Abstract
Purpose
To compare the long-term effects of olmesartan combined with either azelnidipine or amlodipine on central blood pressure (CBP), left ventricular (LV) mass index (LVMI), LV diastolic function (e′ velocity, E/e′ ratio, E/A ratio) and arterial stiffness (brachial-ankle pulse wave velocity [baPWV] and augmentation index normalized for a heart rate of 75 bpm [AIx]).
Patients and methods
Patients with systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg received olmesartan monotherapy (20 mg/day) for 12 weeks. They were then randomly assigned to fixed-dose add-on therapy with azelnidipine (16 mg/day; n = 26) or amlodipine (5 mg/day; n = 26) for a further 2 years. CBP, LVMI, e′ velocity, E/e′ ratio, E/A ratio, baPWV, and AIx were measured at baseline, 6 months, and 2 years.
Results
Baseline characteristics of both groups were similar. The decrease in brachial BP over 2 years was similar in both groups. CBP, LVMI, E/e′ ratio, baPWV, and AIx decreased significantly, and the E/A ratio and e′ velocity increased significantly in both groups. The decreases in CBP (P < 0.001), AIx (P < 0.001), baPWV (P < 0.001), LVMI (P < 0.001), and E/e′ (P = 0.002) as well as the increase in E/A ratio (P = 0.03) over 2 years were significantly greater in the olmesartan/azelnidipine group than in the olmesartan/amlodipine group. Multivariate linear regression analyses showed that the changes in baPWV (β = 0.41, P < 0.001) and CBP (β = 0.47, P = 0.01) were independently associated with the change in LVMI, the change in baPWV (β = 0.25, P < 0.001) was independently associated with the change in E/e′ ratio, and the changes in baPWV (β = 0.21, P = 0.001) and AIx (β = 0.25, P = 0.03) were independently associated with the change in E/A ratio.
Conclusion
Treatment with olmesartan/azelnidipine for 2 years resulted in greater improvements in CBP, LVMI, and LV diastolic function, and arterial stiffness compared with olmesartan/amlodipine. Improvements in LV diastolic function were associated with improvements in arterial stiffness.
Introduction
When blood pressure (BP) control is inadequate with a single antihypertensive drug, the use of two or three drugs in combination is often necessary to achieve the target blood pressure. Combination therapy with an angiotensin II receptor blocker (ARB) and a diuretic or an ARB plus a calcium (Ca2+) channel blocker (CCB) are recommended in the current Japanese Society for Hypertension guidelines.Citation1
Several studies,Citation2–Citation4 including the Conduit Artery Function Evaluation substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT-CAFE),Citation3 have emphasized the importance of targeting central blood pressure (CBP) rather than brachial BP in terms of cardiovascular disease outcomes. Additionally, it was reported that an ARB in combination with a CCB significantly improves CBP more than an ARB in combination with a diuretic.Citation4 We previously reported that 24 weeks of combination therapy with olmesartan plus azelnidipine had greater effects on CBP and left ventricular (LV) mass index (LVMI) than did olmesartan plus amlodipine, even though the reduction in brachial BP was similar in both groups.Citation5 We also detected a strong correlation between the change in CBP and the change in LVMI (R 2 = 0.626, P < 0.001). However, it was unknown whether the observed effects of olmesartan/azelnidipine on CBP and LVMI were long-lasting.
Our earlier study assessed LV hypertrophy but not LV diastolic function. LV hypertrophyCitation6 and LV diastolic dysfunctionCitation7 are often associated with hypertension, and arterial stiffness is often aggravated in hypertensive patients.Citation8 Considering this issue, it is important that the effects of antihypertensive drugs on LV hypertrophy and LV diastolic dysfunction are fully evaluated and compared among different classes of antihypertensive drugs.
To date, very few studies have compared the long-term outcomes of different antihypertensive drug regimens on LV diastolic function or arterial stiffness. Therefore, we hypothesized that the combination of olmesartan plus azelnidipine would have greater long-term effects on cardiac function than the combination of olmesartan plus amlodipine. To test this hypothesis, we conducted a 2-year, prospective, randomized, open-label, parallel-group study (AORTA II study, Azelnidipine plus OlmesaRTAn versus amlodipine plus olmesartan) to compare the effects of olmesartan/azelnidipine and olmesartan/amlodipine on clinic-measured BP, heart rate (HR), CBP, LV hypertrophy, LV diastolic function, and arterial stiffness. Hypertensive patients who failed to achieve a target blood pressure following 12 weeks of olmesartan monotherapy were randomized to receive either olmesartan/azelnidipine or olmesartan/amlodipine for 2 years. We also sought to identify the factors associated with improvements in LV hypertrophy and LV diastolic function. As indices of arterial stiffness and wave reflections, we measured the brachial-ankle pulse wave velocity (baPWV) and the augmentation index (AIx, normalized for a HR of 75 bpm). LV hypertrophy was determined as the LVMI, while LV dysfunction was measured in terms of e′, E/e′ ratio, and E/A ratio. All of these factors are known to be altered in hypertensive patients and predict strokeCitation9,Citation10 or other serious clinical outcomes.Citation11,Citation12
Methods
Patients
As previously described,Citation5 hypertensive outpatients (with or without current therapy) aged 36–75 years were consecutively recruited at the Department of Internal Medicine at Clinic Jingumae (Kashihara, Japan) between March 2007 and January 2010. Hypertension was defined as clinic-measured systolic BP (SBP) ≥140 mmHg and/or diastolic BP (DBP) ≥90 mmHg on two different occasions. Patients on current antihypertensive therapy were also defined as having hypertension. Patients were excluded for any of the following reasons: current treatment with olmesartan, secondary hypertension, arrhythmia, current treatment for congestive heart failure, history of stroke or coronary artery disease, clinically significant valvular heart disease, renal insufficiency (serum creatinine ≥ 2 mg/dL), mental disorders, severe noncardiovascular disease (eg, cancer or liver cirrhosis), or chronic inflammatory disease. Patients with clinic-measured SBP > 200 mmHg and/or DBP > 115 mmHg at any time during the run-in period were withdrawn from the study. All of the patients provided written informed consent. The study protocol was approved by the Institutional Ethical Committee of Nara Medical University.
Study design
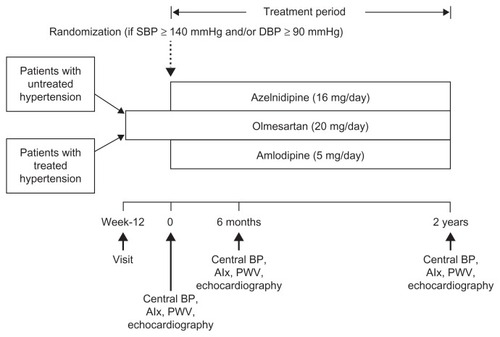
The AORTA II study was designed as a 2-year, prospective, randomized, open-label parallel-group study (). It consisted of a 12-week run-in period and a 2-year randomized treatment period. During the run-in period, patients were treated with olmesartan alone (20 mg/daily). Patients already on antihypertensive drugs were switched to olmesartan monotherapy at the start of the run-in period. After the 12-week run-in period, patients with a clinic-measured SBP ≥ 140 mmHg and/or DPB ≥ 90 mmHg were randomized (1:1) using the permuted block method to receive either azelnidipine (16 mg/day) or amlodipine (5 mg/day) as add-on to ongoing olmesartan. Both regimens were continued at fixed doses, without dose titration, for 2 years. The patients were instructed to take their medications after breakfast and were not permitted to use any other antihypertensive drugs. Drugs (eg, azole antifungal drugs and protease inhibitors) that had the potential to interfere with the safety or efficacy of the study drugs were not permitted during the study. The following were measured at baseline, at 6 months, and at 2 years of treatment: brachial BP, HR, CBP, AIx, baPWV, LVMI, and LV diastolic function (e′, E/e′ ratio, and E/A ratio). There were no modifications to the methods or outcomes after starting the study. The randomization list was prepared by a medical secretary at Clinic Jingumae and was sent to the investigators in opaque envelopes to conceal randomization order and allocation.
Figure 1 Study protocol.
Modified with permission from Takami T, Saito Y. Effects of azelnidipine plus olmesartan versus amlodipine plus olmesartan on central blood pressure and left ventricular mass index: the AORTA study. Vasc Health Risk Manag. 383–390. © 2012 Dove Medical Press.Citation5
Measurement of CBP, AIx, and baPWV
CBP, AIx, and baPWV were measured as previously described.Citation5 An automated tonometry system (HEM-9000AI; Omron Healthcare, Kyoto, Japan) was used to record the pulse pressure waveform of the radial artery with the patient in the sitting position after a ≥5 min rest. The first and second systolic pressure components (SBP1 and SBP2) were determined from the pulse pressure waveform. As SBP2 is similar to invasively measured aortic CBP,Citation13 it was used as an estimate of CBP. The HEM9000-AI-measured CBP was similar to CBP estimated using a generalized aorta–radial transfer function.Citation14–Citation16 The AI was calculated using the formula (SBP2 − DBP)/(SBP1 − DBP) × 100, and was normalized for an HR of 75 bpm (AIx) because it is influenced by the HR.Citation17
Arterial stiffness was measured as baPWV, as previously described.Citation5,Citation18 Pulse waveforms of both forearms and both ankles were obtained after the patient had rested in the supine position for ≥5 min and were used to determine the baPWV. The deviation between two repeated measurements of baPWV was <5%. The mean value on the right side was used for each patient.
Measurement of LVMI and LF diastolic function
Standard M-mode echocardiography with two-dimensional guidance and a 3-MHz transducer (Vivid S6 ultrasound system; GE Healthcare, Milwaukee, WI, USA) was performed to determine LVMI using the formula reported by Devereux et al.Citation18 From the mitral flow velocity pattern, we measured the peak velocities of the E and A waves on mitral flow velocity, and calculated the ratio of their peak velocities (E/A ratio). Doppler tissue integration of longitudinal mitral annular velocity was recorded at the septal annulus in the apical four-chamber view throughout the cardiac cycle. The peak early diastolic (e′) myocardial velocities, e′ velocity, and the ratio of mitral inflow velocity to e′ velocity (E/e′ ratio) were measured as previously described.Citation19
Sample size calculation
The AORTA II study was designed to show a difference in the decrease in central blood pressure (measured in mmHg) between olmesartan/azelnidipine and olmesartan/amlodipine treatment. With the assumption of a standard deviation (SD) of 5.0 mmHg and mean difference of 4.0 mmHg between the two groups and a power of 0.8 at a one-sided significance level of 0.025, at least 30 patients were required in each treatment group. To account for possible withdrawals, we planned to enroll at least 60 patients (30 per group).
Statistical analysis
Data are shown as means ± SD. Differences between the two groups at baseline were analyzed using unpaired t tests for continuous variables and χ2 tests for categorical variables. Repeated-measures ANOVAs with Dunnett’s adjustment were used for within-group comparisons. A between-group time course comparison was performed using an analysis of group-by-time interactions by repeated-measures ANOVA. Pearson’s methods were used to assess correlations among the changes in LVMI, E/e′ ratio, E/A ratio, and other variables. Variables with a P-value <0.05 in univariate analyses were entered into multivariate linear regression analysis with stepwise variable selection to identify the factors that were independently associated with the changes in LVMI ratio, E/e′ ratio, and E/A ratio at 2 years.
Univariate and multivariate correlation and regression analyses were conducted in the total population. Differences were considered statistically significant at values of P < 0.05.
Results
The first patient entered the study in March 2007 and the last patient completed the study in accordance with the study protocol in January 2012. As previously reported,Citation5 113 patients entered the run-in period, of which 54 were randomized to receive add-on azelnidipine (n = 27) or amlodipine (n = 27). One patient in each group was excluded from the assessments performed at 6 months as both of the patients missed the assessment visits (). One patient in the olmesartan/azelnidipine group and one patient in the olmesartan/amlodipine group withdrew from the study between 6 months and 2 years because they did not return to our clinic. However, these patients were followed up at another clinic and did not experience any adverse events. Therefore, 26 patients in each group were included in the present analyses. As shown in , the two groups were well matched, as their baseline characteristics were similar, with no significant differences in any of the factors determined at baseline.
Table 1 Patient characteristics at baseline
shows the changes in parameters assessed at 6 months and 2 years of treatment in the olmesartan/azelnidipine and olmesartan/amlodipine groups. SBP, DBP, CBP, AIx (AI normalized for a heart rate of 75 bpm), baPWV, LVMI, and E/e′ ratio decreased significantly while e′ velocity and E/A ratio increased significantly in both groups from baseline to 6 months and 2 years of treatment. The changes in HR, CBP, AIx, baPWV, LVMI, E/A ratio, and E/e′ ratio over 2 years were significantly greater in the olmesartan/azelnidipine group than in the olmesartan/amlodipine group throughout the 2-year period (P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001, P = 0.03, and P = 0.002 for time × group interactions for HR, CBP, AIx, baPWV, LVMI, E/e′ ratio, and E/A ratio, respectively). shows the results of the univariate and multivariate analyses that were performed to identify the factors associated with the reduction in LVMI in the total population. Univariate analysis showed that the change in LVMI was significantly correlated with changes in HR, CBP, AIx, baPWV, e′ velocity, E/e′ ratio, and E/A ratio. In stepwise multivariate linear regression, the changes in CBP (β = 0.47, P = 0.01) and baPWV (β = 0.41, P < 0.001) were independently associated with the change in LVMI. In analyses stratified by treatment group, the change in baPWV was independently associated with the change in LVMI in both groups (olmesartan/azelnidipine: β = 0.49, P < 0.001; olmesartan/amlodipine: β = 0.44, P < 0.001). Although the change in AIx was also associated with the change in LVMI, multivariate analysis revealed that LVMI was more strongly correlated with baPWV than AIx.
Table 2 Changes in hemodynamic parameters
Table 3 Univariate and multivariate regression analyses for the change in LVMI in all patients and in each treatment group
shows the results of the univariate and multivariate analyses that were performed to detect factors associated with the reduction in E/e′ ratio. Analysis of the entire study population showed that the change in the E/e′ ratio was significantly correlated with the changes in HR, CBP, AIx, baPWV, and LVMI. Stepwise linear regression analysis showed the change in baPWV was independently associated with the change in the E/e′ ratio (β = 0.25, P < 0.001). In analyses stratified by treatment group, the change in baPWV was independently associated with the change in the E/e′ ratio in the olmesartan/azelnidipine group (β = 0.35, P = 0.001) but not in the olmesartan/amlodipine group.
Table 4 Univariate and multivariate regression analyses of the change in E/e′ ratio in all patients and in each treatment group
shows the results of the univariate and multivariate analyses that were performed to identify factors associated with the change in the E/A ratio. Univariate analyses showed that the change in E/A ratio was significantly correlated with the changes in HR, CBP, AIx, baPWV, and LVMI. Stepwise linear regression analysis showed that changes in baPWV (β = 0.21, P = 0.001) and AIx (β = 0.25, P = 0.03) were independently associated with the change in the E/A ratio. In analyses stratified by treatment group, the change in baPWV was independently associated with the change in E/A ratio in both groups (olmesartan/azelnidipine: β = 0.34, P = 0.002, olmesartan/amlodipine: β = 0.27, P = 0.01).
Table 5 Univariate and multivariate regression analyses of the change in E/A ratio in all patients and in each treatment group
No serious adverse events requiring study discontinuation were reported in either treatment group. One patient experienced mild hepatic dysfunction and another experienced vertigo (both in the olmesartan/amlodipine group). Both patients recovered after appropriate therapies.
Discussion
In the present study, we extended on our previous findings by showing that the improvements in CBP, AIx, baPWV, LVMI, E/e′, and E/A ratio were maintained at 2 years, and continued to be significantly greater in the olmesartan/azelnidipine group than in the olmesartan/amlodipine group, despite similar reductions in brachial BP in both groups. The results of stepwise multivariate linear regression analysis showed that the reduction in LVMI after 2 years of treatment was directly correlated with the reduction in CBP. Increased CBP causes an increase in LV afterload that can lead to LV hypertrophy. The improvement in LVMI observed in this study appears to have resulted from the reduction in CBP. The reduction in baPWV was also independently associated with the reduction in LVMI. The results of this study suggest that the reduction in baPWV led to a delay in pulse wave reflection and hence reduced cardiac afterload. The results of a previous study revealed associations of AI and baPWV with LVMI.Citation20 Specifically, AI as an index of aortic sclerosis is related to aortic distensibility or reflecting waves; however, in the present study, baPWV was found to be an independent factor from LVMI. Therefore, the reduction in baPWV appears to be related to the improvement in LVMI. Although our study included many patients with LV diastolic dysfunction, our current findings are consistent with those of the earlier study showing a correlation between baPWV and LVMI, E/A ratio.Citation20
A follow-up study that monitored 1839 hypertensive patients for up to 11 years (mean: 4.4 years) for the onset of cardiovascular events showed that patients with LV diastolic dysfunction were at increased risk for cardiovascular events compared with patients without diastolic dysfunction.Citation21 Our study also detected improvements in LV diastolic function and LVMI. The E/e′ ratio, defined as the ratio of peak early LV filling velocity to peak early diastolic mitral annular velocity, is considered to reflect pulmonary arterial wedge pressure regardless of the severity of LV systolic dysfunction. E/e′ is used as an index for LV end-diastolic pressure. It is reported that in cases of E/e′ ≤ 8, LV end-diastolic pressure is low and in cases of E/e′≥ 15, LV end-diastolic pressure is high.Citation22 Thus, the E/e′ ratio is widely used as a prognostic predictor in clinical treatment.Citation23 We previously reported that olmesartan/azelnidipine antihypertensive therapy achieved greater reductions in HR, baPWV, and AIx than did olmesartan/amlodipine therapy at 24 weeks, and thus achieved greater improvements in arterial stiffness.Citation5 The first new finding of the present study is that the E/e′ ratio showed greater improvements in the olmesartan/azelnidipine group than in the olmesartan/amlodipine group throughout the 2-year study period. The second new finding is that baPWV, an indicator of arterial stiffness, was independently associated with the reduction in the E/e′ ratio (P < 0.001), suggesting baPWV is a useful indicator of LV diastolic function. In analyses stratified by treatment group, the reduction in baPWV was independently associated with the reduction in the E/e′ ratio in the olmesartan/azelnidipine group, but not the olmesartan/amlodipine group. In brief, this is thought to be attributable to the greater improvement in arterial stiffness in the olmesartan/azelnidipine group than in the olmesartan/amlodipine group. A recent cohort studyCitation24 that assessed asymptomatic LV diastolic dysfunction by tissue Doppler imaging revealed that aggravated arterial stiffness was linked to aggravated LV diastolic dysfunction.
The present study also revealed that a reduction in the E/A ratio was an indicator of LV diastolic dysfunction, and that the change in baPWV was independently associated with the change in E/A ratio. It was previously reported that, even if the E/A ratio is corrected for age in hypertensive patients, baPWV is an independent predictor for LV diastolic dysfunction, as is E/e′, so that increases in baPWV may be a risk factor for diastolic heart failure.Citation25 baPWV was also reported to be an independent risk factor for LV diastolic dysfunction in untreated hypertensive patients.Citation26 Additionally, a cohort study of Chinese patients with LV diastolic dysfunction showed a significant correlation between PWV and the E/A ratio based on multivariate linear regression analysis.Citation27
An experimental study in mice suggested that a reduction in HR may improve vascular stiffness and consequently lead to improvements in LV diastolic function.Citation28 An experimental study also showed that azelnidipine dose-dependently reduces HR.Citation29 In another study, azelnidipine suppressed cardiac hypertrophy, fibrosis, NADPH oxidase, and superoxide levels in stroke-prone spontaneously hypertensive rats more potently than did amlodipine, and was associated with lower HR than was amlodipine.Citation30
Hypertension is associated with oxidative stress, which impairs vascular endothelial function and, together with other factors, may increase arterial stiffness. However, increasing arterial stiffness, which may be caused by a variety of factors, could be a cause of hypertension in some patients.Citation31 Drugs that reduce cardiac afterload are thought to effectively control hypertension by reducing cardiac load. Dihydropyridine CCBs that have vasodilating and antioxidant activities are representative medications that can reduce the cardiac load by dilating the peripheral capillary vessels, although the effects of their antioxidant activities in patients with hypertension are largely unknown.
In patients who switched from amlodipine to azelnidipine, BP and HR decreased significantly, and these reductions were associated with an increase in the e′ velocity.Citation32 On the basis of this finding, regression of LVMI may be associated with the improvements in LV diastolic function elicited by azelnidipine. Furthermore, aortic stiffness increases central SBP and LV afterload, leading to LV myocyte hypertrophy. Diastolic dysfunction may be due to impaired relaxation resulting from the cardiac changes described above. Alternatively, aortic stiffness reduces central DBP and coronary blood flow, resulting in subendocardial ischemia. Diastolic dysfunction may also occur because of myocardial fibrosis arising from subendocardial ischemia occurring via the pathway described above.Citation33 Therefore, olmesartan/azelnidipine antihypertensive therapy appears to play a valuable role in these pathological pathways.
The results of the present study indicate that olmesartan/azelnidipine is significantly superior to olmesartan/amlodipine in reducing HR and in improving vascular stiffness and diastolic function. Vascular stiffness alone, however, was independently associated with improved diastolic function.
Some limitations of this study should be discussed. First, the present study enrolled a relatively small number of patients. Moreover, the study was not conducted in a double-blind manner. However, all measurements (AI, LVMI, E/A ratio, e′, E/e′ ratio, and PWV) were performed by an investigator who was blinded to treatment allocation to avoid bias in these measurements.
Conclusion
Treatment with olmesartan/azelnidipine for 2 years achieved significantly greater improvements in HR, arterial stiffness, and cardiac hypertrophy compared with olmesartan/amlodipine therapy. Therefore, the two therapies differed in their effects on improving cardiac hypertrophy and arterial stiffness. Compared with olmesartan/amlodipine, olmesartan/azelnidipine also achieved significantly greater improvements in LV diastolic dysfunction, which were maintained for 2 years. These results indicate that olmesartan/azelnidipine steadily improves LV diastolic dysfunction during long-term therapy and that the change in arterial stiffness was independently associated with improvements in LV diastolic dysfunction.
Acknowledgments
We wish to thank N Mune for technical support and A Takemura for secretarial support.
Disclosure
The authors have no conflicts of interest to declare.
References
- Ogihara T Kikuchi K Matsuoka H The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH2009) Hypertens Res 2009 32 3 107 19300436
- Roman MJ Devereux Kizer JR Central pressure more strongly relates to vascular disease and outcome than dose brachial pressure: the Strong Heart Study Hypertension 2007 50 197 203 17485598
- Williams B Lacy PS Thom SM Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduct Artery Function Evaluation (CAFE) study Circulation 2006 113 1213 1225 16476843
- Matsui Y Eguchi K O’Rourke MF Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients Hypertension 2009 54 716 723 19667251
- Takami T Saito Y Effects of azelnidipine plus olmesartan versus amlodipine plus olmesartan on central blood pressure and left ventricular mass index: the AORTA study Vasc Health Risk Manag 2011 7 383 390 21796252
- Massie BM Tubau JF Szlachcic J O’Kelly BF Hypertensive heart disease: the critical role of left ventricular hypertrophy J Cardiovasc Pharmacol 1989 13 Suppl 1 S18 S24 2468972
- Bonow RO Udelson JE LV diastolic dysfunction as a cause of congestive heart failure. Mechanism and management Ann Intern Med 1992 117 502 510 1503353
- Asmar R Rudnichi A Blacher J London GM Safar ME Pulse pressure and aortic pulse wave are markers of cardiovascular risk in hypertensive populations Am J Hypertens 2001 14 91 97 11243313
- Laurent S Boutouyrie P Arterial stiffness and stroke in hypertension: therapeutic implications for stroke prevention CNS Drugs 2005 19 1 11 15651901
- Blacher J Safar ME Large-artery stiffness, hypertension and cardiovascular risk in older patients Nat Clin Pract Cardiovasc Med 2005 2 450 455 16265585
- Udelson JE Konstam MA Relation between left ventricular remodeling and clinical outcomes in heart failure patients with left ventricular systolic dysfunction J Card Fail 2002 8 S465 S471 12555159
- Wachtell K Palmieri V Gerdts E Prognostic significance of left ventricular diastolic dysfunction in patients with left ventricular hypertrophy and systemic hypertension (the LIFE Study) Am J Cardiol 2010 106 999 1005 20854964
- Takazawa K Kobayashi H Shindo N Tanaka N Yamashina A Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave Hypertens Res 2007 30 219 228 17510503
- Richardson CJ Maki-Petaja KM McDonnell BJ Comparison of estimates of central systolic blood pressure and peripheral augmentation index obtained from the Omron HEM-9000AI and SphygmoCor systems Artery Res 2009 3 24 31
- Hickson SS Butlin M Mir FA The accuracy of central SBP determined from the second systolic peak of the peripheral pressure waveform J Hypertens 2009 27 1784 1788 19702000
- Hirata K Kojima I Momomura SI Noninvasive estimation of central blood pressure and the augmentation index in the seated position: a validation study of two commercially available methods J Hypertens 2013 31 508 515
- Wilkinson IB Mohammad NH Tyrrell S Heart rate dependency of pulse pressure amplification and arterial stiffness Am J Hypertens 2002 15 24 30 11824855
- Devereux RB Palmieri V Sharpe N Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (PRESERVE) trial Circulation 2001 104 1248 1254 11551875
- Safar ME London GM Therapeutic studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. The Clinical Committee of Arterial Structure and Function. Working Group on Vascular Structure and Function of the European Society of Hypertension J Hypertens 2000 18 1527 1535 11081763
- Xu L Jiang CQ Lam TH Arterial stiffness and left-ventricular diastolic dysfunction: Guangzhou Biobank Cohort Study-CVD J Hum Hypertens 2011 25 152 158 20428193
- Kim MN Park SM Shim WJ The relationship between aortic stiffness and left ventricular dyssynchrony in hypertensive patients with preserved left ventricular systolic function Clin Exp Hypertens 2012 34 410 416 22471755
- Schillaci G Pasqualini L Verdecchia P Prognostic significance of left ventricular diastolic dysfunction in essential hypertension J Am Coll Cardiol 2002 39 2005 2011 12084601
- Ommen SR Nishimura RA Appleton CP Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study Circulation 2000 102 1788 1794 11023933
- Hillis GS Møller JE Pellikka PA Noninvasive estimation of left ventricular filling pressure by e/e′ is a powerful predictor of survival after acute myocardial infarction J Am Coll Cardiol 2004 43 360 367 15013115
- Yambe M Tomiyama H Hirayama Y Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure Hypertens Res 2004 27 625 631 15750255
- Jaroch J Łoboz Grudzień K Bociąga Z The relationship of carotid arterial stiffness to left ventricular diastolic dysfunction in untreated hypertension Kardiol Pol 2012 70 223 231 22430399
- Xu L Jiang CQ Lam TH Arterial stiffness and left-ventricular diastolic dysfunction: Guangzhou Biobank Cohort Study-CVD J Hum Hypertens 2011 25 152 158 20428193
- Reil JC Hohl M Reil GH Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction Eur Heart J 7 24 2012 [Epub ahead of print.]
- Fujisawa M Yorikane R Chiba S Koike H Chronotropic effects of azelnidipine, a slow- and long-acting dihydropyridine-type calcium channel blocker, in anesthetized dogs: a comparison with amlodipine J Cardiovasc Pharmacol 2009 53 325 332 19295444
- Yamamoto E Lai ZF Yamashita T Enhancement of cardiac oxidative stress by tachycardia and its critical role in cardiac hypertrophy and fibrosis J Hypertens 2006 24 2057 2069 16957567
- Tomiyama H Yamashina A Arterial stiffness in prehypertension: a possible vicious cycle J Cardiovasc Transl Res 2012 5 280 286 22223090
- Ito H Ishii K Iwakura K Impact of azelnidipine treatment on left ventricular diastolic performance in patients with hypertension and mild diastolic dysfunction: multi-center study with echocardiography Hypertens Res 2009 32 895 900 19680260
- Mottram PM Haluska BA Leano R Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease Heart 2005 91 1551 1556 16287739