Abstract
Introduction
Vascular ulcers constitute a serious global public health problem, responsible for causing a significant social and economic impact due to their recurrent, disabling nature and the need for prolonged therapies to cure them.
Objective
To evaluate the use and efficacy of the rhEGF in the epithelialization of patients with a diagnosis of CEAP stage 6 venous insufficiency, in the two regimes of the health system in Colombia, the contributive (equivalent to a health system where citizens with payment capacity contribute a percentage of their salary) and the subsidized (equivalent to a health system where the state covers the vulnerable population and low socioeconomic level) versus the other treatments used.
Methodology
Observational, descriptive, retrospective, multicenter study, in which 105 medical records with 139 ulcers were reviewed, in 2 centers, one belonging to the subsidized system and the other to the contributive system in Colombia.
Results
The association with the epithelialization variable of the different treatment groups for ulcers according to the application of the mixed effect model test, for both regimes was for the Biologicals (EC 34.401/p = 0.000), Bioactive Agents (Hydrogels) (EC 24.735/p = 0.005) groups; for the rest of the treatment groups, the results were neither associated nor statistically significant.
Conclusion
Intra- and perilesional therapy with rhEGF expands the therapeutic spectrum in patients with venous ulcers, regardless of the type of health system in which it will be applied, shortening the healing time and reaching a possible therapeutic goal, which according to this study there is an association with epithelialization regardless of the regime applied.
Introduction
Venous vascular ulcers are a pathology difficult to treat. They are a serious public health problem with major health and socio-economic repercussions, venous ulcers are the most common type of chronic ulcers on the lower extremities, and their prevalence is between 1% and 3% if active and cured ulcers are considered.Citation1 The prevalence of active venous ulcer alone ranges between 0.2% and 0.3% in the adult population of Western countries.Citation2
On average, 33–60% of these ulcers persist for more than 1 year.Citation3 Venous ulcers are usually recurrent and generate high costs in their treatment, which directly impacts annual health care budgets. Furthermore, in most cases this type of ulcers occur in patients with a low socioeconomic and educational level.Citation4
The current standard of care for venous ulcers implies the use of compression bandages to counter venous hypertension. However, these approaches only cure 50–60% of venous ulcers within 6 months of treatment.Citation5 Nevertheless, with the acquired knowledge about the proteomic physiology that causes this complication, local adjuvant treatments have been developed such as biologic dressings and biomolecular engineering products such as growth factors and therapies with tissues. These products interact directly with the wound to accelerate the healing process and reduce the time to complete its cure.Citation6
Epidermal growth factors (EGF) are endogenous signaling molecules that regulate cellular responses to the wound healing process, which are secreted by platelets, leukocytes, fibroblasts and epithelial cells,Citation7 when there is an imbalance between pro- and anti-inflammatory cytokines, wounds tend to become chronic, remaining in an uncontrolled and persistent inflammatory phase,Citation8 the (EGF) exerts a specific function that results in the regulation of metabolism, differentiation, growth, proliferation and cell survival,Citation9 thus stimulating the migration and proliferation of fibroblasts, suggesting a useful therapeutic strategy for wound healing,Citation10 genetic recombination allows recombinant human Epidermal Growth Factor (rhEGF) to be available in pure, precise and stable concentrations as a drug in 75 mg vials for intra- and perilesional administration,Citation11 with a theoretical basis for its use as a promoter of the healing of wounds such as venous ulcers, which due to its high prevalence and requirement for prolonged treatments, it is necessary to find treatment alternatives capable of shortening the healing time that are cost effective and safe,Citation12–14 with granulation and epithelialization results in less time.Citation15,Citation16
In Colombia, there is the Colombian Guide for the Diagnosis and Management of Chronic Venous Disorders (GCDMDCV), which has been developed and updated by Angiology and Vascular Surgery – Asovascular which, since 2009, determines the type of therapeutic management that this type of pathology should have,Citation17 in addition, a series of recommendations were also generated by a group of experts for the use of rhEGF, with intralesional and perilesional application in venous ulcers, both in daily practice and in the generation of new studies with this medication, which are base documents for the treatment of this pathology in the Colombian health system.Citation18
The Colombian health system is a solidarity system where, in addition to taxes, citizens contribute a percentage of resources according to their work activity and is divided into two regimes based on its financing form. In the contributive regime, which has broad coverage, citizens contribute a percentage of their salary and their access to health services is broader. This regime is equivalent worldwide to health systems that are financed with contributions from citizens for their work activity, with coverage and service according to their contribution. On the other hand, there is the subsidized regime, which although having similar coverage, the beneficiary citizens do not work, are in a state of poverty, vulnerability, and low socioeconomic status, therefore they do not contribute any money to the health system. This regime is financed by taxes and contributions from the salaries of the rest of the citizens of the contributive regime. The subsidized regime is equivalent worldwide to health systems financed by the state through taxes, its accessibility to health services is limited and most of the population belongs to this regime.Citation19,Citation20
For the local management of ulcers, this type of treatment is very variable and dynamic considering the different states of the natural healing process: necrotic, fibrinous, exudative, infectious, granulation and/or epithelialization. The protocols managed by the two regimes of the Health System are based on the aforementioned guidelines using different types of dressings depending on the injury,Citation6 varying the time between healing sessions, depending on the group of dressings used, which can range from 3 to 7 days.Citation21
In our country, EGF therapy is already used in the treatment of recalcitrant and complex venous ulcers,Citation22 and in the Colombian health system (which has the two regimens mentioned above), it seeks to provide the most complete coverage possible to the entire population, which is why the EGF is within the list of medications covered by the system in the two regimes, called the Benefit Plan (PBS), but due to the administrative nature, the authorization processes, the vulnerability of the population, the geographical situation of the citizens in the subsidized regime, many of them cannot access specialized services including EGF medication.Citation19
In a previous study that evaluated the impact on the results of epithelialization of venous ulcers between patients of both regimes, it was found that in the contributive regime there was 5.8 times higher probability of ulcer epithelialization than in the subsidized regime group (p = 0.0000), which we believe may be due to the ease of prescription and administrative access by users, specifically to rhEGF therapy in contrast with other evaluated dressings, creams, and tinctures.Citation23 For this reason, we defined for this study how effective this type of therapy is with respect to an outcome variable such as epithelialization, in addition to believing that the specific administrative barriers for the rhEGF influence the statistically significant result of the contributive regime compared to the subsidized regime and other dressings included in the treatment of venous ulcer.
Objective: Evaluate the use and efficacy of rhEGF in the epithelialization of patients with a diagnosis of CEAP stage 6 venous insufficiency, in the two regimes of the health system in Colombia (Contributive and Subsidized) versus the other treatments used.
Methodology: Observational, descriptive, retrospective, multicenter study, in which 105 medical records of patients with a diagnosis of CEAP stage 6 chronic venous insufficiency were reviewed, having 139 ulcers, who consulted for vascular surgery in 2 centers, one belonging to the subsidized system and the another to the contributive system in Colombia in a period of 4 years, with the use of dressings, creams, gels and medications specifically rhEFG for intralesional and perilesional administration, for the treatment of ulcers to evaluate them, in terms of epithelialization and closure.
Inclusion Criteria
Adult patients over 18 years of age.
Patients diagnosed with venous ulcers of the lower limbs secondary to venous disease, evaluated by a specialist doctor (vascular surgeon) and treated according to GCDMDCV.
Patients with at least one wound that met CEAP stage 6 venous classification criteria, either diagnosed by clinical evaluation or the use of diagnostic aids such as venous duplex ultrasound of the lower extremities.
Patients with time between healing sessions ranging between 3 and 7 days.
Patients for whom the ulcer linear measurement has been reported in their clinical record at all visits.
Ankle-brachial index greater than 0.8.
Patients with information from the complete clinical record for each variable.
Patients who have received compression therapy as part of their treatment.
Exclusion Criteria
Patients with an ankle-brachial index with values less than 0.8.
Patients with diagnosed arterial occlusive disease.
Patients with a diagnosis of arterial, mixed, neuropathic, or diabetic foot ulcer.
Patients for whom the ulcer linear measurement has not been reported in their clinical record.
Patients without complete information in the clinical record for the variables to be studied.
Patients who were not receiving compression therapy as part of their treatment.
Variables
The variables considered were classified as follows: demographic variables (age, sex, socioeconomic level, and diet), variables related to the characteristics of the ulcer (number of ulcers, time of evolution, anatomical location and size), variables related to the treatment, (dressings used, creams used, frequency of healing, patients treated) and outcome variables such as epithelialization and closure percentage.
Collection Techniques and Instruments
The main source of clinical information for each patient was their medical history. Data were collected using the pre-established EXCEL form for the predetermined period of 4 years, evaluating the inclusion and exclusion criteria. Medical records that did not meet the criteria were excluded, those that met the inclusion criteria were reviewed and the database was filled with parameterized and adjusted records. A demographic and descriptive analysis of the database was carried out. For the analysis, the test for mixed effect models was used and for this analysis program R-4.3.3 was used.
Due to the great variability of effects that the different groups of dressings have, among the groups and the dressings within the same group, also adding the two regimes, in contrast to the epithelialization of venous ulcers, the mixed effect model was used, grouping the dressings, subsequently recoding the variables dichotomously, dividing the results into Subsidized Regime, Contributory Regime and in the total of the two regimes to evaluate the association of each of the groups with the epithelialization variable.
Results
The characteristics of the study population as well as the results obtained are described below.
Characterization of the Study Population
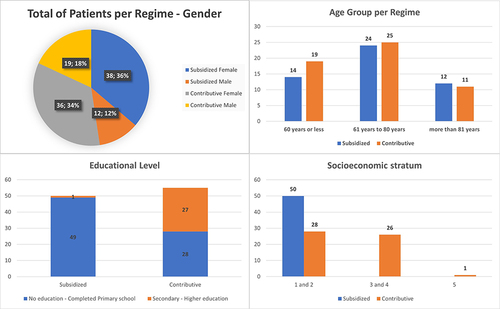
Of the 105 patients, 50 (47.62%) were in the subsidized regime, and 55 (52.38%) in the contributive regime. Other demographic variables are shown in .
Ulcer Characterization
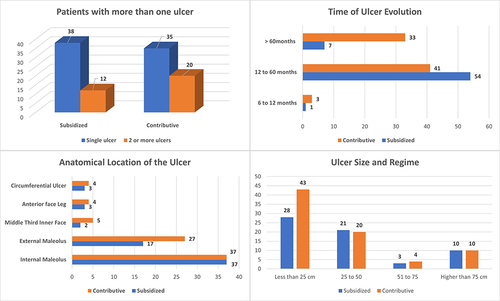
In the 105 patients, 139 ulcers were identified, of which 62 (44.60%) are from patients in subsidized regime, and 77 (55.4%) are from patients in contributive regime patients. describes the number of ulcers per patient, the evolution time, the anatomical location, and size of the ulcer.
Among the treatments found in the review, there are dressings and other technologies such as hydrocolloid type bioactive agents, alginates, and hydrogels. Mixed dressings such as antimicrobial and biocellulose dressings, and interactive dressings such as hydrophilic foams and other elements such as creams, tinctures, and biological drugs, specifically rhEFG, were also found. ()
Table 1 Use in Patients and Frequency of Use of Dressings and Other Technologies / Gel - Tinctures and Creams - Contributory and Subsidized Regime
According to the previous table and for analysis purposes, the different types of treatment were grouped, as can be seen in : bioactive agents (hydrocolloids) with 81.9%; mixed agents (antimicrobials) with 61.9%; biological agents (rhEFG) with 47.62%; bioactive agents with 24.76%; bioactive agents (alginates) with 18.1%, interactive agents (hydrophilic foams) with 12.38%; passive agents with 11.42%; mixed agents (biocellulose dressings) with 9.53%; and creams and tinctures with 139%. However, this last group was not considered in the analysis due to its difficulty in standardization.
Table 2 Number of Patients Treated for Each of the Dressing Groups, Number of Cures Performed - Contributive and Subsidized Regime
We can see the relationship between ulcer size and epithelialized ulcers for each treatment group and by regimen, as well as a correlation with the time of evolution. ()
Table 3 Dressing Groups Vs Epithelialization Groups and Initial Ulcer Size
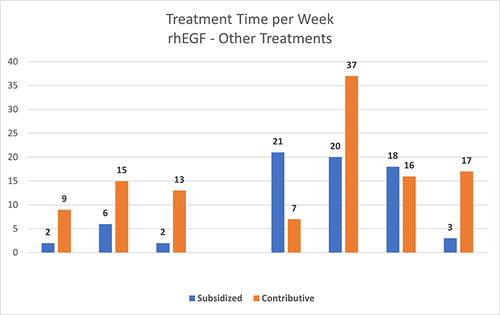
shows the comparison of treatment time used in the 139 ulcers according to the medical history, between rhEGF and the other therapies.
Figure 3 The comparison of treatment time used in the 139 ulcers between rhEGF and the other therapies.
Regarding the results of the application of the mixed effect model, in the Subsidized Regime it was seen that for the Biologicals (Estimated Coefficient (EC) of 1.053/p = 0.2519), Bioactive Agents (Hydrocolloids) (EC of 1.957/p = 0.1328); Bioactive Agents (Alginates) (EC of 39.87/p = 0.9977) groups, they are associated with the epithelialization of ulcers but are not statistically significant. The Bioactive Agents (Hydrogels), Mixed Agents (Antimicrobial Agents), Interactive Agents (Hydrophilic Foams), Mixed Agents (Biocellulose Dressings), Passive Agents and Creams and Tinctures groups are not associated with epithelialization for the Subsidized Regime.Citation9
In the Contributory Regime it was seen that for the Biologicals (EC of 408.162/p = 0.998), Bioactive Agents (Hydrogels) (EC of 398.451/p = 0.998); Mixed Agents (antimicrobial agents) (EC of 211.157/p = 0.999), Passive Agents (EC of 167,608)/p = 1000), Mixed Agents (Biocellulose Dressings) (EC of 47,350/p = 1000), Creams and Tinctures (EC of 27,992/p = 1000) groups are associated with ulcer epithelialization but are not statistically significant. The Bioactive Agents (Hydrocolloids), Bioactive Agents (Hydrogels), Interactive Agents (Hydrophilic Foams) groups are not associated with epithelialization for the Contributory Regime.
And for both regimes in the Biologicals (EC of 34.401/p = 0.000), Bioactive Agents (Hydrogels) (EC of 24.735/p = 0.005) groups, they are associated with epithelialization and for both regimes and are statistically significant. The other groups are not associated with epithelialization. See .
Table 4 Results of the Application of the Mixed Effect Model
Therefore, among all the treatment and dressing groups for the treatment of this type of patients, the Biologicals group showed a statistically significant difference associated with epithelialization in the calculation of the entire 2 regimes in conjunction with the Bioactive Agents (Hydrogels) group.
Discussion
Regarding the demographic data found in this study, we can see that these are consistent with global studies, such as the predominance in the female sex,Citation24 the average age is in the ≥60 years range, consistent with what has been reported by most authors. In his study, KaranicolicCitation25 mentions that this range was 63 years and Beltrán reports an average of 65 years;Citation26 which are similar data and confirm that most cases of venous ulcers occur in this age range. Regarding the educational level it was found that more than 50% of the people with this pathological entity have a low or medium socioeconomic level and a lowest educational level, according to the research data considered.Citation27–29
The literature shows that these ulcers can be of variable size, from small to very extensive, and sometimes circumferential, surrounding the entire leg. They can also be single or multiple (tending to join) and can be bilateral.Citation30 Regarding the wound area, time of evolution and size, our study found that the predominant area was the internal and external perimalleolar area, the wound time of evolution was within the range of 6 to 12 months, and the most frequent diameters were greater than 25 cm2. This is consistent with the literature reporting that wounds larger than 5 cm2, lasting about 12 months, are poor healing diagnosis factors.Citation31 This is directly related to the chronic nature of these wounds and, therefore, a longer healing time. The literature mentions that only 50% heal within the first 4 months, 20% up to 2 years and 8% heal completely after 5 years.Citation32
The treatment of these wounds is a dynamic process that depends on systematic evaluations, different frequency prescriptions and the type of cure or coverage necessary, which can be variable depending on the evolutionary movement of the healing process.Citation33 Compression therapy is crucial for the healing of venous leg ulcers. Different researchers have agreed that it is an essential therapeutic measure for the treatment of venous ulcers.Citation34,Citation35 In addition, these ulcers require a prolonged treatment time to achieve total healing, along with topical treatment that combines compression and topical therapy;Citation33 however, the literature is prolific when it comes to determining the best dressing to achieve the healing process. In the meta-analysis published by Cochrane in 2017, which included 78 RCTs (7014 participants) with topical agents and dressings, it was not possible to determine whether dressings or topical agents improve the probability of healing of venous leg ulcers.Citation36 Taking into account that this type of ulcers involves many complex factors, such as local and systemic factors, and the variability of organs and species in response to the wound, there is the Health System factor (reference), which, as we see in this study, in health systems like the one in Colombia, although it has a broad coverage that includes all the elements studied in this study, there is limited accessibility in the subsidized regime for high-tech treatments such as the rhEFG.
Seeking to obtain a quick cure, the use of growth factors has been promoted in the last decade, and based on the literature, experts suggest that EGF can induce healing of various types of skin wounds, including venous ulcers. At first, the topical administration of EGF was recommended for different types of ulcer, but the state of evidence is moderate for its use in venous ulcers, although with a weak degree of recommendation.Citation37 However, a review and recommendation of experts specifies that perilesional and intralesional application is the most appropriate recommendation for the treatment of venous ulcers.Citation18
Our study showed that all 105 patients had 139 ulcers. When analyzing the association with epithelialization, 66 ulcers were found in 50 patients treated with the Biologicals group (rhEGF), with 47 ulcers being epithelialized, finding a relationship between epithelialization and this treatment that was 34.4 times greater, being statistically significant for the group when the two regimes are evaluated at the same time, like the Bioactive Agents (Hydrogels) group, which gives a probability of 24.7 times, but when we see them in the evaluation by each of the (Contributory and Subsidized) regimes, despite the fact that no statistical significance is found in the Biologicals (rhEGF) group, it shows an association in both regimes with more strength in the Contributory regime with respect to the Subsidized one that the Bioactive Agents (Hydrogels) group does not show. This allows us to conclude that the contribution of rhEGF to epithelialization in these patients is statistically significant compared to other treatment groups and corroborates the conclusions of the study by Cacua et alCitation23 which states that belonging to the Contributory Regime provides a protective factor for epithelialization of the ulcer, which may be due to greater access to rhEGF by patients, but rhEGF also has an important role in the epithelialization of this type of ulcers.
Another aspect that must be considered is the treatment time. This variable is a determining factor in the treatment of this pathology, mainly from an economic point of view. For rhEGF, treatment time was achieved in no more than 16 weeks for 31.92%, 8 weeks for 44.68% and less than 8 weeks for 23.41% of the 47 ulcers that were epithelialized with this therapy.
Our study demonstrates that therapy with recombinant human epidermal growth factor has a positive impact on the closure of venous ulcers, regardless of the type of health system in which it is applied, due to its same efficacy in complex ulcers, but its result is more significant in a health system that provides adequate accessibility to this type of technology with adequate follow-up in its use and application, benefiting patients with this type of ulcers.
Limitations
The main limitation of the study was the underreporting of medical records, which meant that many medical records were not included in the study due to missing data. Furthermore, due to its retrospective nature, it was not possible to perform standardized comparisons to define the association with the outcome variable more clearly; however, many comparative therapies could be included that would have been expensive and complicated to include in a prospective study. It can be concluded that since it is a real-world study, its results can help to determine recommendations for treatment options for this pathology.
Conclusions
Intra- and perilesional therapy with rhEGF expands the therapeutic spectrum in patients with venous ulcers, regardless of the type of health system in which it will be applied, shortening the healing time and reaching a possible therapeutic goal, which according to this study there is an association in epithelialization regardless of the regime applied. This result influences the conclusion of the benefit of belonging to the Contributory Regime in the first part of this study regarding epithelialization.Citation23 However, it is necessary to delve deeper into these types of studies to determine from such a wide variability how different types influence health systems in the therapeutic results of different treatments.
In health systems that have the characteristics of the Colombian subsidized regime (population with low socioeconomic level, financed with public resources, monitored by the state) it is more complicated to have clinical health results, which can be verified with the application of the rhEGF, which despite its efficacy, its result was more significant in a health system that is equivalent, worldwide, to a health system financed by salaried citizens and by working people with payment capacity.
Ethics Committee
The CEDIFF Research Ethics Committee is an Ethics Committee independent of the study institutions in Bogotá, which is governed by the national regulations of Colombia, as well as international guidelines applicable to research with human beings. The approval of this study by this Ethics Committee is based on ethical and legal regulations; and the information management is part of purely statistical processes and does not pose a risk to the integrity or confidentiality of the research subjects.
Disclosure
The authors report no conflicts of interest in this work.
References
- Cornwall JV, Doré CJ, Lewis JD. Úlceras de pierna: epidemiología y etiología. Br J Surg. 1986;73(9):693–696. doi:10.1002/bjs.1800730905
- Fowkes FGR. Epidemiology of venous ulcer. Phlébologie. 1999;52:377–382.
- Briggs M, Flemming K. Viviendo con úlceras en las piernas: una síntesis de la investigación cualitativa. J Adv Nurs. 2007;59(4):319–328. doi:10.1111/j.1365-2648.2007.04348.x
- González Gabriela O, Norstrom Caroline A, Asuaga Miguel M. Úlceras de miembros inferiores: características clínico-epidemiológicas de los pacientes asistidos en la unidad de heridas crónicas del Hospital de Clínicas. Rev Méd Urug. 2012;28(3):182–189.
- Lyon RT, Veith FJ, Bolton L, Machado F. Clinical benchmark for healing of chronic venous ulcers. Venous ulcer study collaborators. Am J Surg. 1998;176(2):172–175. doi:10.1016/S0002-9610(98)00136-6
- Senet P. Local treatment of venous leg ulcers. Phlebolymphology. 2010;17(2):87–94.
- Han C-M, Biao C, Pan W. Clinical guideline on topical growth factors for skin wounds. Burns Trauma. 2020;8:tkaa035. doi:10.1093/burnst/tkaa035
- Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care. 2015;4(9):560–582. doi:10.1089/wound.2015.0635
- Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019;7(10). doi:10.1186/s41038-019-0148-1
- You DH, Nam MJ. Effects of human epidermal growth factor gene-transfected mesenchymal stem cells on fibroblast migration and proliferation. Cell Prolif. 2013;46(4):408–415. doi:10.1111/cpr.12042
- Esquirol Caussa J, Herrero Vila E. Un enfoque para el tratamiento de las úlceras de origen vascular: revisión y papel del factor de crecimiento epidérmico. Angiología. 2016;68(4):322–330. doi:10.1016/j.angio.2015.10.002
- Acosta Reyes M, López A, Álvarez O, González A, Acosta Altamirano G. Evaluación económica del uso de factor de crecimiento epidérmico recombinante humano (FCErh) en población Mexicana con pie diabético en el Sector Salud. Rev Hosp Jua Mex. 2014;81(3):147–153.
- Romero Prada M, Roaa C, Alfonso P, Acero G, Huerfano D, Vivas C. Cost-effectiveness analysis of the human recombinant epidermal growth factor in the management of patients with diabetic foot ulcers. Diabetic Foot Ankle. 2018;9(1):1480249. doi:10.1080/2000625X.2018.1480249
- Osorio D, Yanilke J. Cost-utility analysis of recombinant human epidermal growth factor versus negative pressure therapy in the treatment of complicated diabetic foot ulcers in the Colombian setting. 2021.
- Sánchez María Teresa C. Giraldo Luis Fernando Vascular; Experience With the Use of Perilesional and Intralesional Recombinant Human Epidermal Growth Factor (Nepidermin) in the Treatment of Patients With Chronic Venous Ulcers. Vascular Dis Manage Dis Management. 2019;16(1):E3–E8.
- Cacua Sanchez MT, Giraldo LF, Díaz JA. Efficacy of Human Recombinant Epidermal Growth Factors vs Conventional Therapy for the Treatment of Chronic Venous Ulcers: a Retrospective Case Series. Wounds. 2021;33(2):41–49.
- Pizano N, et al. Guías Colombianas para el diagnóstico y el manejo de los desórdenes crónicos de las venas. Asociación Colombiana de Angiología y Cirugía Vascular. 2009.
- Cacua Sanchez MT, Vargas Abello LM, Á O, et al. Use of Intralesional and Perilesional Human Recombinant Epidermal Growth Factor (hrEGF) in the Local Treatment of Venous Ulcer - Review Article – expert Recommendation. Vasc Health Risk Manag. 2023;19:595–603. doi:10.2147/VHRM.S417447
- Guerrero R, Gallego AI, Becerril-Montekio V, Vásquez J. The health system of Colombia. Salud Publica Mex. 2011;53(2):S144–S155.
- Seguí Gómez M, Toledo Atucha EA, Juan Jiménez Moleón J. Sistemas de salud. Modelos: Elsevier España, S.L; 2013.
- Gneaupp; Apósitos para el Tratamiento de Úlceras y Heridas Cutaneas y Crónicas; Available from: https://gneaupp.info/apositos-para-el-tratamiento-de-ulceras-y-heridas-cutaneas-y-cronicas/. Accessed May 22, 2024.
- Guerrero R, Gallego AI, Becerril-Montekio V, Vásquez J. Sistema de salud de Colombia. Salud Pública de México. 2011;53(Supl. 2):s144–s155.
- Cacua M, Buenahora G. Socio-Demographic Characteristics and Associated Factors of Morbidity in Patients with Venous Ulcers Treated in Two Institutions of Contributive and Subsidized Regime in Colombia: retrospective, Multicenter. Observational Study Vascular Health Risk Management. 2022;18.
- Abbade LPF, Lastória S. Management of patients with venous leg ulcer. An Bras Dermatol. 2006;81(6):509–522. doi:10.1590/S0365-05962006000600002
- Karanikolic V, Karanikolic A, Petrovic D, Stanojevic M. Prognostic factors related to delayed healing of venous leg ulcer treated with compression therapy. Dermatologica Sinica. 2015;33(4):206–209. doi:10.1016/j.dsi.2015.04.005
- Beltrán V, Bulla A, Espitia N, Vargas D, Vargas L. Characteristics of patients with recurrence of ulcer due to chronic venous. Ciencia e Innovación en Salud. 2021;118:088–098. doi:10.17081/innosa.118
- Moffatt CJ, Franks PJ, Doherty DC, Smithdale R, Martin R. Sociodemographic factors in chronic leg ulceration. Br J Dermatol. 2006;155(2):307–312. doi:10.1111/j.1365-2133.2006.07265
- Otero González G, Agorio Norstrom C. Miguel Martínez Asuaga. Úlceras de miembros inferiores Características clínico-epidemiológicas de los pacientes asistidos en la unidad de heridas crónicas del Hospital de Clínicas. Rev Méd Urug. 2012;28(3):182–189.
- Heinen MM, Persoon A, Kerkhof PV, Otero M, Achterberg TV. Ulcer-related problems and health care needs in patients with venous leg ulceration: a descriptive, cross-sectional study. Int J Nurs. 2007;44(8):1296–1303. doi:10.1016/j.ijnurstu.2006.05.001
- Minguez M, Lizundia S, Sáenz E. Manejo de la úlcera vascular de los miembros inferiores Servicio Central de Publicaciones del Gobierno Vasco. Vitoria. 1996.
- Nicolaides AN. Cardiovascular Disease Educational and Research Trust; European Society of Vascular Surgery; The International Angiology Scientific Activity Congress Organization; International Union of Angiology; Union Internationale de Phlebologie at the Abbaye des Vaux de Cernay. Investigation of chronic venous insufficiency: a consensus statement. Circulation. 2000;102(20):E126–63. doi:10.1161/01.cir.102.20.e126
- Kantor J, Margolis DJ. Management of leg ulcers. Semin Cutan Med Surg. 2003;22(3):212–221. doi:10.1016/S1085-5629(03)00043-9
- Guimarães Barbosa JA, Nogueira Campos LM. Directrices para el tratamiento de úlcera venosa. Enfermería Global. 2010;(20). doi:10.4321/S1695-61412010000300022.
- Ávila P C. Terapéutica de la Compresión en el tratamiento de la úlcera de etiología venosa. Rev Todoheridas. 2010;1(1):4–16.
- O’Donnell TF, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery_ and the American Venous Forum. J Vascular Surgery. 2014;60(2S):3S–57S. doi:10.1016/j.jvs.2014.04.049
- Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev. 2018;(6). doi:10.1002/14651858.CD012583.pub2
- Han C-M. Grupo de redacción de la guía del factor de crecimiento en nombre de la Asociación China de Quemaduras, Guía clínica sobre factores de crecimiento tópicos para heridas en la piel. Burns Trauma. 2020;8:tkaa035. doi:10.1093/burnst/tkaa035