toll-like receptors (TLRs), play a vital role in the innate immune system, which could recognized extracellular antigen and activated downstream inflammatory signalling pathways.Citation34 Especially in the development of DN, high glucose will promote the expression and activation of TLR4 strikingly. Previous research indicated that increased the TLR4 expression could promote the pathogenesis of DN by enhancing the inflammatory cell proliferation.Citation35 In addition, Lin et al showed the high expression of TLR4 and other inflammatory mediators in DN rats.Citation36 The kidney inflammatory response and fibrosis will be effectively attenuated via knocking out TLR4.Citation37 Similarly, in our results, the expression of TLR4 in the DN group was strikingly higher than that in the control group. After treatment with APS, the condition was notably improved and these results were consistent with previous findings.
In podocytes, HG promoted the activation of TLR4, subsequently stimulates the NF-κB pathway to the production of inflammatory cytokines, including TGF-β1, TNF-α, MCP-1 and IL-1β, which will exacerbate the inflammatory response and fibrosis. Meanwhile, the over-expressed inflammatory factors will further stimulate the activation of NF-κB.Citation38 Specifically, NF-κB as a downstream effector of TLR4, is a crucial nuclear transcription factor that mediates a variety of inflammatory processes and facilitates the expression of cellular inflammation genes.Citation39 Furthermore, APS ameliorated collagen deposition and attenuated pulmonary fibrosis by inhibiting NF-κB pathway.Citation40 IκBα, as an inhibitor protein of NF-κB, was combined as a form of an inactive complex in the cytoplasm.Citation41 Along with the external stimuli of response, IκBα was degraded after phosphorylation and ubiquitylation. Afterwards, NF-κB transfers from the cytoplasm to the nucleus, combines with the inflammation genes and mediates inflammatory responses.
It was reported that IκBα had the low expression, p-IκBα and NF-κB/p65 performed higher expression in glomeruli and proximal tubules with DN condition.Citation42 Consistently, we found that the expression of TLR4, p-IκBα and p65 were increased in both STZ-induced DN rats and HG-treated podocytes, which was consistent with previous researches. The treatment of HG-induced podocytes with TAK-242, a TLR4 inhibitor, decreased the levels of inflammatory cytokines, including IL-1β, IL-6 and MCP-1. It was demonstrated that TLR4/NF-κB played critical roles in regulating podocytes injury. Furthermore, APS administration has significantly reduced the expression of TLR4, p-IκBα and p65. The results suggested that APS ameliorated the progression of DN by restraining the TLR4/NF-κB pathway in vivo and in vitro.
In conclusion, our research provides strong evidence that APS effectively ameliorates renal injury in DN rats by decreasing the production of inflammatory cytokines. We further uncovered the underlying mechanism of APS in HG-induced podocytes via suppressed TLR4/NF-κB pathway in glomeruli and proximal tubules. These results suggested that APS might be a potential therapeutic agent for the therapy of DN.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
Additional information
Funding
References
- Sever B, Altıntop M, Demir Y, et al. A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity. Open Chem. 2021;19(1):347–357. doi:10.1515/chem-2021-0032
- Sever B, Altıntop MD, Demir Y, et al. Design, synthesis, in vitro and in silico investigation of aldose reductase inhibitory effects of new thiazole-based compounds. Bioorg Chem. 2020;102:104110. doi:10.1016/j.bioorg.2020.104110
- DeFronzo RA, Bakris GL. Modifying chronic kidney disease progression with the mineralocorticoid receptor antagonist finerenone in patients with type 2 diabetes. Diabetes Obes Metab. 2022;24(7):1197–1205. doi:10.1111/dom.14696
- Wang J, Liu F, Kong R, Han X. Association between globulin and diabetic nephropathy in type 2 diabetes mellitus patients: a cross-sectional study. Front Endocrinol. 2022;13:890273. doi:10.3389/fendo.2022.890273
- Han H, Chen Y, Yang H, et al. Identification and verification of diagnostic biomarkers for glomerular injury in diabetic nephropathy based on machine learning algorithms. Front Endocrinol. 2022;13:876960. doi:10.3389/fendo.2022.876960
- Pereira PR, Carrageta DF, Oliveira PF, Rodrigues A, Alves MG, Monteiro MP. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med Res Rev. 2022;42(4):1518–1544. doi:10.1002/med.21883
- Forst T, Mathieu C, Giorgino F, et al. New strategies to improve clinical outcomes for diabetic kidney disease. BMC Med. 2022;20(1):337. doi:10.1186/s12916-022-02539-2
- Tokalı FS, Demir Y, Demircioğlu İH, et al. Synthesis, biological evaluation, and in silico study of novel library sulfonates containing quinazolin-4(3H)-one derivatives as potential aldose reductase inhibitors. Drug Dev Res. 2022;83(3):586–604. doi:10.1002/ddr.21887
- Sever B, Altıntop MD, Demir Y, et al. An extensive research on aldose reductase inhibitory effects of new 4H-1,2,4-triazole derivatives. J Mol Struct. 2021;1224:129446. doi:10.1016/j.molstruc.2020.129446
- Demir Y, Ceylan H, Türkeş C, et al. Molecular docking and inhibition studies of vulpinic, carnosic and usnic acids on polyol pathway enzymes. J Biomol Struct Dyn. 2022;40(22):12008–12021. doi:10.1080/07391102.2021.1967195
- Sever B, Altıntop MD, Demir Y, et al. Identification of a new class of potent aldose reductase inhibitors: design, microwave-assisted synthesis, in vitro and in silico evaluation of 2-pyrazolines. Chem Biol Interact. 2021;345:109576. doi:10.1016/j.cbi.2021.109576
- Demir Y, Köksal Z. Some sulfonamides as aldose reductase inhibitors: therapeutic approach in diabetes. Arch Physiol Biochem. 2022;128(4):979–984. doi:10.1080/13813455.2020.1742166
- Akda M, Özçelik AB, Demir Y, Beydemir Ş. Design, synthesis, and aldose reductase inhibitory effect of some novel carboxylic acid derivatives bearing 2-substituted-6-aryloxo-pyridazinone moiety. J Mol Struct. 2022;1258:132675. doi:10.1016/j.molstruc.2022.132675
- Li J, Zhang J, Yang M, et al. Kirenol alleviates diabetic nephropathy via regulating TGF-β/Smads and the NF-κB signal pathway. Pharm Biol. 2022;60(1):1690–1700. doi:10.1080/13880209.2022.2112239
- Wang M, Liu X, Wang Z, Xu Q. The extract of Polygala fallax Hemsl. slows the progression of diabetic nephropathy by targeting TLR4 anti-inflammation and MMP-2/9-mediated anti-fibrosis in vitro. Phytomedicine. 2022;104:154251. doi:10.1016/j.phymed.2022.154251
- Jiang T, Shen S, Wang L, Zhao M, Li Y, Huang S. Grifola frondosa polysaccharide ameliorates early diabetic nephropathy by suppressing the TLR4/NF-κB pathway. Appl Biochem Biotechnol. 2022;194(9):4093–4104. doi:10.1007/s12010-022-03976-8
- Qi MY, He YH, Cheng Y, et al. Icariin ameliorates streptozocin-induced diabetic nephropathy through suppressing the TLR4/NF-κB signal pathway. Food Funct. 2021;12(3):1241–1251. doi:10.1039/d0fo02335c
- Aghamiri SH, Komlakh K, Ghaffari M. The crosstalk among TLR2, TLR4 and pathogenic pathways; a treasure trove for treatment of diabetic neuropathy. Inflammopharmacology. 2022;30(1):51–60. doi:10.1007/s10787-021-00919-3
- Bian Z, Wang X, Zhu R, Chen S. miR-21-5p in extracellular vesicles obtained from adipose tissue-derived stromal cells facilitates tubular epithelial cell repair in acute kidney injury. Cytotherapy. 2023;25(3):310–322. doi:10.1016/j.jcyt.2022.08.002
- Li CX, Liu Y, Zhang YZ, Li JC, Lai J. Astragalus polysaccharide: a review of its immunomodulatory effect. Arch Pharm Res. 2022;45(6):367–389. doi:10.1007/s12272-022-01393-3
- Dong N, Li X, Xue C, et al. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J Cell Physiol. 2020;235(7–8):5525–5540. doi:10.1002/jcp.29452
- Luo MJ, Wang Y, Chen SY, Yang ZM. Astragalus polysaccharides alleviate type 2 diabetic rats by reversing the expressions of sweet taste receptors and genes related to glycolipid metabolism in liver. Front Pharmacol. 2022;13:916603. doi:10.3389/fphar.2022.916603
- Sun J, Wei S, Zhang Y, Li J. Protective effects of astragalus polysaccharide on sepsis-induced acute kidney injury. Anal Cell Pathol. 2021;2021:7178253. doi:10.1155/2021/7178253
- Özaslan MS, Demir Y, Aksoy M, et al. Inhibition effects of pesticides on glutathione- S -transferase enzyme activity of Van Lake fish liver. J Biochem Mol Toxicol. 2018;32(9):e22196. doi:10.1002/jbt.22196
- Alım Z, Kılıç D, Demir Y. Some indazoles reduced the activity of human serum paraoxonase 1, an antioxidant enzyme: in vitro inhibition and molecular modeling studies. Arch Physiol Biochem. 2019;125(5):387–395. doi:10.1080/13813455.2018.1470646
- Türkeş C, Arslan M, Demir Y, et al. N -substituted phthalazine sulfonamide derivatives as non-classical aldose reductase inhibitors. J Mol Recognit. 2022;35(12):e2991. doi:10.1002/jmr.2991
- Anil DA, Aydin BO, Demir Y, et al. Design, synthesis, biological evaluation and molecular docking studies of novel 1H-1,2,3-Triazole derivatives as potent inhibitors of carbonic anhydrase, acetylcholinesterase and aldose reductase. J Mol Struct. 2022;1257:132613. doi:10.1016/j.molstruc.2022.132613
- He X, Liu L, Luo X, et al. Astragalus polysaccharide relieves inflammatory responses in guinea pigs with allergic rhinitis via ameliorating NF-kB-mediated Treg/Th17 imbalance. Am J Rhinol Allergy. 2022;36(5):638–648. doi:10.1177/19458924221098847
- Meng X, Wei M, Wang D, et al. Astragalus polysaccharides protect renal function and affect the TGF-β/Smad signaling pathway in streptozotocin-induced diabetic rats. J Int Med Res. 2020;48(5):300060520903612. doi:10.1177/0300060520903612
- Zhou L, Liu Z, Wang Z, et al. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep. 2017;7(1):44822. doi:10.1038/srep44822
- Chen X, Chen C, Fu X. Hypoglycemic activity in vitro and vivo of a water-soluble polysaccharide from Astragalus membranaceus. Food Funct. 2022;13(21):11210–11222. doi:10.1039/d2fo02298b
- Zheng W, Guo J, Lu X, et al. cAMP-response element binding protein mediates podocyte injury in diabetic nephropathy by targeting lncRNA DLX6-AS1. Metabolism. 2022;129:155155. doi:10.1016/j.metabol.2022.155155
- Cheng Q, Pan J, Zhou ZL, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin. 2021;42(6):954–963. doi:10.1038/s41401-020-00525-z
- Feng Q, Liu D, Lu Y, Liu Z. The interplay of renin-angiotensin system and toll-like receptor 4 in the inflammation of diabetic nephropathy. J Immunol Res. 2020;2020:6193407. doi:10.1155/2020/6193407
- Zhu LL, Wang HY, Tang T. Effects of miR-195 on diabetic nephropathy rats through targeting TLR4 and blocking NF-κB pathway. Eur Rev Med Pharmacol Sci. 2021;25(3):1522–1529. doi:10.26355/eurrev_202102_24860
- Lin L, Lin H, Wang D, Bao Z, Cai H, Zhang X. Bone marrow mesenchymal stem cells ameliorated kidney fibrosis by attenuating TLR4/NF-κB in diabetic rats. Life Sci. 2020;262:118385. doi:10.1016/j.lfs.2020.118385
- Yang J, Dong H, Wang Y, et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int J Biol Macromol. 2020;163:442–456. doi:10.1016/j.ijbiomac.2020.06.153
- Han X, Zhang J, Zhou L, et al. Sclareol ameliorates hyperglycemia-induced renal injury through inhibiting the MAPK/NF-κB signaling pathway. Phytother Res. 2022;36(6):2511–2523. doi:10.1002/ptr.7465
- Liu Y, Hu Z, Xing H, et al. Renoprotective effects of oleanolic acid and its possible mechanisms in rats with diabetic kidney disease. Biochem Biophys Res Commun. 2022;636(Pt1):1–9. doi:10.1016/j.bbrc.2022.10.074
- Zhang R, Xu L, An X, Sui X, Lin S. Astragalus polysaccharides attenuate pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition and NF-κB pathway activation. Int J Mol Med. 2020;46(1):331–339. doi:10.3892/ijmm.2020.4574
- Zhu MM, Wang L, Yang D, et al. Wedelolactone alleviates doxorubicin-induced inflammation and oxidative stress damage of podocytes by IκK/IκB/NF-κB pathway. Biomed Pharmacother. 2019;117:109088. doi:10.1016/j.biopha.2019.109088
- Sierra-Mondragon E, Molina-Jijon E, Namorado-Tonix C, Rodríguez-Muñoz R, Pedraza-Chaverri J, Reyes JL. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-κB during initiation of diabetic nephropathy. J Nutr Biochem. 2018;60:47–60. doi:10.1016/j.jnutbio.2018.06.002