Abstract
Background
Type 2 Diabetes mellitus (T2DM) is a common comorbidity in patients after heart transplantation (HTx) and is associated with adverse long-term outcomes.
Methods
The retrospective study reported here analyzed the effects of vildagliptin therapy in stable patients post-HTx with T2DM and compared these with control patients for matched-pairs analysis. A total of 30 stable patients post-HTx with T2DM were included in the study. Fifteen patients (mean age 58.6 ± 6.0 years, mean time post-HTx 4.9 ± 5.3 years, twelve male and three female) were included in the vildagliptin group (VG) and 15 patients were included in the control group (CG) (mean age 61.2 ± 8.3 years, mean time post-HTx 7.2 ± 6.6 years, all male).
Results
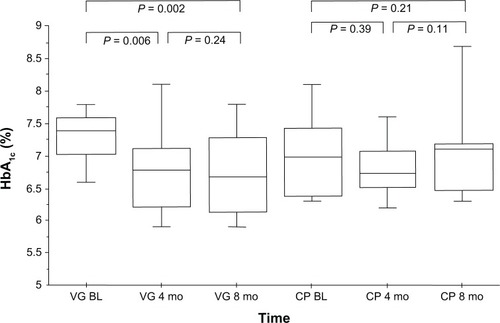
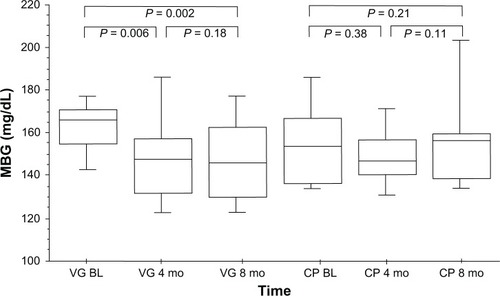
Mean glycated hemoglobin (HbA1c) in the VG was 7.4% ± 0.7% before versus 6.8% ± 0.8% after 8 months of vildagliptin therapy (P = 0.002 vs baseline). In the CG, HbA1c was 7.0% ± 0.7% versus 7.3% ± 1.2% at follow-up (P = 0.21). Additionally, there was a significant reduction in mean blood glucose in the VG, from 165.0 ± 18.8 mg/dL to 147.9 ± 22.7 mg/dL (P = 0.002 vs baseline), whereas mean blood glucose increased slightly in the CG from 154.7 ± 19.7 mg/dL to 162.6 ± 35.0 mg/dL (P = 0.21). No statistically significant changes in body weight (from 83.3 ± 10.8 kg to 82.0 ± 10.9 kg, P = 0.20), total cholesterol (1.5%, P = 0.68), or triglyceride levels (8.0%, P = 0.65) were seen in the VG. No significant changes in immunosuppressive drug levels or dosages were observed in either group.
Conclusion
Vildagliptin therapy significantly reduced HbA1c and mean blood glucose levels in post-HTx patients in this study with T2DM and did not have any negative effects on lipid profile or body weight. Thus, vildagliptin therapy presented an interesting therapeutic approach for this selected patient cohort.
Introduction
Several studies have shown that type 2 diabetes mellitus (T2DM) is a common comorbidity in patients after heart transplantation (HTx), especially due to the immunosuppressive therapy (eg, steroids, tacrolimus), and is associated with adverse long-term outcomes.Citation1–Citation3 However, metformin is often contraindicated due to renal insufficiency, whereas other conventional antidiabetic drugs such as sulfonylureas and thiazolidinediones cause adverse effects like weight gain and hypoglycemia.Citation4,Citation5 A novel approach in the therapy of T2DM is targeting the incretin system.
The incretin hormone glucagon-like peptide 1 (GLP-1) has demonstrated antidiabetic effectsCitation6,Citation7 and is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4).
Vildagliptin increases active levels of the incretin hormone GLP-1 by inhibiting DPP-4.Citation8–Citation10 It improves glucose-dependent functioning of pancreatic islet beta and alpha cells, addressing a central deficit in T2DM.Citation10,Citation11 Vildagliptin increases beta-cell sensitivity to glucose, causing an increased insulin secretory rate relative to glucose levels in both postprandial and fasting states. There is evidence that long-term vildagliptin treatment may slow underlying deterioration of beta-cell function in T2DM.Citation12,Citation13 This improvement of glycemic control is observed when vildagliptin is given as monotherapy,Citation14–Citation17 in combination with metformin,Citation18–Citation20 sulfonlyureas,Citation20,Citation21 thiazolidinediones,Citation22,Citation23 or insulin.Citation24,Citation25
Vildagliptin treatment has also been associated with beneficial extra-pancreatic effects,Citation8 including improved peripheral insulin sensitivity and improved postprandial triglyceride-rich lipoprotein metabolismCitation26 and absence of risk for weight gain.Citation27 Additionally, vildagliptin does not inhibit, induce, or undergo metabolism by cytochrome P450 enzymes and does not exhibit drug–drug interactions with other commonly prescribed agents,Citation28,Citation29 an advantage in patients with T2DM after HTx, who are typically receiving multiple concomitant medications.
A search of the PubMed database using the keywords “vildagliptin,” “heart transplantation,” and “diabetes” did not reveal any study examining the effects of vildagliptin therapy in adult HTx patients; only one case report about sitagliptin was found.Citation30
The aim of this study was to investigate the effects of clinically indicated vildagliptin therapy in chronic stable patients after HTx with T2DM in comparison to a matched control group on conventional antidiabetic therapy consisting of metformin and/or insulin. The focus was on glucose metabolism and diabetes control (glycated hemoglobin [HbA1c] and mean blood glucose [MBG]). Further, effects on concomitant laboratory values (eg, renal function, lipid profile), adverse events (eg, hypoglycemic episodes), and clinical parameters (eg, body weight) were analyzed.
Subjects and methods
Study design
This was a single-center, retrospective, nonrandomized, trial of an 8-month treatment of patients with vildagliptin (Galvus®, Novartis Pharmaceuticals, Nuremberg, Germany) versus matched controls to analyze diabetes control in heart transplant recipients. Vildagliptin was given as co-medication with oral antidiabetics (OADs) according to the manufacturer’s recommendations. All patients treated with metformin, thiazolidinediones, or insulin were given vildagliptin 50.0 mg twice daily whereas patients treated with sulfonylureas received vildagliptin 50.0 mg once daily, according to the manufacturer’s recommendation.
Inclusion criteria were stable chronic (>6 months) HTx patients with T2DM, age > 18.0 years, with HbA1c of at least 6.5%. Prior to study entry, all patients had to have been free of acute rejection or infection for at least 8 weeks. Additionally, all patients had to be followed locally at the Heidelberg HTx center. Patients with type 1 diabetes, diabetes resulting from pancreatic injury, or with secondary forms of diabetes and acute metabolic diabetic complications were excluded, as were those participating in any other study, receiving multiple solid organ transplants or who were pregnant. According to clinical routine, follow-up examinations were performed at baseline, 4, and 8 months after initiation of vildagliptin therapy.
Control subjects were matched according to the following clinical parameters: diabetes duration, diabetes therapy, HbA1c, indication for HTx, age, body weight, and time post-HTx.
Study population
The patient population consisted of 30 subjects. Fifteen patients were in the vildagliptin group (VG) and 15 were selected as control patients for matched-pairs analysis. The control patients were treated with conventional therapy including oral antidiabetics (OADs) and/or insulin. Standard diet recommendations were given to all patients (ie, Mediterranean diet) in both groups.
The primary outcome parameters were changes in HbA1c and MBG after 8 months of vildagliptin therapy. Additionally, vital signs, laboratory analyses, adverse events, body weight, acute rejections, and concomitant medication were documented during clinical routine assessments.
Immunosuppression and routine myocardial biopsies
Myocardial biopsies were performed on week 1, 2, 3, 4, 6, and 8 post-HTX, as well as on month 3, 4, 5, 6, 8, 10, and 12 post-HTX. Thereafter, biopsies were performed at biyearly intervals until year 5 (ie, at year 3 and 5 post-HTX).
All patients received a combination of a calcineurin inhibitor and mycophenolate mofetil as baseline immunosuppression (cyclosporine A target trough levels: month 1–2, 175–225 μg/L; month 3–6, 125–175 μg/L; month 7–12, 110–140 μg/L, month 13–24, 90–110 μg/L, month 24 and beyond, 70–90/50–70 μg/L [depending on rejection profile]. Tacrolimus target trough levels: month 1–2, 12–14 μg/L; month 3–6, 10–12 μg/L; month 7–12, 8–10 μg/L; month 13–24, 6–8 μg/L; month 24 and beyond, 4–6 μg/L [depending on rejection profile]. Mycophenolate mofetil target pre-dose levels: month 1–12, 2.0–4.0 mg/L and month 12 and beyond 1.5–2.5 mg/L). In combination with mammalian target of rapamycin inhibitors, different target levels were applied according to standards at the Heidelberg HTX center. Steroids were routinely administered for 6 months post-HTx (complete withdrawal was according to investigators’ discretion). All patients received post-transplantation induction therapy using anti-thymocyte globulin. Dosage and duration of therapy were adjusted according to cluster of differentiation (CD) 4 T-cell counts monitored daily during the first week post-HTx by flow cytometry, with the aim of absolute CD4 T-cell numbers below 50/μL.Citation31
Ethics and good clinical practice
The protocol was approved by the Ethics Committee of the University of Heidelberg, Germany. All patients gave written informed consent before entering the study. The trial was conducted according to good clinical practice and in compliance with the 2008 Declaration of Helsinki.
Statistical analysis
Analysis was performed using SPSS statistical software (v 14.0; IBM, Armonk, NY, USA) using the Wilcoxon signed-rank test; a two-sided P value of <0.05 was considered statistically significant. Categorical variables were compared with the Chi-square test.
Results
Study population
Between March 2010 and May 2011, 15 patients were recruited to the study for vildagliptin therapy (mean age 58.6 ± 6.0 years, mean time post-HTx 4.9 ± 5.3 years, twelve male/three female, P = nonsignificant [ns]) and 15 control patients for matched-pairs analysis (mean age 61.2 ± 8.3 years, mean time post-HTx 7.2 ± 6.6 years, 15 male) (all P = ns).
The main indications for HTx in the VG were dilated cardiomyopathy in eight patients (53.3% of group), ischemic cardiomyopathy in five patients (33.3%), amyloidosis in one patient (6.7%), and severe valvular disorder in one patient (6.7%). In the control group (CG), HTx was performed due to dilated cardiomyopathy in nine patients (60.0%) and ischemic cardiomyopathy in six patients (40.0%). Mean donor age was 38.2 ± 13.4 years in the VG versus 35.3 ± 11.0 years in the CG (P = 0.39). Donors were predominantly female (66.7% in the VG vs 53.3% in the CG, P = ns). Mean ischemic time was 228.5 ± 82.2 minutes in the VG versus 221.7 ± 65.3 minutes in the CG (P = 0.67). Additional patient baseline characteristics, including diabetic status, are described in .
Table 1 Baseline characteristics of the study population
Antidiabetic therapy
Due to the matched-pairs analysis, both groups received comparable antidiabetic therapy. In the VG group, six patients (40.0%) were given an OAD (metformin) and nine patients (60.0%) insulin, which was comparable to the antidiabetic therapy received in the CG except for one patient who received both metformin and insulin. In the VG, eight patients (53.3%) received intensive insulin therapy (IIT). In the CG, seven patients (46.7%) were treated with IIT. This basal-bolus therapy, which consists of a combination of rapid-acting and long-acting insulin, requires four to five injections per day and is considered the most physiologic way to substitute insulin. Further details regarding patients’ insulin therapy are given in .
Table 2 Antidiabetic therapy in vildagliptin and control patients
Diabetes control
During the follow-up time of 8 months, HbA1c levels in the VG decreased significantly from 7.4% ± 0.7% at baseline to 6.8% ± 0.8% at follow-up (P = 0.002, ). In the CG, HbA1c levels were 7.0% ± 0.7% at baseline and 7.3% ± 1.2% at follow-up (P = 0.21, ). Moreover, a significant reduction in MBG levels was found in the VG (165.0 ± 18.8 mg/dL at baseline vs 147.9 ± 22.7 mg/dL after 8 months [P = 0.002, ]) whereas, in the CG, no statistically significant changes in MBG levels were seen (from 154.7 ± 19.7 mg/dL at baseline to 162.6 ± 35.0 mg/dL at follow-up [P = 0.21, ]). No significant reduction in fasting plasma glucose was observed (all P = ns). No hypoglycemic event occurred in either group.
Body weight
In the VG, a trend toward a reduction in body weight was seen, from 83.3 ± 10.8 kg at baseline to 82.0 ± 10.9 kg after 8 months (P = 0.20). In contrast, in the CG, body weight was 88.3 ± 13.6 kg at baseline and 89.0 ± 12.9 kg at follow-up (P = 0.14).
Lipid profiles
There was a small reduction in triglyceride and in total cholesterol levels in the VG (triglyceride level 216.0 ± 94.0 mg/dL at baseline and 198.5 ± 122.8 mg/dL after 8 months [P = 0.65], total cholesterol 177.5 ± 25.7 mg/dL at baseline and 174.9 ± 23.7 mg/dL at follow-up [P = 0.68]) and a slow rise in high-density lipoprotein levels from 42.5 ± 7.3 mg/dL to 43.4 ± 10.3 mg/dL after 8 months in the VG (P = 0.59), but statistical significance was not reached. In the CG, there was a trend toward increased triglyceride levels (211.1 ± 125.1 mg/dL at baseline vs 295.3 ± 250.2 mg/dL after 8 months [P = 0.16]).
Other laboratory parameters
There were no significant changes in any of the remaining laboratory parameters, except for in hemoglobin levels, which increased significantly in the VG (12.4 ± 1.2 g/dL at baseline vs 13.1 ± 1.2 g/dL at follow-up [P = 0.02]).
Immunosuppression
In the VG, combination tacrolimus and mycophenolate mofetil therapy was most common (nine patients [60.0% of subgroup]) followed by an immunosuppressive regimen consisting of tacrolimus and everolimus (two patients, 13.3%). Four patients (26.7%) were treated with steroids.
Likewise, in the CG, six patients (40.0%) were treated with tacrolimus and mycophenolate mofetil combination therapy, followed by cyclosporine A and mycophenolate mofetil in two patients (13.3%), and everolimus and mycophenolate mofetil in two patients (13.3%). Three patients (20.0%) were treated with steroids (). In the VG and CG, there were no statistically significant differences regarding immunosuppressive regimens or steroids (all P = ns).
Table 3 Immunosuppression regimen in vildagliptin and control patients
Adverse events and safety
In the current study, no adverse events were observed under vildagliptin therapy; of particular note, no hypoglycemia (blood glucose < 70 mg/dL) occurred. No significant changes in immunosuppressive drug levels or dosages were found in either group (all P = ns). Additionally, no rejection episodes requiring therapy were observed during the study period.
Discussion
T2DM is a serious comorbidity in patients after HTx – especially due to the immunosuppressive therapy (eg, steroids, tacrolimus) these patients require and the increasing number of older patients with pre-existing diabetes prior to transplantation — and is associated with adverse long-term outcomes.Citation1–Citation3 Previously published studies have shown that vildagliptin increases active levels of the incretin hormone GLP-1 by inhibiting the DPP-4 enzyme.Citation8–Citation10 Moreover, it improves glucose-dependent functioning of pancreatic islet beta and alpha cells, addressing a central deficit in T2DM.Citation10,Citation11 However, as far as the authors are aware, there are no existent data on the effects of vildagliptin in adult heart transplant recipients.
In the present study, in stable HTx patients during a follow-up period of 8 months, vildagliptin reduced HbA1c and MBG levels significantly (P = 0.002), in line with previous studies.Citation15,Citation18,Citation32 Additionally, as already indicated, no hypoglycemic events occurred during the course of our study. Also in line with previously published studies, no negative effects on body weightCitation27,Citation28 or lipid profileCitation8,Citation26 were observed in our population.
Almost all patients received a combination of two immunosuppressive agents in addition to a multitude of co-medications. It has been demonstrated previously that vildagliptin does not inhibit, induce, or undergo metabolism by cytochrome P450 enzymes and does not exhibit drug–drug interactions with other commonly prescribed agents.Citation29,Citation33 As expected, no significant changes in immunosuppressive drug levels or dosages were observed. Generally, vildagliptin was well tolerated, as no adverse events or acute rejection episodes requiring therapy were observed during the study period.
As such, our study has demonstrated for the first time that vildagliptin can reduce HbA1c and MBG levels significantly in the selected population of stable heart transplant recipients with T2DM without negative effects on body weight.
Limitations
The results of this single-center pilot study are promising. However, future blinded large multicenter studies are required to confirm these findings and to evaluate the long-term effects in terms of survival. In this pilot study, only patients having had HTx were evaluated, but insufficient diabetes control is a general problem after solid organ transplantation and similar results may be anticipated in different patient populations.
Conclusion
The present study shows that vildagliptin is effective and safe in reducing HbA1c and MBG levels in adult heart transplant recipients with T2DM. Moreover, it has advantageous extra-pancreatic effects regarding body weight and lipid profile. Thus, in HTx patients with T2DM, the use of vildagliptin should be considered, particularly due to the absence of CYP3A4 interaction and the lack of negative effects on body weight in this special patient population.
Disclosure
Andreas O Doesch has received a research grant from Novartis Pharmaceuticals, Nuremberg, Germany. The authors declare no other conflicts of interest in this work.
References
- Higgins J Pflugfelder PW Kostuk WJ Increased morbidity in diabetic cardiac transplant recipients Can J Cardiol 2009 25 4 e125 e129 19340357
- Hjelmesaeth J Hartmann A Leivestad T The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events Kidney Int 2006 69 3 588 595 16395250
- Munoz E Lonquist JL Radovancevic B Long-term results in diabetic patients undergoing heart transplantation J Heart Lung Transplant 1992 11 5 943 949 1420243
- Inzucchi SE Oral antihyperglycemic therapy for type 2 diabetes: scientific review JAMA 2002 287 3 360 372 11790216
- Nesto RW Bell D Bonow RO Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association Diabetes Care 2004 27 1 256 263 14693998
- Holst JJ Orskov C The incretin approach for diabetes treatment: modulation of islet hormone release by GLP-1 agonism Diabetes 2004 53 Suppl 3 S197 S204 15561911
- Holst JJ Deacon CF Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus Curr Opin Pharmacol 2004 4 6 589 596 15525549
- Azuma K Radikova Z Mancino J Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes J Clin Endocrinol Metab 2008 93 2 459 464 18042650
- Ahrén B Landin-Olsson M Jansson PA Svensson M Holmes D Schweizer A Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes J Clin Endocrinol Metab 2004 89 5 2078 2084 15126524
- D’Alessio DA Denney AM Hermiller LM Treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin improves fasting islet-cell function in subjects with type 2 diabetes J Clin Endocrinol Metab 2009 94 1 81 88 18957505
- Mathieu C The scientific evidence: vildagliptin and the benefits of islet enhancement Diabetes Obes Metab 2009 11 Suppl 2 9 17 19385979
- Mari A Sallas WM He YL Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes J Clin Endocrinol Metab 2005 90 8 4888 4894 15886245
- Mari A Scherbaum WA Nilsson PM Characterization of the influence of vildagliptin on model-assessed-cell function in patients with type 2 diabetes and mild hyperglycemia J Clin Endocrinol Metab 2008 93 1 103 109 17925336
- Pi-Sunyer FX Schweizer A Mills D Dejager S Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes Diabetes Res Clin Pract 2007 76 1 132 138 17223217
- Ahrén B Vildagliptin: an inhibitor of dipeptidyl peptidase-4 with antidiabetic properties Expert Opin Investig Drugs 2006 15 4 431 442
- Pratley RE Jauffret-Kamel S Galbreath E Holmes D Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes Horm Metab Res 2006 38 6 423 428 16823726
- Ristic S Byiers S Foley J Holmes D Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response Diabetes Obes Metab 2005 7 6 692 698 16219012
- Bosi E Camisasca RP Collober C Rochotte E Garber AJ Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin Diabetes Care 2007 30 4 890 895 17277036
- Bosi E Dotta F Jia Y Goodman M Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus Diabetes Obes Metab 2009 11 5 506 515 19320662
- Rosenstock J Fitchet M Vildagliptin: clinical trials programme in monotherapy and combination therapy for type 2 diabetes Int J Clin Pract Suppl 2008 159 15 23 18269437
- Garber AJ Foley JE Banerji MA Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea Diabetes Obes Metab 2008 10 11 1047 1056 18284434
- Rosenstock J Kim SW Baron MA Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes Diabetes Obes Metab 2007 9 2 175 185 17300593
- Garber AJ Schweizer A Baron MA Rochotte E Dejager S Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study Diabetes Obes Metab 2007 9 2 166 174 17300592
- Fonseca V Schweizer A Albrecht D Baron MA Chang I Dejager S Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes Diabetologia 2007 50 6 1148 1155 17387446
- Fonseca V Baron M Shao Q Dejager S Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus Horm Metab Res 2008 40 6 427 430 18401832
- Matikainen N Mänttäri S Schweizer A Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes Diabetologia 2006 49 9 2049 2057 16816950
- Mathieu C Degrande E Vildagliptin: a new oral treatment for type 2 diabetes mellitus Vasc Health Risk Manag 2008 4 6 1349 1360 19337548
- Foley JE Jordan J Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience Vasc Health Risk Manag 2010 6 541 548 20730070
- Ayalasomayajula SP Dole K He YL Evaluation of the potential for steady-state pharmacokinetic interaction between vildagliptin and simvastatin in healthy subjects Curr Med Res Opin 2007 23 12 2913 2920 17931461
- Pinelli NR Nemerovski CW Koelling TM Successful long-term use of sitagliptin for the treatment of new-onset diabetes mellitus after solid organ transplantation: a case report Transplant Proc 2011 43 5 2113 2115 21693339
- Koch A Daniel V Dengler TJ Schnabel PA Hagl S Sack FU Effectivity of a T-cell-adapted induction therapy with anti-thymocyte globulin (Sangstat) J Heart Lung Transplant 2005 24 6 708 713 15949731
- Ahrén B Gomis R Standl E Mills D Schweizer A Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes Diabetes Care 2004 27 12 2874 2880 15562200
- He YL Ligueros-Saylan M Sunkara G Vildagliptin, a novel dipeptidyl peptidase IV inhibitor, has no pharmacokinetic interactions with the antihypertensive agents amlodipine, valsartan, and ramipril in healthy subjects J Clin Pharmacol 2008 48 1 85 95 17986525