Abstract
Antibiotic resistance is a major health problem; therefore, new antibacterial agents will need to be continuously developed. A series of novel bichalcophenes has been tested and found to have antimicrobial activity against selected bacteria. Due to the promising antimicrobial effects of these 4-substituted phenyl bichalcophene derivatives, the study reported here was launched to examine the interaction between novel bichalcophenes and tetracycline. The minimum inhibitory concentration values for all bichalcophenes were between 8 and 64 μM. Many of the bichalcophenes had synergistic activity that increased the inhibitory effect of tetracycline against bacterial growth, as indicated by the fractional inhibitory concentration index. The post-antibiotic effects of the novel bichalcophenes were determined. Many bichalcophenes were able to elongate the period required for bacteria to recover and grow after a brief exposure to tetracycline. Escherichia coli did not develop resistance to many bichalcophenes over a period of 7 days. A structural activity relationship could be characterized, as monocationic derivatives were more active than the corresponding mononitriles. The presence of a pyridyl group and/or furan ring reduced the activity, while the presence of a phenyl or thiophene ring enhanced the antibacterial activity. Our results suggest that bichalcophenes could be useful to elevate the shelf life of many antibiotics.
Introduction
Since the discovery of penicillin, many antibiotics have been developed to control bacterial infections.Citation1 The use of antibacterial agents along with improved sanitary conditions suggested that industrialized nations had won the war against pathogenic microbes. However, over the past few years, bacterial resistance to antibiotics has developed and resistance to multiple antimicrobial agents has become a major health problem.Citation2 Many factors affect the rate of development of resistance, including excessive use and misuse of antibiotics.Citation3 Due to the development of antibiotic resistance in virtually all clinically important pathogens, new antibacterial agents will need to be continuously developed.
Aromatic dicationic compounds, such as pentamidine, furamidine, and their analogs, have been reported to have broad-spectrum antimicrobial effects against fungi and bacteria.Citation4,Citation5 The antimicrobial activity of pentamidine has been recognized for over 50 years and pentamidine remains the only aromatic diamidine that has been significantly used in the drug industry.Citation6 However, due to the poor oral bioavailability and unfavorable side-effects of many diamidines, numerous new compounds have been synthesized aimed at improving these deficiencies.Citation7 Thiophene-, furan-, and nitrile-containing compounds have been synthesized and shown to exhibit a broad-spectrum antimicrobial activity.Citation8–Citation12 Recently, a series of type I dicationic bichalcophenes () and aza-analogs of furamidine have been reported to be antiprotozoal agents.Citation11,Citation13
Figure 1 (A) Structures of some biologically important cationic bichalcophene compounds. (B) 4-substituted phenyl-2,2′-bichalcophenes and aza-analogs as potent antibacterial agents.
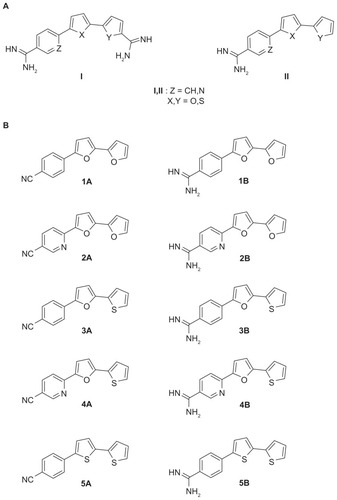
A series of type II 4-substituted phenyl bichalcophenes () have been synthesized and tested for their antimicrobial activity against the growth of several bacteria.Citation14 Due to the promising antimicrobial effects of these 4-substituted phenyl bichalcophene derivatives, the study reported here was launched to examine the interaction between type II novel bichalcophenes and tetracycline by calculating the fractional inhibitory concentration (FIC) of each combination against selected bacteria. Moreover, the effect of the bichalcophenes on the suppression of bacterial growth that persists after a brief exposure of Escherichia coli to tetracycline (known as “post-antibiotic effect” [PAE]) was determined. The increased PAE in the presence of bichalcophenes could offer an alternative way for extension of the useful lifespan of antibiotics.Citation15 The rationale behind the selection of tetracycline was to test the potency of novel bichalcophenes to enhance the efficacy of an antibiotic that has lost efficiency and against which many bacteria have acquired resistance. In addition, to investigate the stability of the tested compounds as antimicrobial agents, the development of spontaneous resistance to bichalcophenes was investigated.
Materials and methods
Chemicals
All reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) except where indicated in the specified methods. Ten bichalcophene derivatives were used throughout the study (). Bichalcophenes were sterilized by filtration through a 0.2 μm filter (Nalgene, Rochester, NY, USA).
Bacterial strains
The tested bacterial strains were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The antibacterial assays were performed against standard strains of Gram-positive (Staphylococcus aureus ATCC 25923 and Bacillus megaterium ATCC 14591) and Gram-negative (E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Klebsiella pneumoniae ATCC 700603) bacteria. All procedures were approved by the Committee of Scientific Research Ethics of King Faisal University.
Minimum inhibitory concentration (MIC) of the novel bichalcophene derivatives
The MIC of the novel bichalcophenes was determined according to the Clinical and Laboratory Standards Institute guidelines.Citation16 An inoculum density of 5 × 105 colony forming units (CFU)/mL of each organism was dispensed into the wells of a 96-well microtiter plate. The bichalcophenes were serially (1–128 μM) diluted in the wells followed by the addition of bacteria. The plates were incubated at 37°C for 18 hours and the absorbance was recorded at 600 nm. The MIC value was recorded as the lowest concentration at which no growth was observed. All MIC values were determined on three independent experiments.
Synergic interaction between the tested bichalcophenes and tetracycline
A combination of each bichalcophene at 0.25 or 0.5 × MIC and tetracycline (0.25–128 μg/mL) was prepared and inoculated with 5 × 105 CFU/mL of each strain. The plates were incubated at 37°C for 18 hours, and the lowest antibiotic concentration in combination with bichalcophene that prevented the development of growth was regarded as the MIC*. The FIC was calculated as follows:Citation17 FIC of tetracycline = MIC* (combination of tetracycline + bichalcophene)/MIC of tetracycline alone. An FIC ≤ 0.5 indicated synergy, an FIC > 0.5–4.0 indicated indifference, and an FIC > 4 indicated antagonism.
PAE
The E. coli inocula equivalent to 106 CFU/mL were incubated at 37°C for 30 minutes with tetracycline (1 × MIC), each bichalcophene (0.25 × MIC), or a combination of each bichalcophene and tetracycline (0.25 × MIC + 1 × MIC, respectively). After 30 minutes, bacteria were diluted 1000-fold with Müeller-Hinton broth (MHB) to eradicate the antibiotic and/or bichalcophene. A 100 μL sample of each treatment was obtained at time zero and every hour thereafter until turbidity developed, and spread onto nutrient agar for total viable cell count after incubation at 37°C for 24 hours. The PAE was calculated as described by Craig and Gudmundsson:Citation18 PAE = T – C, in which T is the time required for the viable cell count of the exposed bacteria to increase by a factor of log10 from the initial time immediately after washing, and C is the corresponding time for cells unexposed to either antibiotic or bichalcophenes. Each assay was carried out in triplicate. To avoid overgrowth of the control culture, a 100-fold dilution was prepared using MHB at every time interval.
Detection of bichalcophenes- resistant variants
To evaluate the development of spontaneous resistance in E. coli against bichalcophenes or tetracycline, growth assays were performed using E. coli in the presence or absence of each bichalcophene or tetracycline. Twenty milliliters of cells in the logarithmic growth phase were centrifuged, and the cells were resuspended in 2 mL of MHB. The inoculum density was adjusted to 108 CFU/mL. Different concentrations of each bichalcophene (3, 2, 1 × MIC) were added. All plates were incubated at 37°C and the absorbance was recorded at 600 nm daily for 7 days.Citation19
Statistical analysis
Statistical analyses were performed using analysis of variance followed by Fisher’s protected least significant difference multiple-range test. Differences were considered significant at P values < 0.05.
Results
The bichalcophenes had broad-spectrum activities against the tested bacteria. Compound 5B was the most effective against all bacteria, with an MIC value at 8 μM (2.7 μg/mL). Compounds 1A and 2A were alike in inhibiting the growth of bacteria, with an MIC value at 32 μM. Gram-positive bacteria were more sensitive to Compounds 3A, 4A, and 5A (MIC 32 μM) than Gram-negative strains (MIC 64 μM). Similarly, Compounds 3B and 4B were most effective in inhibiting the growth of Gram-positive bacteria (MIC 16 μM), while the MIC value against Gram-negative bacteria was 32 μM. Compounds 1B and 2B showed the same pattern, with MIC values at 16 μM against S. aureus, B. megaterium, and E. coli and 32 μM against P. aeruginosa and K. pneumoniae ().
Table 1 Minimum inhibitory concentration (MIC) of the bichalcophene derivatives against different bacterial strains
The interaction of each bichalcophene at a concentration of 0.25 or 0.5 × MIC with various concentrations of tetracycline (0.25–128 μg/mL) was examined (). The reported MIC values for tetracycline were 4 μg/mL against B. megaterium, 8 μg/mL against S. aureus, E. coli, and P. aeruginosa, and 16 μg/mL against K. pneumoniae (). With the exception of Compound 2A, all bichalcophenes at 0.25 × MIC had a synergic effect on the activity of tetracycline against K. pneumoniae and antagonized the activity of tetracycline against the growth of all other tested bacteria (FIC > 4). With the exception of Compounds 2A and 3A against S. aureus and P. aeruginosa and 4A and 4B against P. aeruginosa, all bichalcophenes at 0.5 × MIC acted synergistically with tetracycline against the growth of all bacterial strains under investigation (). Compounds 1A, 1B, and 3B at 0.25 × MIC were similar in potentiating the activity of tetracycline against all tested bacteria except S. aureus (FIC = 2). Indifference (FIC > 0.5–4.0) was shown with Compound 5A at the lowest concentration against S. aureus and B. megaterium, while it increased the activity of tetracycline against Gram-negative bacteria. Compounds 2B and 5B at both concentrations investigated were the most effective in inhibiting the growth of all tested bacteria when used in combination with tetracycline, with both achieving additive effects, as indicated by the FIC values (0.063–0.500). The effect of Compound 3A on the activity of tetracycline varied with the tested bacteria, with indifferent effects reported with S. aureus (at both concentrations) and E. coli (only at low concentration), and an antagonistic activity against the growth of P. aeruginosa at both concentrations and a synergic effect was shown against B. megaterium and K. pneumoniae. Synergic effect was reported with Compound 4B against the growth of B. megaterium, E. coli, and K. pneumoniae, and an antagonistic effect against P. aeruginosa. Compound 4A did not affect the tetracycline activity against B. megaterium, E. coli, or S. aureus only at 0.25 × MIC and antagonized the tetracycline activity when used against P. aeruginosa at both 0.25 and 0.5 × MIC ().
Table 2 Synergic interaction between the bichalcophene derivatives and tetracycline against the tested bacterial strains
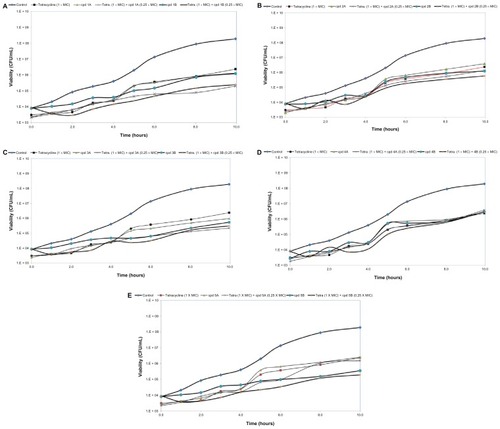
The estimated PAE for tetracycline was 2 hours. Using bichalcophenes 1A–4A and 4B resulted in PAEs of 2 hours. The recorded PAE for 5A, 1B, and 2B was 3 hours. Compounds 3B and 5B were more effective in suppressing E. coli growth for 5 and 6 hours, respectively. The combination of Compound 1A or 5A with tetracycline resulted in a PAE of 3 hours. The combination of 3A, 2B, or 4B with tetracycline had similar effects, elevating the PAE value to 4 hours. Compounds 1B, 3B, and 5B seemed the most effective at extending the suppression time of bacterial growth, with PAEs of 6, 7, and 7 hours, respectively. However, Compounds 2A and 4A had no effect on PAE value as compared to unexposed control cells ().
Figure 2 The post-antibiotic effect of bichalcophenes (0.25 × MIC), tetracycline (1 × MIC), or a combination of bichalcophenes and tetracycline on Escherichia coli American Type Culture Collection 25922. The control curve represents the growth of bacteria unexposed to either bichalcophenes or tetracycline. (A) Compounds 1A and 1B; (B) Compounds 2A and 2B; (C) Compounds 3A and 3B; (D) Compounds 4A and 4B; (E) Compounds 5A and 5B.
Abbreviations: CFU, colony forming unit; cpd, compound; MIC, minimum inhibitory concentration.
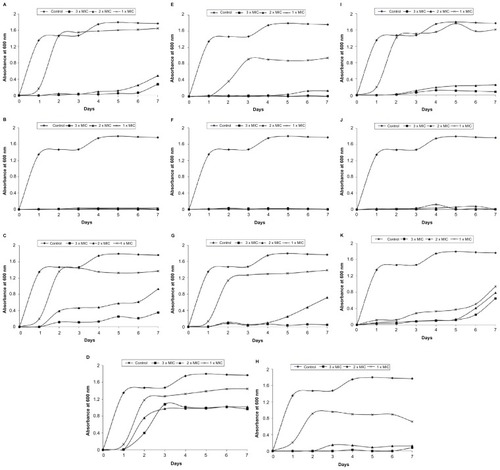
On investigation of the development of resistant variants, a high frequency of resistance development was detected with tetracycline after 2 days of incubation at 1 × MIC (8 μg/mL). Using higher concentrations of tetracycline (2 and 3 × MIC) delayed the bacterial growth until day 5. Compounds 1B, 3B, and 5B were effective at preventing the growth of bacteria even after 7 days of incubation at all concentrations examined. In contrast, the development of resistance against Compounds 2A and 2B was reported after day 2 (). Compounds 3A, 4A, 5A, and 4B were only potent at preventing the bacterial growth at the highest concentration (3 × MIC) investigated and cells failed to develop resistance after day 7. However, at the lowest concentration (1 × MIC), the growth of bacterial cells was detected at day 2. The development of resistance against Compounds 4A, 5A, and 4B at 2 × MIC occurred at days 5, 4, and 3, respectively. Exposing E. coli cells to Compound 3A delayed the emergence of resistance until day 6. Similarly, Compound 1A was effective at preventing the growth of E. coli at the high concentrations used – 2 and 3 × MIC – until 5 and 6 days post-incubation, respectively ().
Figure 3 Development of spontaneous resistant variants of Escherichia coli toward bichalcophenes or tetracycline (3, 2, and 1 × MIC) over 7 days. (A) Compound 1A; (B) Compound 1B; (C) Compound 2A; (D) Compound 2B; (E) Compound 3A; (F) Compound 3B; (G) Compound 4A; (H) Compound 4B; (I) Compound 5A; (J) Compound 5B; (K) tetracycline.
Abbreviation: MIC, minimum inhibitory concentration.
Discussion
The emergence of antibiotic resistance in bacteria is a serious problem facing humanity. The rate at which this resistance develops exceeds the rate of antibiotic development and production. Many antibiotics are now ineffective and the efficacy of many others is declining. Therefore, there must be a continuous and extensive effort to develop novel antimicrobial agents or increase the efficacy of the antibiotics currently in use by reducing the development of resistance in bacteria. Thus, five phenyl bichalcophene derivatives and their corresponding aza-analogs were synthesized and found to have significant antimicrobial activity.Citation14 These novel bichalcophenes were designed to achieve maximum antimicrobial activity, have low toxicity, and have better pharmacological properties than other biologically active amidines. These bichalcophenes contain furan and thiophene rings, which are known for their efficient biological activity,Citation20,Citation21 and through introducing either a monoamidine or cyano group, this activity could be enhanced. In addition, the ability of these novel compounds to act synergistically with tetracycline was also investigated, a property that can be used to enhance or revive many antibiotics that have lost their efficiency.
All monocationic bichalcophenes were more effective than their corresponding mononitrile derivatives. The MIC values of monocationic derivatives used in this study were between 8 and 32 μM, while those of mononitriles were between 32 and 64 μM (). The reported antimicrobial activity of the bichalcophenes can be attributed to the presence of sulfur and amidine functions.Citation22 Compound 5B showed exceptional activity by inhibiting the bacterial growth at 8 μM. The only difference between Compound 5B and 3B is the extra thiophene ring contained in the former compound. The possibility that this extra thiophene ring is responsible for this remarkable antibacterial activity cannot be ruled out. In a previous study, compounds containing a thiophene ring were found to be more active than those containing furan or pyrrole rings.Citation20 Many sulfur-containing compounds have been reported to have antimicrobial activity against bacteria.Citation23,Citation24 Similarities in the inhibition pattern could be noted between each of the following groups of compounds: 1A and 2A; 1B and 2B; 3B and 4B; and 3A, 4A, and 5A. In killing the bacteria, the bichalcophenes behaved like many broad-spectrum antibiotics and were equally effective against both Gram-negative and Gram-positive bacteria; their mechanism may be through inhibition of the synthesis of nucleic acids and/or protein.Citation25 In a previous study, bichalcophenes were found to cause the degradation of bacterial DNA,Citation14 but further studies are required to determine the mechanism(s) of action through which these antibacterial agents may act.
In a dose-dependent manner, all bichalcophenes under investigation, except Compounds 2A, 3A, and 4A, had synergic interactions with tetracycline against almost all strains tested except S. aureus (). Compounds 2B and 5B were the most effective and significantly elevated the tetracycline activity against all organisms, while 2A, 3A, and 4A were the least effective. These compounds are all mononitriles and have at least one furan ring and two of them have pyridyl rings. Compound 2A (mononitrile bifuran with pyridyl ring) antagonized the actions of tetracycline against almost all bacterial strains investigated. Similar to our MIC findings, the monocationic bichalcophenes (2B, 3B, 4B, and 5B) were more effective than the corresponding mononitrile derivatives (2A, 3A, 4A, and 5A). This differentiation in action was evident in P. aeruginosa, where 2A, 3A, and 4A antagonized the inhibitory actions of tetracycline (). Taken together, the bichalcophenes used in this study can be arranged in descending order according to their ability to potentiate tetracycline activity against bacteria: 2B and 5B >> 1B, 3B, and 1A >> 4B and 5A >> 3A >> 4A >> 2A. From this, it is clear that the monocationic derivatives were the most effective.
The in vitro pharmacodynamic properties of bichalcophenes were investigated by studying the PAE against E. coli. Any agent that induces a long antibiotic PAE may secure longer dosing intervals without losing the antibiotic’s efficacy.Citation26 E. coli exposed to either tetracycline or most bichalcophenes was able to recover and grow 2–3 hours post-exposure. The low value obtained in the present study after exposure to tetracycline has been previously reported.Citation27 Two compounds (3B and 5B) resulted in a remarkable delay in bacterial growth (PAE) of up to 5–6 hours after exposure; not surprisingly, these compounds were monocationic phenyl bichalcophenes. With the exception of 3A, all mononitrile bichalcophene (phenyl or pyridyl) derivatives when combined with tetracycline had no significant effect on the PAE. Combining monocationic phenyl bichalcophenes (1B, 3B, and 5B) with tetracycline resulted in a significant 6–7-hour delay in bacterial growth. The presence of a pyridyl ring in Compounds 2B and 4B reduced the ability of these monocationic derivatives, resulting in a 4-hour delay in PAE ().
In the search for new antibacterial agents, it is not enough to prove their efficacy; it is also necessary to investigate the rate of resistance development toward these new agents. In our study, after only 1 day of incubation, E. coli developed resistance to all mononitrile derivatives (1A–5A), monocationic pyridyl bichalcophenes (2B and 4B), and tetracycline at the low concentration of 1 × MIC. Similar findings have previously been reported for tetracycline.Citation28 At the highest concentration of 3 × MIC, the bacteria were not able to develop resistance against almost all bichalcophenes after 7 days of incubation, but overcame tetracycline and started to grow after 5 days. In contrast, the bacteria failed to develop resistance toward the monocationic phenyl bichalcophenes (1B, 3B, and 5B), even after 7 days, at all concentrations investigated ().
In conclusion, in our study, a structural activity relationship was evident, as the presence of mononitrile or pyridyl groups and, to a lesser extent, the furan ring, reduced antibacterial activity, while the presence of a phenyl, monocationic, or thiophene ring clearly enhanced antibacterial activity. Recently, the National Cancer Institute Developmental Therapeutics Program examined these bichalcophenes for anticancer activity in 58 cell lines and found that monocationic bichalcophenes were more active than the corresponding aza-analogs and that 1B, 4B, and 5B were the most active compounds (unpublished data). These bichalcophenes not only have antibacterial properties but can also potentiate the activity of antibiotics currently in use. As it is unlikely that bacteria could develop resistance to these novel compounds, their possible clinical effectiveness is suggested. Two of these bichalcophenes (1A and 1B) have been tested in mice and found to be not toxic.Citation29 Treatment of methicillin-resistant Staphylococcus aureus-infected mice with these bichalcophenes resulted in significant reductions in the viable bacterial count in blood, liver, kidney, and spleen. The structural activity relationship in the present study was also confirmed in vivo, as Compound 1B was superior to 1A and sometimes both provided better anti-staphylococcal agents than vancomycin against methicillin-resistant S. aureus pathogenesis.Citation29
Acknowledgment
This project was supported by the Deanship of Scientific Research at King Faisal University (grant no 130143).
Disclosure
The authors declare no conflicts of interest in this work.
References
- Trémolières F When the antibiotic miracle turns into a nightmare Med Sci (Paris) 2010 26 925 929 French 21106173
- Kennedy B O’Connor B Korn B Gibbons N O’Connor T Keane J Multi-drug resistant tuberculosis: experiences of two tertiary referral centres Ir Med J 2011 104 6 182 185 22111396
- Kutty N Treating children without antibiotics in primary healthcare Oman Med J 2011 26 5 303 305 22125722
- Checchi F Barrett MP African sleeping sickness BMJ 2008 336 7646 679 680 18321959
- Soeiro MN de Castro SL de Souza EM Batista DG Silva CF Boykin DW Diamidine activity against trypanosomes: the state of the art Curr Mol Pharmacol 2008 1 2 151 161 20021429
- Libman MD Miller MA Richards GK Antistaphylococcal activity of pentamidine Antimicrob Agents Chemother 1990 34 9 1795 1796 2285292
- Werbovetz K Diamidines as antitrypanosomal, antileishmanial and antimalarial agents Curr Opin Investig Drugs 2006 7 2 147 157
- Taylor R Thiophene and its derivatives Gronowitz S The Chemistry of Heterocyclic Compounds Hoboken, NJ Wiley 1985 261 323
- Soeiro MN De Souza EM Stephens CE Boykin DW Aromatic diamidines as antiparasitic agents Expert Opin Invest Drugs 2005 14 8 957 972
- Wilson WD Nguyen B Tanious FA Dications that target the DNA minor groove: compound design and preparation, DNA interactions, cellular distribution and biological activity Curr Med Chem Anticancer Agents 2005 5 4 389 408 16101490
- Wenzler T Boykin DW Ismail MA Hall JE Tidwell RR Brun R New treatment option for second-stage African sleeping sickness: in vitro and in vivo efficacy of aza analogs of DB289 Antimicrob Agents Chemother 2009 53 10 4185 4192 19620327
- Del Poeta M Schell WA Dykstra CC In vitro antifungal activities of a series of dication-substituted carbazoles, furans, and benzimidazoles Antimicrob Agents Chemother 1998 42 10 2503 2510 9756748
- Ismail MA El Bialy SA Brun R Dicationic phenyl-2,2′-bichalcophenes and analogues as antiprotozoal agents Bioorg Med Chem 2011 19 2 978 984 21194955
- Youssef MM Al-Omair MA Ismail MA Synthesis, DNA affinity, and antimicrobial activity of 4-substituted phenyl-2,2′-bichalcophenes and aza-analogues Med Chem Res 2012 21 4074 4082
- Spangler SK Lin G Jacobs MR Appelbaum PC Postantibiotic effect and postantibiotic sub-MIC effect of levofloxacin compared to those of ofloxacin, ciprofloxacin, erythromycin, azithromycin, and clarithromycin against 20 pneumococci Antimicrob Agents Chemother 1998 42 5 1253 1255 9593160
- National Committee for Clinical Laboratory Standards (NCCLS) Performance Standards for Antimicrobial Susceptibility Testing: Eleventh Informational Supplement M100-S11 Wayne, PA NCCLS 2001
- Mackay ML Milne K Gould IM Comparison of methods for assessing synergic antibiotic interactions Int J Antimicrob Agents 2000 15 2 125 129 10854808
- Craig WA Gudmundsson S Postantibiotic effect Lorian V Antibiotics in Laboratory Medicine Philadelphia, PA Lippincott Williams & Wilkins 1996 296 329
- Pillai SP Pillai CA Shankel DM Mitscher LA The ability of certain antimutagenic agents to prevent development of antibiotic resistance Mutat Res 2001 496 1–2 61 73 11551481
- Boschi D Guglielmo S Aiello S Morace G Borghi E Fruttero R Synthesis and in vitro antimicrobial activities of new (cyano-NNO-azoxy)pyrazole derivatives Bioorg Med Chem Lett 2011 21 11 3431 3434 21530247
- Xiao ZP He XB Peng ZY Synthesis, structure, molecular docking, and structure-activity relationship analysis of enamines: 3-aryl-4-alkylaminofuran-2(5H)-ones as potential antibacterials Bioorg Med Chem 2011 19 5 1571 1579 21330140
- Thebault P Taffin de Givenchy E Levy R Vandenberghe Y Guittard F Géribaldi S Preparation and antimicrobial behaviour of quaternary ammonium thiol derivatives able to be grafted on metal surfaces Eur J Med Chem 2009 44 2 717 724 18571289
- Kim S Kubec R Musah RA Antibacterial and antifungal activity of sulfur-containing compounds from Petiveria alliacea L J Ethnopharmacol 2006 104 1–2 188 192 16229980
- Dekié BR Radulović NS Dekić VS Vukićević RD Palić RM Synthesis and antimicrobial activity of new 4-heteroarylamino coumarin derivatives containing nitrogen and sulfur as heteroatoms Molecules 2010 15 4 2246 2256 20428040
- Wood K Nishida S Sontag ED Cluzel P Mechanism-independent method for predicting response to multidrug combinations in bacteria Proc Natl Acad Sci U S A 2012 109 30 12254 12259 22773816
- Güzel CB Gerçeker AA In vitro activities of various antibiotics, alone and in combination with colistin methanesulfonate, against Pseudomonas aeruginosa strains isolated from cystic fibrosis patients Chemotherapy 2008 54 2 147 151 18322363
- Athamna A Athamna M Medlej B Bast DJ Rubinstein E In vitro post-antibiotic effect of fluoroquinolones, macrolides, beta-lactams, tetracyclines, vancomycin, clindamycin, linezolid, chloramphenicol, quinupristin/dalfopristin and rifampicin on Bacillus anthracis J Antimicrob Chemother 2004 53 4 609 615 14998982
- Yang H Chen S White DG Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China J Clin Microbiol 2004 42 8 3483 3489 15297487
- El-Sayed WM Hussin WA Ismail MA Efficacy of two novel 2,2′-bifurans to inhibit methicillin-resistant Staphylococcus aureus infection in male mice in comparison to vancomycin Drug Des Devel Ther 2012 6 279 287