Abstract
Background
The Wnt/β-catenin/T cell factor (TCF) signaling pathway is important in the development of nonmelanoma skin cancers (NMSCs). Nitric-oxide-releasing nonsteroidal anti-inflammatory drugs (NO-NSAIDs) are chemopreventive agents consisting of a traditional NSAID attached to an NO-releasing moiety through a chemical spacer. Previously we showed that an aromatic spacer enhanced the potency of a particular NO-NSAID compared to an aliphatic spacer.
Methods
We synthesized an NO-releasing NSAID with an aromatic spacer (flurbiprofen benzyl nitrate, NBS-242), and using the human skin cancer cell line A-431, we evaluated its effects on cell kinetics, Wnt/β-catenin, cyclin D1, and caspase-3.
Results
NBS-242 inhibited the growth of A-431 cancer cells, being ~15-fold more potent than flurbiprofen and up to 5-fold more potent than NO-flurbiprofen with an aliphatic spacer, the half maximal inhibitory concentrations (IC50) for growth inhibition being 60 ± 4 μM, 320 ± 20 μM, and 880 ± 65 μM for NBS-242, NO-flurbiprofen, and flurbiprofen, respectively. This effect was associated with inhibition of proliferation, accumulation of cells in the G0/G1 phase of the cell cycle, and an increase in apoptotic cell population. NBS-242 cleaved β-catenin both in the cytoplasm and the nucleus of A-431 cells. NBS-242 activated caspase-3 whose activation was reflected in the cleavage of procaspase-3. To test the functional consequence of β-catenin cleavage, we determined the expression of cyclin D1, a Wnt-response gene. NBS-242 reduced cyclin D1 levels in a concentration dependent manner.
Conclusion
These findings establish a strong inhibitory effect of NBS-242 in A-431 human epidermoid carcinoma cells. NBS-242 modulates parameters that are important in determining cellular mass.
Introduction
In the USA, nonmelanoma skin cancers (NMSCs) are increasing by about one million new cases annually.Citation1 The Wnt/β-catenin/T cell factor (TCF) signaling pathway has been shown to be important in the development of many cancers including colon, prostate, leukemia, and others. The Wnt/β-catenin pathways are also implicated in the development of malignancy in NMSCs such as squamous cell carcinoma (SCC) of the skinCitation2,Citation3 and basal cell carcinoma (BCC).Citation4 Mouse models of induced skin tumors have provided evidence of constitutive activation of β-catenin/TCF signaling.Citation5 β-catenin is a multifunctional protein that controls a number of cell activities, both at the membrane and the nuclear level. In the nucleus, β-catenin interacts with the TCF family of transcription factors. Elevated β-catenin/TCF-4 signaling is correlated with activation of its downstream target cyclin D1 and accelerated entry from the G1 phase into the S phase of the cell cycle. The upregulation of cyclin D1 is generally accepted as the basis for β-catenin’s proliferative and oncogenic effect.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are considered to be the prototypical cancer chemopreventive agents.Citation6 A number of randomized, double-blind trials of aspirin against colorectal adenomas have established their chemopreventive effect.Citation7–Citation9 Recently, Rothwell et alCitation10 reported that even daily use of low dose aspirin resulted in reductions in cancer incidence and mortality. Moreover, they also reported that aspirin prevented distant metastasis.Citation11 For skin cancer, NSAIDs have been shown to have a protective effect against SCC and BCC.Citation12 Interestingly, the data suggested that a shorter duration of use offered a better protective effect than a longer duration of use. Recently, it was shown that celecoxib, an NSAID which selectively inhibits the enzyme cyclo-oxygenase-2, may be effective for prevention of SCCs and BCCs in individuals who have extensive actinic damage and are at high risk for development of nonmelanoma skin cancers.Citation13 A recent study concluded that long-term use (>5 years) of NSAIDs, especially aspirin, was associated with a significantly decreased risk of developing cutaneous melanoma.Citation14 However, regular NSAID use may lead to serious side effects encompassing the gastrointestinal, renal, and cardiovascular systems.Citation15–Citation17 These potential life-threatening side effects are limiting to the widespread use of NSAIDs as potential chemopreventive agents.
Nitric-oxide-releasing NSAIDs (NO-NSAIDs) are an emerging new class of chemopreventive agents. They consist of a traditional NSAID attached to an NO-releasing moiety through a chemical spacer. AnimalCitation18,Citation19 and humanCitation20,Citation21 studies have shown that many NO-NSAIDs are safer to the gastrointestinal mucosa than the parent NSAID. We had previously shown that an aromatic spacer enhanced the potency of a particular NO-NSAID compared to an aliphatic spacer.Citation22 At the cellular level, our previous studies have also demonstrated that the chemopreventive effects may be mediated through proapoptotic and antiproliferative effects of NO-NSAIDs in various cancer cell lines of different cell types.Citation23,Citation24 Hence, for these studies, we synthesized an NO-releasing arylpropionic acid NSAID, comprising an aromatic spacer and evaluated it against the human skin cancer cell line, A-431 which expresses β-catenin. In this study, we compared the effects of the NSAID flurbiprofen, NO-releasing-flurbiprofen containing an aliphatic spacer, and the newly synthesized flurbiprofen benzyl nitrate (FBN, NBS-242), which has an aromatic spacer on cell growth inhibition. The effect of FBN on cell proliferation, apoptosis, and cell cycle was examined with a focus on the Wnt pathway components.
Materials and methods
Reagents and cell culture
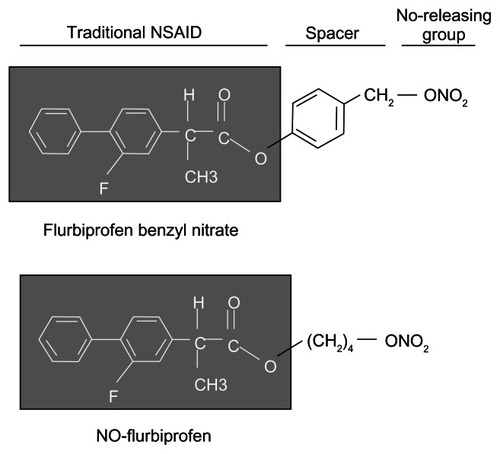
NO-flurbiprofen (4-(nitrooxy)butyl 2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanoate) and flurbiprofen benzyl nitrate (4-O-[2-(3-fluoro-4-phenyl)phenyl propionyl]-4-hydroxybenzyl nitrate, NBS-242) () were synthesized and purified as described previously.Citation25 Stock solutions (100 mM) were made in dimethyl sulfoxide (DDDT); the final DDDT concentration was adjusted in all media to 1%. Flurbiprofen and other fine chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA). A-431 human epidermoid carcinoma (ATCC, Manassas, VA, USA) cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 unit/mL of penicillin, and 100 μg/mL of streptomycin in a humidified atmosphere containing 5% CO2 and 95% air at 37°C.
Determination of cell viability by MTT assay
Cell growth inhibition was measured using an MTT kit (Roche, Indianapolis, IN, USA). A-431 cells were plated at a density of 7000 cells/well in a 96-well plate. After overnight incubation, cells were treated with vehicle or various concentrations of the test compounds for 24 hours after which viable cells were quantified according to the manufacturer’s instructions. Growth inhibition was expressed as percentage of the corresponding control.
Proliferating cell nuclear antigen (PCNA) staining for cell proliferation
A-431 cells (8000 cells/well) were treated for 24 hours with various concentrations of FBN. Cell proliferation was assessed by measuring the levels of PCNA using a PCNA enzyme-linked immunosorbent assay kit (Calbiochem, La Jolla, CA, USA), in accordance with the manufacturer’s protocol.
4,6-diamidino-2-phenylindole (DAPI) staining for cell death analysis
Chromatin condensation for detection of apoptosis was determined by DAPI fluorescence. Cells were cultured in the growth medium in the presence or absence of FBN for 24 hours. Cells were washed three times with phosphate-buffered solution (PBS), fixed in a 3.7% formaldehyde solution for 10 minutes, fixed once in 1 mL of methanol, stained, and mounted in a fluid containing 2 mg/mL DAPI (Oncor Inc, Gaithersburg, MD, USA). Results were determined by visual observation of nuclear morphology via fluorescence microscopy.
Cell cycle analysis
A-431 cells were treated for 24 hours with various concentrations of FBN. Cell cycle phase distributions of control and treated cells were obtained using a Coulter Profile XL, (Beckman Coulter, Inc., Fullerton, CA, USA) equipped with a single argon ion laser. For each subset, >10,000 events were analyzed. All parameters were collected in list mode files. Data were analyzed on a Coulter XL Elite Workstation, (Beckman Coulter, Inc.) using the software programs Multigraph and Multicycle. Cells (0.5 × 106) were fixed in 100% methanol for 10 minutes at −20°C, pelleted (5000 rpm for 10 minutes at 4°C), resuspended, and incubated in PBS containing 1% FBS/0.5% NP-40 on ice for 5 minutes. Cells were washed again in 500 μL of PBS/1% FBS containing 40 μg/mL propidium iodide (used to stain for DNA) and 200 μg/mL RNase type IIA, and analyzed within 30 minutes by flow cytometry. The percentage of cells in the G0/G1, G2/M, and S phases was determined from DNA content histograms.
Cytoplasmic and nuclear protein extraction
To obtain nuclear protein extracts, cell pellets were resuspended in 500-μL lysis-buffer I (10 mM Tris-HCl [ph 7.4], 10 mM NaCl, 3 mM MgCl2, and 0.5% NP-40), incubated for 5 minutes on ice, and centrifuged at 3200 rpm for 5 minutes. Nuclei were then washed with washing buffer (10 mM Tris-HCl [pH 7.4], 10 mM NaCl, and 3 mM MgCl2), resuspended in lysis-buffer II (20 mM Tris-HCl [pH 7.4], 40 mM Na-pyrophosphate, 50 mM NaF, 5 mM MgCl2, 20 mM ethylenediaminetetraacetic acid [EDTA], 1% Triton-X-100, and 0.5% SDS, 0.1 mM phenylmethylsulfonyl fluoride [PMSF]), sonicated, and centrifuged at 14,000 rpm for 10 minutes. The supernatant was diluted by the addition of one vol lysis buffer III (20 mM HEPES-KOH, pH 7.4, 0.2 mM EDTA, 0.5 mM PMSF, and 2 mM dithiothreitol). Glycerol was added to obtain a final concentration of 20%, and aliquots were stored at −80°C. Lastly, 50 μg of soluble extracts and 30 μg of nuclear extracts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting according to standard protocols.
Antibodies
Primary mouse monoclonal antibodies were against the following at the dilutions indicated: β-catenin, 1:1000 (BD Transduction Laboratories, San Jose, CA, USA); TCF-4, 1:500 (Upstate Biotechnology, Lake Placid, NY, USA); α-tubulin, 1:1000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); Cox-2, 1:2000 (Cayman Chemicals, Ann Arbor, MI, USA), and rabbit polyclonal anti-cyclin D1, 1:1000 (Upstate Biotechnology). Secondary antibodies conjugated to horseradish peroxidase (1:4000) were from Sigma-Aldrich. Immunoreactive protein was detected by using chemiluminescence (Pierce Chemicals, Rockford, IL, USA).
Statistics
Data are presented as the mean ± standard error of the mean, with sample sizes as indicated in figure legends. Comparisons between groups were performed using a one-way analysis of variance followed by a Student’s t-test; P < 0.05 was considered significant.
Results
FBN strongly inhibits the growth of A-431 cells
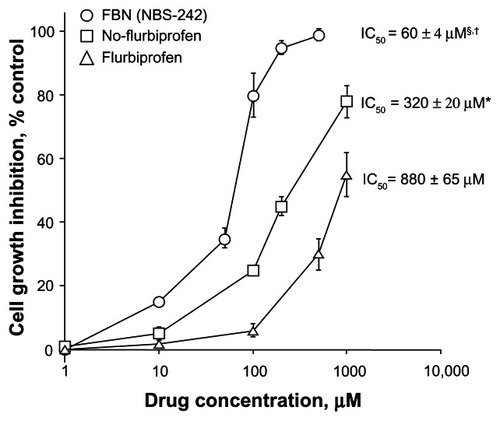
We examined the growth inhibitory activities of FBN, NO-flurbiprofen, and flurbiprofen (their structures are presented in ) by an MTT assay in the A-431 human epidermoid carcinoma cell line. For the initial growth inhibition study, a wide range of doses of the three compounds, including up to 1000 μM for flurbiprofen, were used in order to obtain observable readouts for all the compounds. After treatment of cells for 24 hours, the percent inhibition was determined for the various concentrations examined. Overall, all three compounds inhibited cell growth in a concentration-dependent manner. Among the three compounds, flurbiprofen exhibited a moderate inhibitory effect on cell growth, the aliphatic NO-flurbiprofen strongly inhibited growth, whereas the aromatic NO-flurbiprofen (FBN) was significantly more potent (). The half maximal inhibitory concentration (IC50) values determined for each compound were 880 ± 65 μM, 320 ± 20 μM, and 60 ± 4 μM for flurbiprofen, aliphatic NO-flurbiprofen, and FBN, respectively. The ratio of the IC50s (traditional NSAID/modified NSAID), which reflects the fold increase in potency, indicated that FBN was 15-fold more potent than flurbiprofen and up to 5-fold more potent than NO-flurbiprofen. A lower IC50 of FBN compared to NO-flurbiprofen in A-431 cells strongly suggests that the aromatic spacer may contribute to the strong growth suppressive properties of FBN either alone or by modifying delivery of NO. For further studies we focused on the effects of FBN on cellular kinetics such as proliferation, apoptosis, and cell cycle.
Figure 2 Inhibitory effect of FBN on A-431 cell growth.
P < 0.01 compared to flurbiprofen; §
P < 0.05 compared to NO-flurbiprofen.
Abbreviations: FBN, flurbiprofen benzyl nitrate; IC50, half maximal inhibitory concentration; NO, nitric oxide; NSAID, nonsteroidal anti-inflammatory drug; SEM, standard error of the mean.
FBN affects proliferation and induces apoptosis
Proliferation and apoptosis are two major parameters determining cellular mass. We examined the effects of FBN after 24 hours of treatment on these two parameters. PCNA is an index of the proliferative status of cells. FBN caused a concentration-dependent reduction of PCNA in A-431 cells from 10 to 75 μM (). Compared to the control, the maximal antiproliferative activity of FBN was 15% at 75 μM and remained relatively constant for increasing concentrations, suggesting that inhibition of proliferation may be a partial contributor to the growth inhibitory effect of FBN. On the other hand, the percentage of apoptotic cells, as measured by counting DAPI stained nuclei with apoptotic morphology, increased in a concentration dependent manner from 10 to 100 μM FBN. At 75 μM, approximately 50% of the cells were apoptotic compared to the control ().
Figure 3 Effect of FBN on cell kinetics. FBN causes G0/G1 cell cycle arrest, induces apoptosis, and inhibits proliferation. (A) A-431 cells were treated with FBN at the indicated concentrations for 24 hours followed by PCNA quantification for proliferation or DAPI staining of apoptotic nuclei and counting as described in the Materials and methods section. Results are mean ± SEM of three different experiments. *P < 0.05; † P < 0.01 compared with untreated cells. (B) Asynchronous A-431 cells were treated with increasing concentrations of FBN as indicated for 24 hours.
Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; FBN, flurbiprofen benzyl nitrate; PCNA, proliferating cell nuclear antigen; SEM, standard error of the mean.
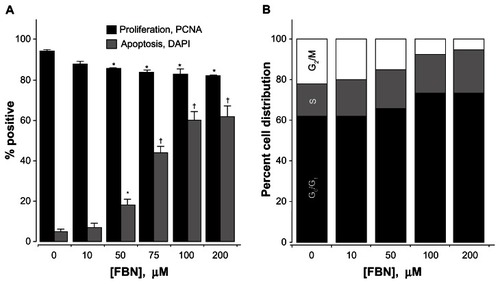
Since the induction of apoptosis may be mediated through the regulation of cell cycle, we examined the effect of FBN on cell cycle perturbations (). Cells were treated with various concentrations of FBN for 24 hours and their DNA content, as assessed by flow cytometric analysis, indicated that FBN-treated cells progressively accumulated in the G0/G1 phase; for example, at 100 μM compared to untreated cells, the proportion of cells in the G0/G1 phase increased from 61% to 73%, and concomitantly decreased the proportion of cells in the G2/M phase from 21% to 9%. Collectively, our data indicate that FBN induces cell cycle arrest and apoptosis in A-431 cells.
FBN targets components of the Wnt pathway and induces caspase-3
According to the canonical Wnt/β-catenin pathway, β-catenin translocates to the nucleus, interacting with TCF and modulating transcription of specific genes. We examined the effect of FBN on β-catenin protein levels in the cytoplasm and nucleus in these cells. FBN cleaved cytoplasmic and nuclear β-catenin as a function of concentration (), with substantial degradation at 75 μM and 100 μM. In the cytoplasm, the functional consequence of reduced levels of β-catenin cleavage was examined by determining the cytoplasmic protein expression of cyclin D1. Cyclin D1 represents an early G1 phase cyclin and is a consequence of β-catenin/TCF-4 transcriptional activity. FBN at 25 μM reduced cytoplasmic cyclin D1 levels and abrogated its expression completely at 75 μM (). This abrogation was associated with β-catenin degradation observed at these concentrations. On the other hand, nuclear expression of cyclin D1, which is a result of protein export from the cytoplasm to the nucleus, was also strongly reduced (). Since these cells were asynchronously dividing, it is understandable that at any time of treatment with FBN cytoplasmic cyclin D1 and its nuclear levels will be affected. We further examined the protein level of TCF-4, the transcription factor in the nucleus that binds to β-catenin. FBN completely reduced the expression of TCF-4 at 75 μM. Thus, FBN targets the two major elements of the Wnt pathway, namely β-catenin and TCF-4. Our data demonstrate that FBN induces G1 cell cycle arrest, which agrees well with the marked downregulation of cyclin D1.
Figure 4 FBN degrades β-catenin, inhibits cyclin D1, and activates caspase-3.
Abbreviations: FBN, flurbiprofen benzyl nitrate; TCF, T-cell factor.
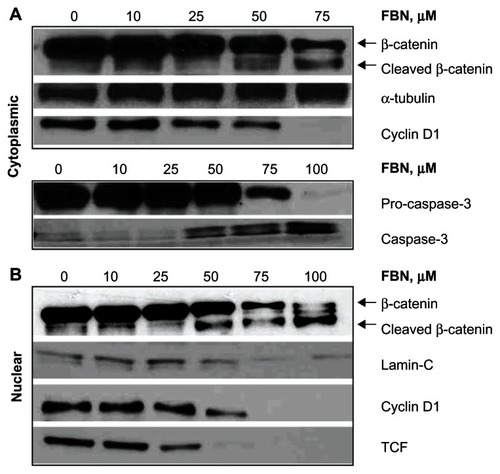
We also examined the activation of caspases by FBN since there was a strong induction of apoptosis in A-431 cells by DAPI staining as shown in . Caspase activation constitutes the hallmark of apoptotic detection and it is well known that β-catenin is a direct target of caspase-3 activity.Citation24 Therefore, the activation of caspase-3 was examined in the cytoplasm fraction of A-431 cells by performing immunoblotting detection for procaspase-3 and its smaller cleaved fragment, signifying the activation of caspase-3. The appearance of activated caspase-3 was observed initially at 50 μM FBN, followed by a substantial increase in activation at 75 and 100 μM FBN, with simultaneous reduction in levels of procaspase-3 (). The activation of caspase-3 coincided with the cleavage of β-catenin at 50 and 75 μM suggesting that there is an association between the events.
Discussion
This study demonstrates the potential of the aromatic NO-releasing compound FBN against skin cancer of the nonmelanocytic type that is known to occur via constitutive activation of β-catenin/TCF signaling. We report, for the first time, the effects of an NO-releasing flurbiprofen that has an aromatic spacer. FBN is more potent than NO-flurbiprofen and flurbiprofen in inhibiting the growth of the A-431 skin cancer cell line.
The data suggest a possible link between the growth inhibitory effects of FBN and the β-catenin pathway as a potential target. Studies in recent years have suggested that β-catenin accumulation has been implicated in tumorigenesis in a wide variety of human cancers including skin cancer.Citation3 These studies suggest, in essence, that degrading nuclear accumulation of β-catenin suppresses tumorigenesis. Our data show the possible molecular mechanism underlying the anti-growth effect of FBN. Taken together, these results suggest that the reduction of β-catenin expression by FBN may contribute to an antiproliferative mechanism in A-431 skin cancer cells. Others have shown that downregulation of β-catenin expression by accelerating its proteasomal degradation led to impaired cell proliferation and abolished the tumorigenic potential of these cells in the nude mice model.Citation26 In this regard, the NSAID R-flurbiprofen was reported to show strong antiproliferative effects in vivo and in vitro.Citation27 However, very high concentrations of this compound, such as 1000 μM R-flurbiprofen, did not affect the protein level of β-catenin or induce its degradation.Citation27 An interesting future goal would be to determine whether proteolytic cleavage of β-catenin is simply an effect of apoptosis or induces the apoptotic program. The versatile functions of β-catenin within the interplay of cell growth and cell death will be an interesting aspect for future studies on FBN.
Since the only difference between NO-flurbiprofen and FBN is the linker joining the NO-releasing group to the NSAID flurbiprofen, it may be concluded that the enhanced potency observed with FBN may be due to the aromatic linker alone, or the way in which NO is delivered or a combination of both.
Previous work by us on the structure–activity properties of NO-aspirin had indicated that NO was pivotal for its anticancer effects.Citation22 However, careful re-examination regarding the contribution to the overall biological effect of each of the three structural components of NO- acetylsalicylate (ASA) in which the spacer joining the ASA to the NO-releasing moiety was aromatic, led us and others to conclude that the NO-releasing moiety was not required for the observed biological effects. Rather, the spacer was responsible for the biological actions of NO-aspirin, with the NO-releasing moiety acting as a leaving group that facilitated the release and activation of the spacer to a quinone methide intermediate which acted as a powerful electrophile.Citation28–Citation30 Here, we have NO-flurbiprofen which was ~2.8-fold more potent than its parent compound flurbiprofen in inhibiting the growth of A-431 cells and FBN which was ~15-fold more potent. On this basis, we may cautiously conclude that with FBN we may have two effects, one as a result of released NO and one due to an active aromatic linker.
Finally, FBN is able to reduce PCNA levels, which is a polymerase accessory protein detected in a cell-cycle-dependent manner,Citation31 with a marked downregulation of cyclin D1. The cyclin D1 proto-oncogene is an important regulator of G1 to S phase transition in numerous cell types from diverse tissues. This series of events results in a G1 phase arrest of the cell cycle, which is an irreversible process that ultimately results in the apoptotic death of cancer cells.
In conclusion our data establish a strong inhibitory effect of FBN in A-431 human epidermoid carcinoma cells, an effect that is modulated through parameters important in determining cellular mass. FBN is more potent than NO-flurbiprofen and flurbiprofen in the inhibition of cell proliferation. Activation of caspase-3 is associated with degradation of β-catenin and reduction in cyclin D1 levels, suggesting that FBN has potential as a chemotherapeutic agent against NMSC.
Two major aspects constitute future directions for this study. First, at the mechanistic level, the degradation of β-catenin by FBN merits further investigation regarding the sequence of events in relation to caspase activity. A second focus is the potential of FBN in topical treatment regimens, which are generally considered to be better alternatives for nonmelanoma skin cancer in minimizing systemic exposure while achieving high local concentrations of the drug. We are currently formulating hydrogel-delivering systems for local application of FBN.
Authors’ contributions
Participated in research design: NN, KK; conducted experiments: NN, LJ, XL; performed data analysis: NN, LJ, XL, KK; wrote or contributed to the writing of the manuscript: NN, LJ, XL, KK.
Acknowledgments
This work was supported in part by NIH grant R24 DA018055. The funding agency had no role in the study design, collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Cancer Society Cancer Facts and Figures 2010 Atlanta American Cancer Society 2010 Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf Accessed March 26, 2013
- Tataroglu C Karabacak T Apa DD Beta-catenin and CD44 expression in keratoacanthoma and squamous cell carcinoma of the skin Tumori 2007 93 3 284 289 17679465
- Doglioni C Piccinin S Demontis S Alterations of beta-catenin pathway in non-melanoma skin tumors: loss of alpha-ABC nuclear reactivity correlates with the presence of beta-catenin gene mutation Am J Pathol 2003 163 6 2277 2287 14633602
- El-Bahrawy M El-Masry N Alison M Poulsom R Fallowfield M Expression of beta-catenin in basal cell carcinoma Br J Dermatol 2003 148 5 964 970 12786827
- Bhatia N Spiegelman VS Activation of Wnt/beta-catenin/Tcf signaling in mouse skin carcinogenesis Mol Carcinog 2005 42 4 213 221
- Thun MJ Henley SJ Patrono C Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues J Natl Cancer Inst 2002 94 4 252 266 11854387
- Baron JA Epidemiology of non-steroidal anti-inflammatory drugs and cancer Prog Exp Tumor Res 2003 37 1 24
- Benamouzig R Deyra J Martin A Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial Gastroenterology 2003 125 2 328 336
- Sandler RS Halabi S Baron JA A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer N Engl J Med 2003 348 10 883 890
- Rothwell PM Price JF Fowkes FG Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials Lancet 2012 379 9826 1602 1612
- Rothwell PM Wilson M Price JF Belch JF Meade TW Mehta Z Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials Lancet 2012 379 9826 1591 1601
- Clouser MC Roe DJ Foote JA Harris RB Effect of non-steroidal anti-inflammatory drugs on non-melanoma skin cancer incidence in the SKICAP-AK trial Pharmacoepidemiol Drug Saf 2009 18 4 276 283 19226541
- Elmets CA Viner JL Pentland AP Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial J Natl Cancer Inst 2010 102 24 1835 1844 21115882
- Curiel-Lewandrowski C Nijsten T Gomez ML Hollestein LM Atkins MB Stern RS Long-term use of nonsteroidal anti-inflammatory drugs decreases the risk of cutaneous melanoma: results of a United States case-control study J Invest Dermatol 2011 131 7 1460 1468 21390049
- Bjorkman DJ Current status of nonsteroidal anti-inflammatory drug (NSAID) use in the United States: risk factors and frequency of complications Am J Med 1999 107 6A 3S 8S discussion 8S–10S 10628588
- Fitzgerald GA Coxibs and cardiovascular disease N Engl J Med 2004 351 17 1709 1711 15470192
- Scheiman JM Fendrick AM Summing the risk of NSAID therapy Lancet 2007 369 9573 1580 1581 17499583
- Davies NM Røseth AG Appleyard CB NO-naproxen vs naproxen: ulcerogenic, analgesic and anti-inflammatory effects Aliment Pharmacol Ther 1997 11 1 69 79 9042976
- Wallace JL Reuter B Cicala C McKnight W Grisham MB Cirino G Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat Gastroenterology 1994 107 1 173 179 8020659
- Fiorucci S Santucci L Gresele P Faccino RM Del Soldato P Morelli A Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study Gastroenterology 2003 124 3 600 607
- Fiorucci S Santucci L Wallace JL Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa Proc Natl Acad Sci U S A 2003 100 19 10937 10941 12960371
- Kashfi K Borgo S Williams JL Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo J Pharmacol Exp Ther 2005 312 3 978 988 15528453
- Rigas B Kashfi K Nitric-oxide-donating NSAIDs as agents for cancer prevention Trends Mol Med 2004 10 7 324 330 15242680
- Nath N Labaze G Rigas B Kashfi K NO-donating aspirin inhibits the growth of leukemic Jurkat cells and modulates beta-catenin expression Biochem Biophys Res Commun 2005 326 1 93 99 15567157
- Penning TD Talley JJ Bertenshaw SR Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem 1997 40 9 1347 1365 9135032
- Cong F Zhang J Pao W Zhou P Varmus H A protein knockdown strategy to study the function of beta-catenin in tumorigenesis BMC Mol Biol 2003 4 10 14516475
- Maier TJ Janssen A Schmidt R Geisslinger G Grösch S Targeting the beta-catenin/APC pathway: a novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cells FASEB J 2005 19 10 1353 1355 15946992
- Kashfi K Rigas B The mechanism of action of nitric oxide-donating aspirin Biochem Biophys Res Commun 2007 358 4 1096 1101 17512900
- Dunlap T Chandrasena RE Wang Z Sinha V Wang Z Thatcher GR Quinone formation as a chemoprevention strategy for hybrid drugs: balancing cytotoxicity and cytoprotection Chem Res Toxicol 2007 20 12 1903 1912 17975886
- Hulsman N Medema JP Bos C Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin J Med Chem 2007 50 10 2424 2431 17441704
- Hsieh T Halicka D Lu X Effects of resveratrol on the G(0)-G(1) transition and cell cycle progression of mitogenically stimulated human lymphocytes Biochem Biophys Res Commun 2002 297 5 1311 1317 12372431