Abstract
Purpose
Sufentanil has been widely used to inhibit the hemodynamic responses caused by tracheal intubation. Using intravenous lidocaine may reduce the dose of sufentanil and better maintain the hemodynamics. This study aimed to determine the effects of intravenous lidocaine on the median effective concentration (EC50) of sufentanil for endotracheal intubation in obese patients.
Patients and Methods
This is a randomized, double-blind, up-and-down sequential allocation study. Fifty obese patients undergoing bariatric surgery were randomly allocated in a 1:1 ratio into the lidocaine group and the saline group. Anesthesia was induced using a target-controlled infusion of propofol and sufentanil. The effect-site concentration (Ce) of propofol was 3.5 μg/mL. The Ce of sufentanil for the first patient was 0.4 ng/mL, and the sufentanil dose for the next patient was determined according to the responses of the previous patient, using Dixon’s up-and-down sequential method with an interval of 0.05 ng/mL. When the target concentration of propofol and sufentanil was reached, lidocaine 1.5 mg/kg or the same volume of normal saline was infused over 3 min. Tracheal intubation was performed 3 min after the end of the lidocaine or normal saline infusion. Probit regression was used to calculate the EC50 and 95% confidence interval (CI) of sufentanil.
Results
Thirty-eight patients completed this study. The EC50 of sufentanil was 0.36 ng/mL (95% CI: 0.31–0.41 ng/mL) in the lidocaine group, which was significantly lower than 0.50 ng/mL (95% CI: 0.43–0.62 ng/mL) in the saline group. In addition, compared with saline group, the dosage of sufentanil in lidocaine group decreased significantly during the test. The hemodynamics of the two groups were stable during the study period.
Conclusion
Intravenous lidocaine 1.5 mg/kg decreased the EC50 of sufentanil required for tracheal intubation in obese patients undergoing bariatric surgery.
Introduction
The prevalence of obesity has been increasing over the past decades.Citation1 Obesity is associated with many comorbidities, such as hypertension, diabetes, and cardiovascular diseases.Citation2 Treatment for obesity includes lifestyle interventions, pharmacotherapy, and bariatric surgery.Citation3 Compared with non-surgical interventions, bariatric surgery is a more effective therapeutic strategy for weight loss and weight-associated comorbidities.Citation4 Tracheal intubation is routinely performed for patients undergoing bariatric surgery and may cause adverse cardiovascular responses.Citation5 In obese patients, the increased sympathetic activity and catecholamine levels contribute to potential cardiovascular risk during tracheal intubation.Citation6,Citation7 Therefore, it is important to maintain hemodynamic stability during tracheal intubation in obese patients.
Many medications, such as dexmedetomidine,Citation8 lidocaine,Citation9 fentanyl,Citation10 esmolol,Citation11 propofol,Citation12 and volatile anesthetic agents,Citation13 have been used to inhibit hemodynamic responses induced by tracheal intubation. Currently, sufentanil is commonly used to attenuate cardiovascular reactions during tracheal intubation.Citation14 However, using sufentanil during anesthesia induction is associated with a reduction in blood pressure and heart rate.Citation15 Previous clinical studies suggested that intravenous injection of 1.5 mg/kg lidocaine before tracheal intubation attenuated the hemodynamic responses without cardiovascular inhibition.Citation9,Citation16 The combination of sufentanil and lidocaine may achieve stable hemodynamics during anesthesia induction and tracheal intubation. However, the effect of lidocaine on the median effective concentration (EC50) of sufentanil for tracheal intubation in obese patients is still unknown. Therefore, we designed this study to determine the EC50 of sufentanil when combined with lidocaine in obese patients for tracheal intubation.
Materials and Methods
Ethical Approval
This is a prospective, randomized, double-blind, and up-and-down sequential allocation trial. The study protocol was approved by the Institutional Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (Approval No. 2022ER385-1) on 14 October 2022 and registered on the Chinese Clinical Trials Registry (available at http://www.chictr.org.cn, identifier: ChiCTR-2200064981) on 25 October 2022. All participants provided their written informed consent.
Participants
From October 25, 2022, to February 28, 2023, fifty patients who underwent laparoscopic bariatric surgery under general anesthesia were enrolled in this study. The inclusion criteria were 18–65 years old, body mass index (BMI) ≥ 30 kg/m2, and American Society of Anesthesiologists (ASA) physical status of II–III. The exclusion criteria were as follows: serious cardiopulmonary disease; abnormal liver and kidney function; body weight >150 kg;Citation17 anticipated difficult airway; known allergy to general anesthetics or lidocaine; or recent use of drugs that affect the sympathetic adrenergic system or hemodynamics (such as atropine and enalapril).
Randomization and Blinding
All eligible patients were randomly allocated, in a 1:1 ratio, to the lidocaine group (n=25) and the normal saline group (n=25) using a computer-generated random number sequence. The allocation details were concealed using opaque sealed envelopes. A research nurse prepared the study solutions (lidocaine and normal saline) in identical 20 mL syringes. Lidocaine was diluted with 0.9% normal saline to a final volume of 20 mL. Patients in the normal saline group received the same volume of saline. All patients, surgeons, and other investigators were masked to the group allocation.
Anesthesia
All patients fasted before the operation. After entering the operating room, patients were monitored with an electrocardiogram, pulse oximetry, non-invasive mean arterial blood pressure (MAP), heart rate (HR) and cerebral state index (CSI). A peripheral venous of the left arm was cannulated, and 5 mL/kg lactated ringer solution was infused before induction. Patients received preoxygenation with 100% oxygen via a facemask for 3 min. General anesthesia was induced with propofol target-controlled infusion (TCI, propofol based on adjusted body weight [ABW], Marsh pharmacokinetic model)Citation18 with effect-site concentration (Ce) of 3.5 μg/mLCitation19 and sufentanil TCI (Gepts pharmacokinetic model)Citation20. The Ce of sufentanil for the first patient in either the lidocaine group or the normal saline group was set at 0.4 ng/mL, based on our preliminary observation and a study by Bidgoli et al.Citation21 Following loss of consciousness, ulnar nerve stimulation was initiated to monitor the train-of-four (TOF) response.
When the targets concentration of propofol and sufentanil was reached, lidocaine 1.5 mg/kg (ABW) or the same volume of normal saline was infused over 3 min. Tracheal intubation was performed 3 min after the end of lidocaine or normal saline infusion. Rocuronium 0.9 mg/kg (ideal body weight [IBW]) was administered to facilitate tracheal intubation. ABW and IBW were calculated as follows: .Citation22 When CSI values was within 40–60 and TOF count was 0, an experienced anesthesiologist performed the tracheal intubation using a video laryngoscope within 30 seconds. Mechanical ventilation was performed after intubation with 100% oxygen (tidal volume of 8 mL/kg, frequency of 10–14 breaths/min). The end-tidal CO2 was monitored. During induction of anesthesia and tracheal intubation, the values of MAP and HR were recorded at 1-min intervals until 3 min after tracheal intubation. Bradycardia (HR < 50 beats/min) and hypotension (MAP < 50 mmHg) were treated with atropine and ephedrine. If these hemodynamic events occurred, the patient was excluded from the study. After the trial, the chief anesthesiologist administered the anesthesia according to his schedule until the end of the operation.
Determination of EC50
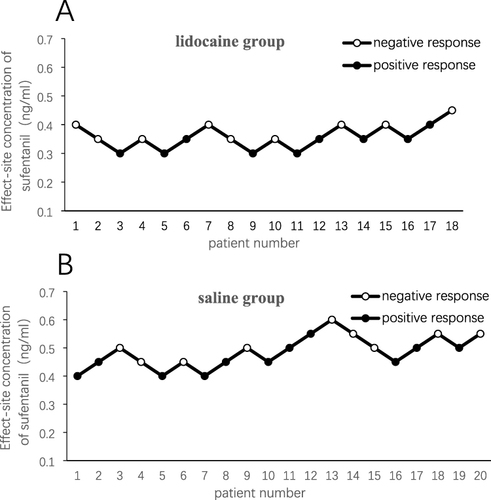
The EC50 of sufentanil was determined using Dixon’s up-and-down sequential allocation method.Citation23 The Ce of sufentanil for the first patient was 0.4 ng/mL. If there was a positive response (an increase of HR or MAP ≥ 20% of the baseline values to tracheal intubation within 3 min), the Ce of sufentanil for the subsequent patient was increased by 0.05 ng/mL. If there was a negative response (HR and MAP values recorded 3 min after intubation did not exceed 20% of the baseline values), the Ce of sufentanil for the subsequent patient was decreased by 0.05 ng/mL. When six “positive versus negative response or negative versus positive response” crossover points were obtained, we considered that a sufficient number of patients was reached for this study.
Statistical Analysis
The non-independence and unknown distribution of data of the sequential methodology study prevent the formulation of theoretical strict rules for calculating sample size.Citation24 Simulation study shows that including at least 20–40 patients will provide a stable estimate of the target dose for most scenarios.Citation25 In our study, we included 25 patients in each group, which can make a stable estimation, and after obtaining six crossovers of a positive or negative response, the measurements were terminated. The normality of data was evaluated using the Shapiro–Wilk test. Continuous data are expressed as mean ± SD or medians (25th to 75th percentiles) where appropriate, and discrete data are presented as numbers and percentages. Continuous data were analyzed by Student’s t-test or rank tests, and categorical data were analyzed by Fisher’s exact test. Changes in HR and MAP over time between the saline and lidocaine groups were analyzed with a two-way repeated measures ANOVA, with the group as the between-subjects factor and time as the within-subjects factor. Probit probability unit regression analysis was used to calculate EC50 and the 95% confidence interval (CI) of sufentanil. The comparison of EC50 values between the two groups was performed using the Mann–Whitney U-test. A two-tailed P value <0.05 was considered statistically significant. Statistical analyses were conducted using the SPSS software (version 26.0).
Results
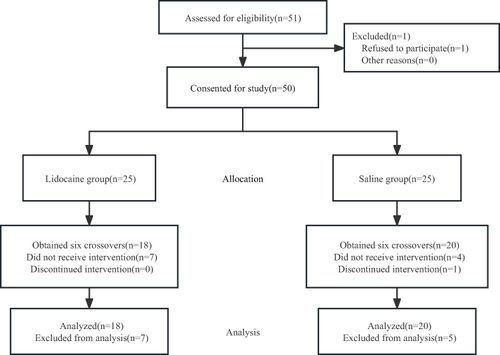
A total of 51 patients were initially approached. Of these, one patient refused to participate, and 50 patients were recruited in this trial, with 25 patients randomized to each group. Finally, 38 patients completed the study, with 18 in the lidocaine group and 20 in the saline group (). In the saline group, one patient was excluded due to MAP < 50 mmHg during the induction of anesthesia. In addition, seven patients in the lidocaine group and four in the saline group were withdrawn from the study after six crossover points were obtained.
There were no significant differences in patient characteristics between the two groups (). The dosages of propofol and rocuronium were comparable between groups. Compared with the saline group, the dose of sufentanil in the lidocaine group was significantly reduced [20.0 (17.8–23.0) μg vs 25.0 (25.0–28.8) μg, P < 0.001] (). The two groups had similar baseline values of MAP and HR. MAP and HR values were not significantly different between the two groups at different time points (). and show the process of determining the EC50 of sufentanil in the two groups. The use of lidocaine significantly reduced the EC50 of sufentanil (0.36 ng/mL; 95% CI: 0.31–0.41 ng/mL) compared to normal saline (0.50 ng/mL; 95% CI: 0.43–0.62 ng/mL), with a 28% of reduction in EC50 by lidocaine ().
Table 1 Patient Characteristics and Medications During Anesthesia Induction
Table 2 HR and MAP Values at Different Time Points for the Two Groups
Table 3 EC50 of Sufentanil and Its 95% CI for the Two Groups
Figure 2 Individual response to the allocated sufentanil effect-site concentration in the lidocaine group (A) and the saline group (B). Positive (closed circle) or negative (open circle) hemodynamic responses to tracheal intubation were assessed using an up-and-down sequential allocation method from consecutive patients with a predetermined concentration of sufentanil. To get six crossovers, 18 and 20 patients were included in the lidocaine and saline groups, respectively.
Discussion
In this study, we found that the adjunct use of lidocaine reduced the EC50 of sufentanil for tracheal intubation in obese patients undergoing bariatric surgery. The EC50 of sufentanil to inhibit responses to tracheal intubation in the normal saline group was 0.50 ng/mL, whereas the intravenous injection of 1.5 mg/kg lidocaine reduced the EC50 of sufentanil to 0.36 ng/mL.
Al-Metwalli et alCitation26 reported that the EC50 of sufentanil required for laryngeal mask insertion during a propofol TCI at Ce of 4.0 μg/mL was 0.16 ng/mL, which was lower than that in our study. That is possibly because the Ce of propofol is higher than ours (3.5 μg/mL), and the hemodynamic response to laryngeal mask insertion is lower than that to tracheal intubation. The main finding of our study is that the dose of sufentanil in the lidocaine group was significantly decreased. Similar to our results, Xu et alCitation27 showed that the administration of lidocaine with the aerosol inhalation approach also reduced the sufentanil dosage for tracheal intubation. Regarding the safety of lidocaine in our patients, we did not observe any serious complications in the two groups.
TCI systems utilize the models of pharmacokinetics and pharmacodynamics to provide the optimal infusion and target concentration of anesthetic drugs. Studies have shown that TCI of propofol and sufentanil could be successfully used for obese patients.Citation28,Citation29 Currently, open TCI systems allow the clinicians to achieve both the plasma and the effect-site concentrations, and the propofol effect-site concentration correlates better with patient response than plasma concentration.Citation30 Echevarría et alCitation31 and Bidgoli et alCitation21 targeted the desired effect-site concentration during TCI of propofol or sufentanil in their patients. In line with the previous studies, we used the effect-site concentration models of propofol and sufentanil to minimize equilibration time between the plasma and effect-site concentrations and to maintain a stable drug concentration.
Lidocaine is an amide-type local anesthetic. Studies have suggested that intravenous lidocaine administration could decrease the catecholamines induced by tracheal intubation.Citation9,Citation32 The potential mechanisms of lidocaine to reduce stress response and maintain hemodynamic stability during tracheal intubation include the inhibition of mediator release, interruption of reflex arcs, suppression of burst discharge activity from neurons within the central nervous system, and the effect on synaptic transmissions.Citation33,Citation34 Moreover, intravenous injection of lidocaine before tracheal intubation maximized this advantage.Citation35 Based on these, 1.5 mg/kg lidocaine was intravenously infused at 3 minutes before endotracheal intubation in our study. The body composition of obese patients is different from that of normal-weight patients, and ABW reflects the modified pharmacokinetic profile of lidocaine in this population.Citation36 Previous report suggested that the serum lidocaine concentration did not exceed the toxic concentration (5 µg/mL) when an i.v bolus of 1.5 mg/kg lidocaine followed by a continuous infusion of 2 mg/kg/h was administered during bariatric surgery.Citation36 Therefore, a single intravenous injection of 1.5 mg/kg lidocaine in obese patients is safe. In addition, we administered rocuronium at the IBW-adjusted dose and propofol at the ABW-adjusted dose.Citation37,Citation38 We applied the Gepts pharmacokinetics model for TCI of sufentanil, and this model does not include body weight as a significant covariate. It has been shown that the Gepts pharmacokinetic parameter could be used for accurate prediction of plasma sufentanil concentration in obese patients.Citation29 Taken together, the anesthesia scheme we used in the obese patients undergoing bariatric surgery is safe and feasible.
In this study, we employed intravenous target-controlled infusion of propofol to achieve a stable effect-site concentration, thus maintaining the anesthesia depth within a CSI range of 40–60.Citation39,Citation40 Additionally, rocuronium, dosed at 0.9 mg/kg and adjusted for ideal body weight, was used to ensure sufficient muscle relaxation for intubation.Citation37,Citation41 The intubation procedure was carried out when the TOF counts reached zero, an approach has been reported to significantly attenuate hemodynamic responses.Citation42 All these above mentioned strategies were combined to achieve the optimal conditions for intubation, ensuring that the MAP and HR remain unaffected by inadequate anesthesia depth or muscle relaxation during the intubation procedure.
This study has several limitations. First, we did not compare the EC50 of sufentanil between normal-weight and obese patients, with or without intravenous lidocaine, to inhibit the responses to intubation. Second, we did not measure the plasma levels of catecholamines during tracheal intubation. Last, this trial included more female patients undergoing bariatric surgery, so our results should be tested on male patients in future studies.
Conclusion
In conclusion, intravenous lidocaine injection at a dose of 1.5 mg/kg significantly reduced the EC50 of sufentanil for tracheal intubation in obese patients undergoing bariatric surgery.
Data Sharing Statement
The data supporting the study findings are available from the corresponding author upon request.
Ethics Approval and Informed Consent
The study was approved by the Institutional Ethics Committee of the Affiliated Hospital of North Sichuan Medical College, China (No. 2022ER385-1) and was registered on the Chinese Clinical Trials. gov (No. ChiCTR-2200064981). All participants provided their written informed consent. We confirm our study complies with the Declaration of Helsinki.
Disclosure
All authors declare that they have no conflicts of interest in this work.
Additional information
Funding
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi:10.1016/S0140-6736(17)32129-3
- Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. doi:10.1159/000442721
- Kahan S. Overweight and obesity management strategies. Am J Manag Care. 2016;22(Suppl 7):s186–s196.
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;2014(8):CD003641. doi:10.1002/14651858
- Edwards ND, Alford AM, Dobson PM, Peacock JE, Reilly CS. Myocardial ischaemia during tracheal intubation and extubation. Br J Anaesth. 1994;73(4):537–539. doi:10.1093/bja/73.4.537
- Grassi G, Biffi A, Seravalle G, et al. Sympathetic neural overdrive in the obese and overweight state. Hypertension. 2019;74(2):349–358. doi:10.1161/HYPERTENSIONAHA.119.12885
- Grassi G, Arenare F, Quarti-Trevano F, Seravalle G, Mancia G. Heart rate, sympathetic cardiovascular influences, and the metabolic syndrome. Prog Cardiovasc Dis. 2009;52(1):31–37. doi:10.1016/j.pcad.2009.05.007
- Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7(1):43–52. doi:10.2165/00126839-200607010-00004
- Wilson IG, Meiklejohn BH, Smith G. Intravenous lignocaine and sympathoadrenal responses to laryngoscopy and intubation. The effect of varying time of injection. Anaesthesia. 1991;46(3):177–180. doi:10.1111/j.1365-2044.1991.tb09403.x
- Adachi YU, Satomoto M, Higuchi H, Watanabe K. Fentanyl attenuates the hemodynamic response to endotracheal intubation more than the response to laryngoscopy. Anesth Analg. 2002;95(1):233–237. doi:10.1097/00000539-200207000-00043
- Ugur B, Ogurlu M, Gezer E, Nuri Aydin O, Gürsoy F. Effects of esmolol, lidocaine and fentanyl on haemodynamic responses to endotracheal intubation: a comparative study. Clin Drug Investig. 2007;27(4):269–277. doi:10.2165/00044011-200727040-00006
- Gore MS, Harnagale KD. Evaluation of intubating conditions with varying doses of propofol without muscle relaxants. J Anaesthesiol Clin Pharmacol. 2011;27(1):27–30. doi:10.4103/0970-9185.76612
- Muzi M, Robinson BJ, Ebert TJ, O’Brien TJ. Induction of anesthesia and tracheal intubation with sevoflurane in adults. Anesthesiology. 1996;85(3):536–543. doi:10.1097/00000542-199609000-00012
- Yeganeh N, Roshani B, Latifi H, Almasi A. Comparison of target-controlled infusion of sufentanil and remifentanil in blunting hemodynamic response to tracheal intubation. J Inj Violence Res. 2013;5(2):101–107. doi:10.5249/jivr.v5i2.325
- Ebert TJ, Ficke DJ, Arain SR, Holtz MN, Shankar H. Vasodilation from sufentanil in humans. Anesth Analg. 2005;101(6):1677–1680. doi:10.1213/01.ANE.0000184119.85400.0E
- Zou Y, Kong G, Wei L, et al. The effect of intravenous lidocaine on hemodynamic response to endotracheal intubation during sufentanil-based induction of anaesthesia. Anaesthesiol Intensive Ther. 2020;52(4):287–291. doi:10.5114/ait.2020.99918
- Nimmo AF, Absalom AR, Bagshaw O, et al. Guidelines for the safe practice of total intravenous anaesthesia (TIVA): joint guidelines from the association of anaesthetists and the society for intravenous anaesthesia. Anaesthesia. 2019;74(2):211–224. doi:10.1111/anae.14428
- Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67(1):41–48. doi:10.1093/bja/67.1.41
- Taylor IN, Kenny GN. Requirements for target-controlled infusion of propofol to insert the laryngeal mask airway. Anaesthesia. 1998;53(3):222–226. doi:10.1046/j.1365-2044.1998.00316.x
- Gepts E, Shafer SL, Camu F, et al. Linearity of pharmacokinetics and model estimation of sufentanil. Anesthesiology. 1995;83(6):1194–1204. doi:10.1097/00000542-199512000-00010
- Bidgoli J, Delesalle S, De Hert SG, Reiles E, Van der Linden PJ. A randomized trial comparing sufentanil versus remifentanil for laparoscopic gastroplasty in the morbidly obese patient. Eur J Anaesthesiol. 2011;28(2):120–124. doi:10.1097/EJA.0b013e3283405048
- Nightingale CE, Margarson MP, Shearer E; Members of the Working Party. Peri-operative management of the obese surgical patient 2015: Association of Anaesthetists of Great Britain and Ireland Society for Obesity and Bariatric Anaesthesia. Anaesthesia. 2015;70(7):859–876. doi:10.1111/anae.13101
- Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50. doi:10.1016/s0149-7634(05)80090-9
- Pace NL, Stylianou MP, Warltier DC. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
- Zhang J, Kong L, Ni J. ED50 and ED95 of propofol combined with different doses of intravenous lidocaine for first-trimester uterine aspiration: a prospective dose-finding study using up-and-down sequential allocation method. Drug Des Devel Ther. 2022;16:3343–3352. doi:10.2147/DDDT.S382412
- Al-Metwalli RR. The optimal effect-site concentration of sufentanil for laryngeal mask insertion during induction with target-controlled propofol infusion at 4.0 μg/mL. Saudi J Anaesth. 2014;8(2):215–219. doi:10.4103/1658-354X.130721
- Xu Q, Zhou Z, Ai L, Liu J, Tian X. Sufentanil EC50 for endotracheal intubation with aerosol inhalation of carbonated lidocaine by ultrasonic atomizer. BMC Anesthesiol. 2021;21(1):144. doi:10.1186/s12871-021-01367-w
- Igarashi T, Nagata O, Iwakiri H, Negishi C, Ozaki M. Reliability of propofol target-controlled infusion in obese patients. Masui. 2009;58(10):1226–1231. Japanese.
- Slepchenko G, Simon N, Goubaux B, Levron JC, Le Moing JP, Raucoules-Aimé M. Performance of target-controlled sufentanil infusion in obese patients. Anesthesiology. 2003;98(1):65–73. doi:10.1097/00000542-200301000-00014
- Struys MM, De Smet T, Depoorter B, et al. Comparison of plasma compartment versus two methods for effect compartment--controlled target-controlled infusion for propofol. Anesthesiology. 2000;92(2):399–406. doi:10.1097/00000542-200002000-00021
- Echevarría GC, Elgueta MF, Donoso MT, Bugedo DA, Cortínez LI, Muñoz HR. The effective effect-site propofol concentration for induction and intubation with two pharmacokinetic models in morbidly obese patients using total body weight. Anesth Analg. 2012;115(4):823–829. doi:10.1213/ANE.0b013e31825d6254
- Yang W, Geng Y, Liu Y, et al. Comparison of effects of thoracic epidural and intravenous administration of lidocaine on target-controlled infusion of propofol and tracheal intubation response during induction of anesthesia. J Cardiothorac Vasc Anesth. 2013;27(6):1295–1300. doi:10.1053/j.jvca.2012.12.020
- Miller KA, Harkin CP, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995;80(1):149–172. doi:10.1097/00000539-199501000-00025
- Ebert TJ, Mohanty PK, Kampine JP. Lidocaine attenuates efferent sympathetic responses to stress in humans. J Cardiothorac Vasc Anesth. 1991;5(5):437–443. doi:10.1016/1053-0770(91)90116-b
- Tam S, Chung F, Campbell M. Intravenous lidocaine: optimal time of injection before tracheal intubation. Anesth Analg. 1987;66(10):1036–1038.
- Carabalona JF, Delwarde B, Duclos A, et al. Serum concentrations of lidocaine during bariatric surgery. Anesth Analg. 2020;130(1):e5–e8. doi:10.1213/ANE.0000000000003905
- De Baerdemaeker LE, Jacobs S, Pattyn P, Mortier EP, Struys MM. Influence of intraoperative opioid on postoperative pain and pulmonary function after laparoscopic gastric banding: remifentanil TCI vs sufentanil TCI in morbid obesity. Br J Anaesth. 2007;99(3):404–411. doi:10.1093/bja/aem164
- Castillo-Monzón CG, Marroquín-Valz HA, Fernández-Villacañas-Marín M, Moreno-Cascales M, García-Rojo B, Candia-Arana CA. Comparison of the macintosh and airtraq laryngoscopes in morbidly obese patients: a randomized and prospective study. J Clin Anesth. 2017;36:136–141. doi:10.1016/j.jclinane.2016.10.023
- Eriksson O, Josephsson R, Långstrom B, Bergström M. Positron emission tomography and target-controlled infusion for precise modulation of brain drug concentration. Nucl Med Biol. 2008;35(3):299–303. doi:10.1016/j.nucmedbio.2007.12.003
- Lenkin AI, Zaharov VI, Lenkin PI, Smetkin AA, Bjertnaes LJ, Kirov MY. Monitoring of anesthetic depth during surgical correction of acquired valvular disorders: single center, randomized trial. J Cardiothorac Vasc Anesth. 2014;28(2):301–307. doi:10.1053/j.jvca.2013.05.032
- Meyhoff CS, Lund J, Jenstrup MT, et al. Should dosing of rocuronium in obese patients be based on ideal or corrected body weight? Anesth Analg. 2009;109(3):787–792. doi:10.1213/ane.0b013e3181b0826a
- Nandi R, Basu SR, Sarkar S, Garg R. A comparison of haemodynamic responses between clinical assessment-guided tracheal intubation and neuromuscular block monitoring-guided tracheal intubation: a prospective, randomised study. Indian J Anaesth. 2017;61(11):910–915. doi:10.4103/ija.IJA_93_17