Abstract
Background
It has been reported that pitavastatin improves endothelial function faster than other statins. Recently introduced reactive hyperemia peripheral arterial tonometry (RH-PAT) provides objective and quantitative assessment of peripheral microvascular function.
Purpose
This study aimed to investigate whether peripheral microvascular function improved 2 hours after pitavastatin in subjects with coronary artery disease (CAD) using RH-PAT, and the results were compared with those of rosuvastatin.
Methods
This study included 94 subjects with CAD, assigned to a group given 2 mg of pitavastatin (n = 36), a group given 2.5 mg of rosuvastatin (n = 38), and a control group (n = 20). RH-PAT examinations were performed before and 2 hours after statin administration.
Results
The RH-PAT index increased 2 hours after pitavastatin administration from 1.82 ± 0.45 to 2.16 ± 0.62 (P = 0.02), whereas there were no differences in the RH-PAT index in the rosuvastatin group (1.79 ± 0.71 to 1.91 ± 0.53, P = 0.09) and the control group (1.68 ± 0.36 to 1.84 ± 0.58, P = 0.4). No significant changes were observed at 2 hours in serum cholesterol levels in each group.
Conclusion
The present study demonstrated that peripheral microvascular function improved 2 hours after a single clinical dose of pitavastatin, but not after rosuvastatin.
Introduction
Evidence from several large, randomized, controlled trials has demonstrated that the decrease in the serum cholesterol concentration produced by the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) reduces the mortality of subjects with coronary artery disease (CAD).Citation1,Citation2 Previous studies have confirmed that statins have pleiotropic effects, including improvement of endothelial function, and that effects other than cholesterol reduction may contribute to reduction of cardiovascular complications.Citation3 These putatively cholesterol-independent effects may be exerted more rapidly than cholesterol lowering itself, ranging from 1 day to several months.Citation4–Citation8 Previous reports have shown that pitavastatin may improve endothelial function faster than other statins.Citation9,Citation10 Therefore, we hypothesized that pitavastatin, but not other statins, improves microvascular function within several hours in subjects with CAD.
Reactive hyperemia peripheral arterial tonometry (RH-PAT) has recently been introduced to evaluate peripheral microvascular function.Citation11–Citation14 RH-PAT is designed to measure volumetric changes in the fingertip by using a probe to quantify pulse amplitude in response to reactive hyperemia. This technique has been reported to be useful in identifying cardiovascular risk factors. Considering it is noninvasive and less operator- and observer-dependent, this method may have the potential to detect subtle differences in serial measurements of microvascular function. This study therefore aimed to investigate the very rapid effects (within 2 hours) of pitavastatin on peripheral microvascular function using RH-PAT in subjects with CAD and compare them with those of rosuvastatin.
Methods
Study population
There were 94 study subjects with hemodynamically stable CAD (68 ± 12 years of age, 73 males) who had serum low-density lipoprotein (LDL)-cholesterol ≥ 100 mg/dL, and/or LDL/high-density lipoprotein (HDL)-cholesterol ratio ≥ 2. CAD was defined as ≥ 50% organic stenosis based on the classification by the American Heart Association on coronary angiography in at least one branch of the coronary artery. Subjects were excluded if they had a history of statin therapy, valvular heart disease, severe hepatic disease (history of liver cirrhosis or alanine aminotransferase > 2.5 times upper normal), or renal failure (serum creatinine > 2.0 mg/dL). The study subjects were assigned to the following three groups: (1) 2 mg of pitavastatin, (2) 2.5 mg of rosuvastatin, and (3) placebo (control group). This study was approved by the Institutional Review Board of the Osaka Ekisaikai Hospital, and all subjects gave their informed consent.
Protocol
A quiet, temperature-controlled (24°C–27°C) room was used for the RH-PAT test. Subjects underwent RH-PAT examinations before and 2 hours after statin intake in the pitavastatin and rosuvastatin groups. Blood samples were taken immediately before each RH-PAT examination. These examinations were performed more than 3 hours after meals and after 30 minutes of rest lying down. In the control group, the RH-PAT tests were performed before and after 2 hours of rest without statin treatment, to investigate the effect of repeated RH-PAT tests on the results.
RH-PAT examination
RH-PAT was performed using ENDO-PAT 2000 (Itamar Medical, Caesarea, Israel). Specially designed finger probes were placed on the middle finger of each subject’s hand. These probes consisted of a system of inflatable latex air cuffs connected by pneumatic tubes to an inflating device controlled through a computer algorithm. Pulsatile volume changes of the distal digit induced pressure alterations in the finger cuff, which were sensed by pressure transducers.
Subjects were instructed to remain at rest for 5 minutes to obtain a baseline measurement. After 5 minutes of baseline recording, a blood pressure cuff was inflated to 60 mm Hg above systolic pressure or at least 200 mm Hg in the test arm. After 5 minutes of occlusion, the cuff was rapidly deflated, with PAT tracings being recorded for a further 6 minutes. The ratio of the PAT signal after cuff deflation compared with baseline was calculated though a computer algorithm automatically normalizing for the baseline signal and indexed to the contralateral arm.
Statistical analysis
Values are expressed as mean ± standard deviation. Comparisons of the RH-PAT index and laboratory and hemodynamic data before and after statin administration were performed using the Mann–Whitney U test. The chi-square test was used for comparison of categorical variables. One-way analysis of variance followed by a post-hoc Bonferroni test was used to compare the three groups (pitavastatin, rosuvastatin, and control groups). Differences were considered significant at P < 0.05.
Inter- and intra-observer variabilities of RH-PAT examination were examined in 10 healthy subjects (8 males, mean age 31 ± 5 years) who had no history of cardiac disease or risk factors. Inter-observer variability for RH-PAT examination was analyzed with two independent blinded observers. Intra-observer variability was analyzed with the same observer at two different time points. The results were analyzed by both least squares fit linear regression analysis and the Bland–Altman method.
Results
Of the 94 subjects, 36 subjects were assigned to the pitavastatin group, 38 subjects to the rosuvastatin group, and 20 subjects to the control group. The clinical characteristics of the three groups are summarized in . There were no correlations between baseline RH-PAT index and medications, including beta-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and aspirin, as shown in . There was no significant difference in the baseline value of the RH-PAT index among the three groups: 1.82 ± 0.45 in the pitavastatin group, 1.79 ± 0.71 in the rosuvastatin group, and 1.68 ± 0.36 in the controls (P = 0.6). Further, the baseline laboratory parameters and hemodynamics did not differ significantly among the three groups ().
Table 1 Clinical characteristics of the pitavastatin, rosuvastatin, and control groups
Figure 1 Baseline RH-PAT index and medications.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; RH-PAT, reactive hyperemia peripheral arterial tonometry.
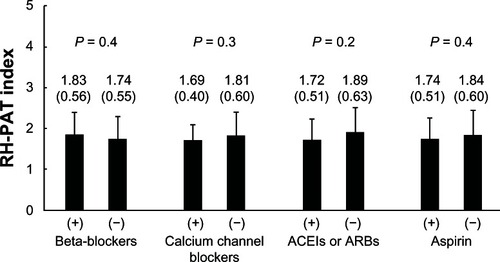
Figure 2 RH-PAT index before and after treatment in the pitavastatin group (left), rosuvastatin group (middle), and control group (right).
Abbreviations: CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; RH-PAT, reactive hyperemia peripheral arterial tonometry.
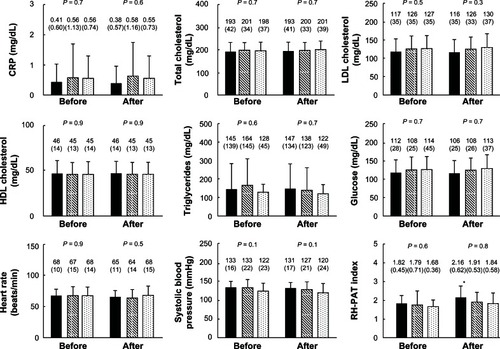
The RH-PAT index increased significantly 2 hours after pitavastatin administration, from 1.82 ± 0.45 to 2.16 ± 0.62 (P = 0.02), as shown in . However, there were no differences in the RH-PAT index in the rosuvastatin group (1.79 ± 0.71 to 1.91 ± 0.53, P = 0.09) and the control group (1.68 ± 0.36 to 1.84 ± 0.58, P = 0.4). Moreover, there were no significant differences in any laboratory parameters before and after statin intake in each group.
Excellent correlations were observed for inter-observer and intra-observer variabilities of RH-PAT examination. Values were r = 0.92 and r = 0.95 for the RH-PAT index. From the Bland–Altman method, inter- and intra-observer variabilities for the RH-PAT index were 0.04 and 0.06, respectively.
Discussion
Advantages of RH-PAT over flow-mediated dilation (FMD)
FMD of the brachial artery is a widely used method for the study of endothelial function in clinical practice.Citation15–Citation17 However, this method requires a certain degree of skill to obtain accurate measurements of brachial artery diameter. Furthermore, sympathetic nervous activity and subsequent hemodynamic changes may affect the FMD result.Citation15,Citation18 The RH-PAT system has the potential to overcome these shortcomings of the FMD test. This method is an automatic, less operator-dependent, quantitative test for digital volume measurement of the hyperemic response, and by normalizing using the contralateral arm measurement, the effect of hemodynamic changes can be eliminated.Citation11–Citation14 Importantly, the relationship between or a comparison of the FMD and RH-PAT index needs careful consideration. Some studies have indicated that FMD and RH-PAT index reflect different pathologies of peripheral vascular endothelial function.Citation19,Citation20 FMD is used to assess large artery reactivity, whereas the RH-PAT index reflects microvascular function. The RH-PAT index has been shown to be at least 50% dependent on endothelial nitric oxide (NO) activity.Citation21 Therefore, the RH-PAT index may be suitable for assessing subtle differences in serial measurements of microvascular function.
Rapid effects of statins on endothelial function
Previous clinical investigations have demonstrated that the effect of statins on peripheral and coronary endothelial function occurs within 24 hours to several months. O’Driscoll et al demonstrated that 4-week simvastatin treatment improved FMD in subjects with dyslipidemia.Citation5 A similar positive effect on microvascular function was recently confirmed by Nonogaki et al using RH-PAT 4 weeks after pitavastatin therapy.Citation6 Tsunekawa et al reported that cerivastatin improved FMD within 3 days in subjects with diabetes, independent of its lipid lowering effect.Citation7 Wassmann et al, who measured coronary flow reserve, also showed that endothelial-dependent coronary vasomotion increased 24 hours after a single treatment of pravastatin in subjects with CAD.Citation8 The present study demonstrated that pitavastatin improved microvascular function rapidly, within 2 hours. This finding may indicate the importance of early and new therapeutic approaches with pitavastatin with respect to improved endothelial function contributing to improved cardiovascular outcomes, such as in the settings of acute coronary syndrome and heart failure.
Potential mechanisms of the difference between pitavastatin and rosuvastatin
The advantage of pitavastatin over rosuvastatin in the rapid improvement of peripheral microvascular function was demonstrated in the present study, as in previous studies. Sakabe et al examined FMD tests 2 and 12 weeks after pitavastatin and atorvastatin, respectively.Citation9 They found that the short-term effect of pitavastatin was superior to that of atorvastatin. An intravascular ultrasonographic study also reported that the positive effect of pitavastatin on coronary plaques was faster than that seen with atorvastatin.Citation10
These results might be explained as follows. First, pitavastatin administered orally reaches a peak plasma concentration (Cmax) in approximately 1 hour. The Cmax at a dose of 2 mg pitavastatin is 26.1 ng/mL, which is higher than that of 5 mg rosuvastatin (3.6 ng/mL).Citation22,Citation23 Faster transfer of the drug to blood vessels may result in increased bioavailability of NO through the inhibition of Rho-kinase in endothelial cells. An experimental study showed that Rho-kinase inhibition rapidly activated Akt/PI3 kinase within 15 to 30 minutes and increased in a concentration-dependent manner.Citation24 Second, the pleiotropic effects of statins may depend on their lipophilicity. Because the cell membrane consists of lipid bilayers, intracellular pathways and possible effects of statins differ between lipophilic and hydrophilic statins. It has been reported that cerivastatin, a lipophilic statin, was more effective with the respect to endothelial NO synthase expression for 12 hours than rosuvastatin, a hydrophilic statin, in subjects with diabetes.Citation25 Furthermore, short-term cardiovascular outcomes after acute myocardial infarction were better in subjects with a lipophilic statin than with a hydrophilic statin.Citation26 Finally, pitavastatin may simply have a stronger effect on endothelial function than other statins. Also, pitavastatin is marginally metabolized by cytochrome P450 enzymes, and is less dependent on organic anion-transporting polypeptides for its uptake into hepatocytes.Citation27,Citation28 Such a unique pharmacological profile of pitavastatin might have novel and wide-ranging effects on endothelial function. The present study demonstrated that pitavastatin resulted in rapid improvement of microvascular function. Since endothelial dysfunction is strongly associated with the onset and development of CAD and considerable mortality despite contemporary therapies,Citation16,Citation29,Citation30 early modification of endothelial function is of crucial importance. The management of CAD in clinical practice could be improved by considering the physicochemical characteristics of statins. Also, a distinctive metabolic pathway of pitavastatin is associated with a favorable drug–drug interaction profile; this supports the use of pitavastatin among the statins currently available.Citation28,Citation31
Study limitations
Several limitations need to be considered. First, the study was initially designed to include 60 subjects (20 subjects in each group). However, because of wider distribution of the RH-PAT index in the rosuvastatin group than in the other groups, 36 subjects were added, randomly assigned to pitavastatin or rosuvastatin groups on the basis of the value of first RH-PAT index. Two subjects with pitavastatin then refused to perform second RH-PAT test due to pain of cuff-inflation. Crossover designed studies with a larger number of subjects would be ideal to assess the acute effect of two different drugs. Another ideal way would be to randomize subjects to the different treatment groups to balance out other factors. Further, although observer variability of RH-PAT values was small, the results of a one-way analysis of variance did not account for observer variability. Second, this study included subjects with CAD, and the application of these results to other populations may be limited. Furthermore, 73 (78%) subjects continued taking one or more antihypertensive drugs during the examinations, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, which might have altered endothelial function and affected the results of this study.Citation32,Citation33 Although no correlations were observed between baseline RH-PAT index and medications, the time of intake of these medications might have altered endothelial function and thus affected the results of this study. Third, RH-PAT index was slightly increased even in the control group, which may be due to 2-hour rest and/or circadian variation of endothelial function.Citation34 The first examination was performed in the morning in all subjects, and this question may be resolved if the subjects were randomly assigned with respect to the time of day when the first study was done. Finally, a dose of 2.5 mg of rosuvastatin was used because this is the recommended starting dose in Japan. It remains to be seen whether different doses will show results similar to those found in this study. The very rapid increase in the RH-PAT index after pitavastatin administration may be associated with a decrease in oxidative stress, which inactivates NO. Measurement of plasma concentrations of oxidative stress markers, thiobarbituric acid reactive substances, isoprostanes, and 8-hydroxydeoxyguanosine may illuminate the presumed underlying mechanisms of this finding, and nitroglycerin-mediated vasodilation, as a marker of endothelium-independent vasodilation, should be measured in a future study.
Conclusion
This RH-PAT study demonstrated that single clinical doses of pitavastatin, but not of rosuvastatin, improved peripheral microvascular function within 2 hours in subjects with CAD. These results may support early intervention of statin therapy in the management of CAD and other atherosclerotic diseases.
Acknowledgemnts
This work was supported in part by a research grant from the Osaka Foundation for the Prevention of Cancer and Cardiovascular Diseases.
Disclosure
The authors report no conflicts of interest in this work.
References
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet 1994 344 8934 1383 1389 7968073
- Shepherd J Cobbe SM Ford I Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group N Engl J Med 1995 333 20 1301 1307 7566020
- Kirmizis D Papagianni A Dogrammatzi F Effects of simvastatin on markers of inflammation, oxidative stress and endothelial cell apoptosis in patients on chronic hemodialysis J Atheroscler Thromb 2010 17 12 1256 1265 20885069
- Takemoto M Liao JK Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors Arterioscler Thromb Vasc Biol 2001 21 11 1712 1719 11701455
- O’Driscoll G Green D Taylor RR Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month Circulation 1997 95 5 1126 1131 9054840
- Nonogaki K Suzuki M Kanai N Sumii M Kaji T Short-term effect of pitavastatin on the reactive hyperemic index in post-menopausal women with high levels in serum LDL-cholesterol Int J Cardiol 2011 150 2 227 228 21636141
- Tsunekawa T Hayashi T Kano H Cerivastatin, a hydroxy-methylglutaryl coenzyme a reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days Circulation 2001 104 4 376 379 11468195
- Wassmann S Faul A Hennen B Scheller B Bohm M Nickenig G Rapid effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition on coronary endothelial function Circ Res 2003 93 9 e98 e103 14551237
- Sakabe K Fukuda N Fukuda Y Comparisons of short- and intermediate-term effects of pitavastatin versus atorvastatin on lipid profiles, fibrinolytic parameter, and endothelial function Int J Cardiol 2008 125 1 136 138 17400311
- Toi T Taguchi I Yoneda S Early effect of lipid-lowering therapy with pitavastatin on regression of coronary atherosclerotic plaque. Comparison with atorvastatin Circ J 2009 73 8 1466 1472 19531899
- Bonetti PO Barsness GW Keelan PC Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease J Am Coll Cardiol 2003 41 10 1761 1768 12767662
- Hamburg NM Keyes MJ Larson MG Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study Circulation 2008 117 19 2467 2474 18458169
- Matsuzawa Y Sugiyama S Sugamura K Digital assessment of endothelial function and ischemic heart disease in women J Am Coll Cardiol 2010 55 16 1688 1696 20394872
- Rubinshtein R Kuvin JT Soffler M Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events Eur Heart J 2010 31 9 1142 1148 20181680
- Corretti MC Anderson TJ Benjamin EJ Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force J Am Coll Cardiol 2002 39 2 257 265 11788217
- Gokce N Keaney JFJr Hunter LM Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease J Am Coll Cardiol 2003 41 10 1769 1775 12767663
- Shimada K Fukuda S Maeda K Aromatherapy alleviates endothelial dysfunction of medical staff after night-shift work: preliminary observations Hypertens Res 2011 34 2 264 267 21107332
- Hijmering ML Stroes ES Pasterkamp G Sierevogel M Banga JD Rabelink TJ Variability of flow mediated dilation: consequences for clinical application Atherosclerosis 2001 157 2 369 373 11472736
- Hamburg NM Palmisano J Larson MG Relation of brachial and digital measures of vascular function in the community: the Framingham heart study Hypertension 2011 57 3 390 396 21263120
- Schnabel RB Schulz A Wild PS Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods Circ Cardiovasc Imaging 2011 4 4 371 380 21551420
- Nohria A Gerhard-Herman M Creager MA Hurley S Mitra D Ganz P Role of nitric oxide in the regulation of digital pulse volume amplitude in humans J Appl Physiol 2006 101 2 545 548 16614356
- Livalo®(pitavastatin) [prescribing information] Tokyo, Japan Kowa Company, Ltd 2010
- Crestor®(rosuvastatin) [prescribing information] Osaka, Japan AstraZeneca 2011
- Wolfrum S Dendorfer A Rikitake Y Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection Arterioscler Thromb Vasc Biol 2004 24 10 1842 1847 15319269
- Jantzen F Konemann S Wolff B Isoprenoid depletion by statins antagonizes cytokine-induced down-regulation of endothelial nitric oxide expression and increases NO synthase activity in human umbilical vein endothelial cells J Physiol Pharmacol 2007 58 3 503 514 17928646
- Kim MC Ahn Y Jang SY Comparison of clinical outcomes of hydrophilic and lipophilic statins in patients with acute myocardial infarction Korean J Intern Med 2011 26 3 294 303 22016590
- Davignon J Pleiotropic effects of pitavastatin Br J Clin Pharmacol 2012 73 4 518 535 22053916
- Catapano AL Statin-induced myotoxicity: pharmacokinetic differences among statins and the risk of rhabdomyolysis, with particular reference to pitavastatin Curr Vasc Pharmacol 2012 10 2 257 267 22022768
- Kitta Y Obata JE Nakamura T Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease J Am Coll Cardiol 2009 53 4 323 330 19161880
- Yoshino S Hamasaki S Ishida S Relationship between bilirubin concentration, coronary endothelial function, and inflammatory stress in overweight patients J Atheroscler Thromb 2011 18 5 403 412 21350306
- Corsini A Ceska R Drug-drug interactions with statins: will pitavastatin overcome the statins’ Achilles’ heel? Curr Med Res Opin 2011 27 8 1551 1562 21682551
- Ghiadoni L Magagna A Versari D Kardasz I Huang Y Taddei S Salvetti A Different effect of antihypertensive drugs on conduit artery endothelial function Hypertension 2003 41 6 1281 1286 12719441
- Wassmann S Hilgers S Laufs U Bohm M Nickenig G Angiotensin II type 1 receptor antagonism improves hypercholesterolemia-associated endothelial dysfunction Arterioscler Thromb Vasc Biol 2002 22 7 1208 1212 12117739
- Fukuda S Shimada K Maeda K Circadian variation in coronary flow velocity reserve and its relation to alpha1-sympathetic activity in humans Int J Cardiol 2012 157 2 216 220 21194761