Abstract
Background
To investigate the effects of nalbuphine on emergency agitation (EA), which affects up to 80% of the children following otolaryngology procedures, in children undergoing cochlear implantation.
Methods
A prospective double-blinded randomized controlled clinical trial was conducted between November 2020 and October 2022. Eligible children, aged 6 months to 3 years old, were randomly assigned to either 0.1 mg/kg, 0.15 mg/kg, 0.2 mg/kg nalbuphine or 0.9% saline groups. EA was defined by the Pediatric Anesthesia Emergence Delirium (PAED) score ≥10. Extubation time, post-anesthesia care unit (PACU) length of stay, severe EA (PAED ≥ 15), peak PAED score, the Faces, Legs, Activity, Cry, and Consolability (FLACC) scale, Ramsay sedation score, and adverse events were also recorded.
Results
A total of 104 children were enrolled, with 26 children in each group. Nalbuphine significantly reduced the EA occurrence from 73.1% in the saline group to 38.5%, 30.8%, and 26.9% in the 0.1 mg/kg, 0.15 mg/kg, and 0.2 mg/kg nalbuphine groups, respectively (P < 0.001), without affecting the extubation time and PACU length of stay. More children (34.6%) in the 0.9% saline group experienced severe EA. Higher dose nalbuphine (0.15 mg/kg, 0.2 mg/kg) showed lower peak PAED score, better analgesia and sedation effect compared with 0.1 mg/kg nalbuphine and saline groups. However, 0.2mg/kg nalbuphine caused undesired over-sedation in two (7.7%) children. No other adverse events were reported.
Conclusion
Young children undergoing cochlear implantation surgery were at a high risk of EA and postoperative pain, while 0.2 mg/kg nalbuphine might be an ideal candidate for EA and pain prevention when used under close monitoring.
Trial Registration
ChiCTR2000040407.
Introduction
As one of the most common postoperative complications in children, emergence agitation (EA) refers to a status of inconsolable crying, agitation, restlessness, disorientation, delusions, and hallucinations, with transient loss of memory and cognition.Citation1 The occurrence of EA ranged from 10 to 80% in children who received general anesthesia,Citation2 which could result in displaced surgical dressings, dislodged drainage tubes or intravenous catheters, patient injury, and delayed recovery,Citation1 as well as an increased risk of long-term maladaptive behaviors.Citation3 Successful prevention and treatment of EA can mitigate perioperative complications and help ensure favorable postoperative outcomes.
The risk factors of EA include young age (≤3 years old), otolaryngology surgeries, inhalation anesthesia, anxiety and postoperative pain.Citation4 Cochlear implantation, which is a common procedure used to treat profound hearing loss affecting about 4.8–6.4% of the young children, was found to frequently cause postoperative EA.Citation5 However, the prevalence and optimal management of EA in children undergoing cochlear implantation remains largely unknown.
In current clinical practice, fentanyl, remifentanil, sufentanil, and alfentanil are commonly used to reduce the occurrence and severity of EA during the surgical operation.Citation6,Citation7 However, these medications have their own limitations. For example, fentanyl prolonged post-anesthesia care unit (PACU) length of stay and increased the occurrence of postoperative nausea and vomiting (PONV).Citation6 Sufentanil could lead to respiratory suppression,Citation7 and remifentanil could cause a high postoperative analgesic requirement.Citation8 As a semi-synthetic opioid, nalbuphine is a κ-opioid receptor agonist and partial μ-opioid receptor antagonist. It has moderate analgesic effects with less frequent side effects, such as respiratory depression, PONV, and pruritus.Citation9 Therefore, nalbuphine is considered as a relatively safe analgesic and commonly used in pediatric patients.Citation10,Citation11 Prior research has shown that 0.1 mg/kg of nalbuphine could significantly decrease the occurrence of EA in children during magnetic resonance imaging (MRI) examination, adenotonsillectomy and ophthalmic surgery.Citation12–14
In the present study, we performed a prospective double-blinded randomized controlled clinical trial with the aim to investigate the dose-dependent effects of nalbuphine on EA in children undergoing cochlear implantation.
Materials and Methods
Study Design and Participant Enrollment
We performed a prospective double-blinded randomized controlled clinical trial in Xijing Hospital, Xi’an, China, between November 2020 and October 2022. This study was approved by the medical ethics committee of Xijing Hospital (KY20202089-F-1) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at Chinese Clinical Trial Registry (ChiCTR2000040407, http://www.chictr.org.cn/showproj.aspx?proj=64844, Principal investigator: Yan Li, Date of registration: 2020.11.28). The parents or healthcare guardians of pediatric patients signed the informed consent. The manuscript adheres to the applicable CONSORT guideline and the study complied with the Declaration of Helsinki.
The inclusion criteria were children aged 6 months to 3 years old, with American Society Anesthesiologists (ASA) grade I or II, and scheduled for cochlear implantation. Children were excluded from the study if they had a history of 1) neurological and psychiatric disease; 2) premature birth; 3) mental retardation; 4) important organ comorbidities; 5) recent use of analgesic or sedative drugs; or 6) serious genetic disease(s).
Randomization and Double-Blinding
Based on a computer-generated randomization table, eligible children were assigned into one of the following four groups: 0.1 mg/kg nalbuphine, 0.15 mg/kg nalbuphine, 0.2 mg/kg nalbuphine, or 0.9% saline group.
The group allocation was concealed in sealed opaque envelopes. An independent nurse opened the sealed envelope. Depending on the group assignment and body weight, the nurse prepared the required dose of nalbuphine solution and diluted it with normal saline to the final volume of 5 mL. The anesthetist, who evaluated the Pediatric Anesthesia Emergence Delirium (PAED) score,Citation15 the Faces, Legs, Activity, Cry and Consolability (FLACC) scale,Citation16 and modified Ramsay sedation score,Citation17 was specially trained and blinded to the allocations. The children and their parents or healthcare guardians were also not aware of the allocations.
Study Protocol
After the informed consent process, the baseline clinical information was recorded. All children were fasted for at least 6 h and received 2 μg/kg of intranasal dexmedetomidine 30 mins before the surgery. After inhalation of 8% sevoflurane (delivered with 6 L/min 100% oxygen) and venous catheterization, dexamethasone (0.1 mg/kg), sufentanil (0.3 μg/kg), propofol (1.5–2.0 mg/kg), and vecuronium (0.1 mg/kg) were injected for the anesthetic induction, which was followed by the endotracheal intubation. The general anesthesia was maintained via inhalation of 2–3% sevoflurane (delivered with 2 L/min mixed oxygen) and infusion of 0.1–0.3 μg/kg/min remifentanil, with 8–10 mL/kg in tidal volume, 18–25 breaths/min to keep ETCO2 at 35–45 mmHg. Non-invasive blood pressure, heart rate, pulse oximetry and end-tidal carbon dioxide were continuously monitored throughout the surgery. At 15–30 mins before the end of the surgery, nalbuphine or saline was given intravenously.
Upon completion of the surgery, sevoflurane and remifentanil were discontinued, and the oxygen flow was increased to 6 L/min. The endotracheal tube was removed when the respiratory, swallowing reflex, and cough reflex were recovered. Then, children were transferred to the PACU for further monitoring while routinely under 2 L/min oxygen supply through a face mask.
Outcome Measurements
The primary outcome was the occurrence of EA, which was defined as PAED ≥ 10 at 10, 20, or 30 mins after extubation.Citation18 When the PAED score ≥15, it was considered severe EA.Citation18 We chose 30 min as the observation duration since most EA has been reported to occur during this time period.Citation19
The secondary outcomes included extubation time (duration from anesthetics cessation to extubation), EA severity (peak PAED scores at each observation time point), pain severity (FLACC scale), and sedation level (modified Ramsay sedation score). Children with a FLACC ≥ 4 were considered insufficient analgesia. Subjects received 1 mg/kg flurbiprofen axetil intravenously as “rescue analgesia” in all groups. We also documented the duration of surgery and anesthesia, the PACU length of stay and the adverse events, including PONV, pruritus, laryngospasm, and oxygen desaturation (peripheral oxygen saturation <92%).Citation20 PONV was evaluated by experienced PACU nurses and defined as an active attempt to vomit with or without expulsion of gastric contents.Citation21 All children were observed for 6 h after the surgery, while the FLACC scale and Ramsay score were assessed at 10, 20, and 30 min, as well as 1, 3, and 6 h after extubation.
Statistical Analysis
The sample size was calculated using Power Analysis and Sample Size (PASS) Software (version 15, Kaysville, Utah, USA). According to a previous study, the occurrence of EA in preschool children was 40% with sevoflurane anesthesia.Citation22 Our preliminary data suggested that the occurrences of EA were approximately 20%, 10%, and 5% in the 0.1 mg/kg, 0.15 mg/kg, and 0.2 mg/kg nalbuphine groups, respectively. According to the overall differences of multi-group rates, a sample size of at least 26 children in each group would have a power (1-β) of 80% and a two-sided-type I error of 5%, considering a dropout rate of 10%.
Data were analyzed using SPSS statistical software version 26.0 (IBM, USA) and Prism 6 software (GraphPad Software, Inc, La Jolla, CA, USA). The Kolmogorov–Smirnov test was used to test the normality distribution. The continuous data were presented as mean ± standard deviation (SD) or median and interquartile range (IQR) according to the normality test. The categorical data were reported as the number (percentage, %). The continuous data with normal distribution were analyzed by one-way analysis of variance followed by Tukey post hoc test. Non-normally distributed continuous data were analyzed by the Kruskal–Wallis rank-sum test. Generalized linear mixed (GEE) model was used in repeated measurement data. Ordered categorical data were analyzed using the Kruskal–Wallis H-test, whereas disordered categorical data were analyzed using the Chi-square test or Fisher’s exact test with P value adjusted according to the Bonferroni method. A P < 0.05 was considered statistically significant.
Results
Participant Enrollment and Baseline Characteristics
One hundred and twenty children were assessed. Finally, 104 children were allocated randomly into four groups (26 in each group). The recruitment details are shown in . There were no significant differences in patient characteristics, including age, sex distribution, height, and weight among the four groups (). The extubation time, operating duration, and PACU length of stay were also comparable among these groups.
Table 1 Clinical Characteristics of the Participating Children
Emergency Agitation
As shown in , the occurrences of EA (PAED ≥ 10) for the 0.15 mg/kg and 0.2 mg/kg nalbuphine groups were 30.8% (P < 0.05) and 26.9% (P < 0.05), respectively, which was significantly lower than that (73.1%) in the 0.9% saline group. Furthermore, the occurrence of EA decreased with the increasing dose of nalbuphine, as indicated by the statistically lower occurrence of EA in the 0.2 mg/kg nalbuphine group compared with those in the 0.1 mg/kg (P < 0.05) and 0.15 mg/kg nalbuphine groups (P < 0.05).
Table 2 Emergence Agitation and Postoperative Pain Comparisons Among Different Groups
The occurrence of severe EA (PAED ≥ 15) and peak agitation scores show a similar trend. Nine (34.6%) children in the 0.9% saline group suffered from severe EA, whereas only three (11.5%) children in the 0.1 mg/kg nalbuphine group and one (3.8%) child in the 0.15 mg/kg nalbuphine group were affected. There were no children with severe EA in the 0.2 mg/kg nalbuphine group (P = 0.001).
The peak PAED scores in the 0.15 mg/kg nalbuphine group (9.5 ± 4.2) and 0.2 mg/kg nalbuphine group (5.5 ± 3.2) were significantly lower than that in the 0.9% saline group (13.0 ± 4.7) (P = 0.003, P < 0.001, respectively). Meanwhile, the peak PAED score was lower in the higher dose of nalbuphine group, (0.2 mg/kg nalbuphine group, 5.5 ± 3.2) than those in the 0.1 mg/kg nalbuphine group (10.5 ± 3.2) and 0.15 mg/kg nalbuphine group (9.5 ± 4.2) (P = 0.001, P = 0.032, respectively).
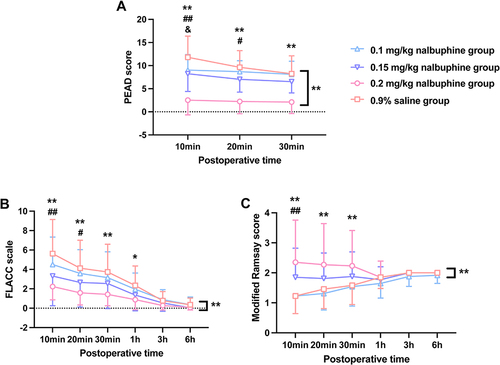
The results of agitation scores at different time points were also in consistent with previous findings (P < 0.001, ). The significant differences of PAED score between low-dose nalbuphine and control groups gradually disappeared after 10 mins in the 0.1 mg/kg nalbuphine group and 20 mins in the 0.15 mg/kg nalbuphine group. Only the 0.2 mg/kg nalbuphine group had a persistent and significantly lower PAED score.
Figure 2 Comparisons of PAED, FLACC, and modified Ramsay score among different groups at different time points after extubation (A-C).
Postoperative Analgesia
We studied the FLACC score to evaluate the analgesic effect of nalbuphine. Seventeen children (65.4%) in the 0.9% saline group had insufficient analgesia (FLACC ≥ 4), which was significantly higher than those in children who received nalbuphine, with sixteen (61.5%), nine (34.6%), and two (7.7%) children in the 0.1 mg/kg, 0.15 mg/kg, and 0.2 mg/kg nalbuphine groups, respectively (P < 0.001, ). The analgesic effect of nalbuphine also showed the dose-dependent effect, since the occurrence of insufficient analgesia in the 0.2 mg/kg nalbuphine group was significantly reduced compared with the 0.9% saline and 0.1 mg/kg nalbuphine groups (P < 0.001, ).
Notably, 0.15 mg/kg nalbuphine caused significant reduction in FLACC score during the first 20 mins postoperatively, while the analgesic effect of 0.2 mg/kg nalbuphine could last 1 h after the procedure (P < 0.001, ). There was no difference in FLACC score between nalbuphine groups and 0.9% saline group at 3 and 6 h after the surgery (P > 0.05, ).
Postoperative Sedation
According to the modified Ramsay score, an anxious child was scored as 1, which was more frequently observed in the 0.1 mg/kg nalbuphine group (84.6%) and 0.9% saline group (84.6%) than the 0.15 mg/kg nalbuphine group (46.2%) and 0.2 mg/kg nalbuphine group (42.3%, P < 0.01. ). Meanwhile, the percentages of children with satisfactory sedation (Ramsay = 2–4) was higher in the 0.15 mg/kg nalbuphine group (53.9%) and 0.2 mg/kg nalbuphine group (50.0%) than those in the 0.1 mg/kg nalbuphine group (15.4%) and 0.9% saline group (15.4%, P < 0.05. ). There was no difference in over-sedation (Ramsay > 4) between nalbuphine groups and the 0.9% saline group (P > 0.05, ).
Table 3 Comparisons of Postoperative Sedation Among Different Groups
Specifically, 0.2 mg/kg nalbuphine significantly elevated Ramsay score to achieve relatively satisfactory sedative effect during the first 30 mins after recovery, while 0.15 mg/kg nalbuphine improved the Ramsay score within 10 mins after extubation (P < 0.001, ). However, 0.2 mg/kg nalbuphine caused undesired over-sedation in two (7.7%) children within 30 mins after extubation without statistical significance. There was no difference in Ramsay score between nalbuphine groups and the 0.9% saline group at 1, 3, and 6 h after surgery (P > 0.05, ).
Side Effects
Although 0.2 mg/kg nalbuphine caused undesired oversedation in two children within 30 mins after extubation, neither of them had hypoxemia, apnea, laryngospasm, PONV, or pruritus, suggesting 0.1–0.2 mg/kg nalbuphine was safe in the population studied. In addition, no side effects, hypoxemia, apnea, laryngospasm, PONV, or pruritus, were observed in the other groups.
Discussion
In the present study, we demonstrated that the occurrence of EA and postoperative pain was 73.1% and 65.4% in children undergoing cochlear implantation surgery without nalbuphine administration, respectively. Nalbuphine applied 15–30 mins before the end of the procedure could alleviate EA and postoperative pain and provide satisfactory postoperative sedation. A higher dose of nalbuphine could have stronger efficacy, without affecting extubation time, PACU length of stay, or serious adverse side effects.
EA is one of the most common complications in pediatric anesthesia, especially in toddler and preschool children receiving sevoflurane-based anesthesia. The occurrence of EA has been attributed to several factors, including age, postoperative pain, surgery type, and operative duration.Citation4 Previous studies have mainly focused on children undergoing adeno-tonsillectomy and found the occurrence of EA was up to 72%.Citation23 In the present study, we found the occurrence of EA was 73.1% in children undergoing cochlear implantation because of difficulties in communication, which is similar to adeno-tonsillectomy. In a retrospective anesthesia chart review, the occurrence of EA was reported as 2.63% in children undergoing cochlear implantation.Citation24 The difference in EA occurrence might be due to the study design, older age, medications used during the procedure, and/or surgical techniques. Nevertheless, our results further emphasized the importance of EA management after cochlear implantation.
As an agonist-antagonist opioid analgesic, nalbuphine has been widely used in pediatric anesthesia because of its moderate analgesic effect and safety.Citation9–11 The reported onset and peak action time after intravenous nalbuphine administration are 1–2 min and 30 mins, respectively. Meanwhile, EA typically presents in the early recovery phase with an average of 14 minutes after the general anesthesia.Citation25 Therefore, nalbuphine was applied 15–30 min before the end of surgery in our study.
Most recently, a retrospective cohort analysis revealed that 0.1 mg/kg nalbuphine in preschool children undergoing ophthalmic surgery reduced PAED score to ≤6 and FLACC scale to ≤3 during the recovery period.Citation12 In addition, 0.1 mg/kg of nalbuphine showed a significant EA reduction in children during MRI examination, which showed a better effect than 0.25 mg/kg of ketamine.Citation13 However, ophthalmic surgery or MRI examination only cause mild pain or discomfort during and after the procedures. Another multi-center RCT study investigated the effect of nalbuphine on EA occurrence in children, aged 3–9 years, that underwent adenotonsillectomy under general anesthesia. In consistent with our results, they confirmed that 0.1 mg/kg nalbuphine could significantly alleviate EA and postoperative pain.Citation14 Given the older age, the incidence of EA decreased to 28.39% in control group, indicating surgery and nalbuphine may exhibit different influence in EA in different age groups. Further, the above research only investigated a single-dose formula of nalbuphine, whether higher dosage is more effective and safer needs to be addressed. To fill in the gap, we conducted the present study in children aged 6 months to 3 years old, who are most sensitive to general anesthesia associated neurological impairs, and explored the effect of 3 increasing dosages from 0.1 mg/kg to 0.2 mg/kg of nalbuphine. Intriguingly, nalbuphine dose-dependently reduced the occurrence and severity of EA, as well increased the duration of EA reduction effect, which provided an important extension of nalbuphine’s clinical application in young children undergoing otorhinolaryngologic surgery.
As demonstrated previously, postoperative pain is an important risk factor of EA in children. The risk of EA increased 1.32 times for every 1-point increase in the pain intensity.Citation26 It has been reported that the occurrence of acute moderate and severe postoperative pain in children is as high as 60%.Citation27 Further, 68.9% of children require analgesics after cochlear implantation.Citation28 Our findings further confirmed that most children (65.4%) suffer from postoperative pain after cochlear implantation surgery. A higher dose of nalbuphine could provide favorable analgesic effect. Unlike fentanyl,Citation6 nalbuphine did not influence the extubation time and PONV even at a high dose of 0.2 mg/kg. Our results were also consistent with the study of Liaqat et al, which reported a satisfactory analgesic effect of nalbuphine at a dose of 0.2 mg/kg.Citation27 Taken together, the available evidence suggests that nalbuphine might be an attractive candidate for postoperative pain prevention. Notably, the postoperative PAED score and FLACC scale shared a similar trend, further supporting that the postoperative pain intensity is highly correlated with EA.
Satisfactory sedation is a mitigation for EA. Brenner et al reported that 0.1 mg/kg nalbuphine with midazolam could provide satisfactory sedation in infants and children under caudal anesthesia.Citation29 Animal studies also revealed that 1–2 mg/kg nalbuphine promoted mild sedation,Citation30 while 0.3–1 mg/kg nalbuphine decreased the degree of sedation induced by morphine and acepromazine due to its partial antagonism effect.Citation31 Our results showed that 0.1mg/kg nalbuphine was not enough to promote sedation, whereas 0.15 and 0.2 mg/kg nalbuphine could achieve satisfactory sedation. However, 2 children in 0.2 mg/kg nalbuphine group showed excessive sedation without prolonged PACU length of stay, suggesting the oversedation of 0.2 mg/kg nalbuphine might not influence postoperative recovery. Further research on the safety of high-dose nalbuphine usage is required.
The main concern of opioid application during the surgical procedure is delayed recovery, respiratory distress, PONV, and pruritus. However, we found no difference in the PACU length of stay and no adverse events reported in the study groups. We hypothesize that the safety profile may be derived from the following: Firstly, we administered nalbuphine at 15–30 min before the end of surgery, which minimized the possibility of prolonged extubation time. Secondly, dexamethasone was applied as a premedication, which has been confirmed to be effective to prevent PONV in patients undergoing cochlear implant surgery.Citation24 Thirdly, intravenous administration of nalbuphine (3 and 10 mg) has been shown to be an effective treatment of opioid-induced pruritus due to its partial antagonism effect.Citation32 Future large sample studies should be performed to validate our findings.
Limitations of our study included small sample size and single-center research. Large scale, multi-center clinical trials are necessary to confirm our findings. We only studied the effects of nalbuphine on EA after sevoflurane inhalation anesthesia for cochlear implantation. Future studies are required to investigate the nalbuphine effects under other types of general anesthesia.
Conclusions
In conclusion, young children undergoing cochlear implantation surgery were at a high risk of EA and postoperative pain, which could be minimized by nalbuphine administration. Compared with 0.1 mg/kg and 0.15 mg/kg nalbuphine, 0.2 mg/kg nalbuphine could have more powerful and prolonged effect on EA, postoperative pain, and sedation, might be an ideal dose when used under close monitoring.
Abbreviations
ASA, American Society Anesthesiologists; EA, emergency agitation; FLACC, the Faces, Legs, Activity, Cry, and Consolability; GEE, Generalized linear mixed; MRI, magnetic resonance imaging; PAED, Pediatric Anesthesia Emergence Delirium; PACU, post-anesthesia care unit; PONV, postoperative nausea and vomiting; SD, standard deviation.
Ethics Approval and Informed Consent
The study protocol was reviewed and approved by the ethics committee of Xijing Hospital (KY20202089-F-1) and was registered at the Chinese Clinical Trial Registry (ChiCTR2000040407) on November 28, 2020. The parents or healthcare guardians signed the informed consent.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors appreciate the staff in the operating room and PACU for their assistance during data collection.
Data Sharing Statement
The raw data are available upon reasonable requests to the corresponding authors.
Additional information
Funding
References
- Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138–1145. doi:10.1097/00000542-200405000-00015
- Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104(1):84–91. doi:10.1213/01.ane.0000250914.91881.a8
- Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99(6):1648–1654. doi:10.1213/01.ANE.0000136471.36680.97
- Mason KP. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth. 2017;118(3):335–343. doi:10.1093/bja/aew477
- Kanaya A. Emergence agitation in children: risk factors, prevention, and treatment. J Anesth. 2016;30(2):261–267. doi:10.1007/s00540-015-2098-5
- Kim N, Park JH, Lee JS, Choi T, Kim MS, Lerman J. Effects of intravenous fentanyl around the end of surgery on emergence agitation in children: systematic review and meta-analysis. Paediatr Anaesth. 2017;27(9):885–892. doi:10.1111/pan.13181
- Tan Y, Shi Y, Ding H, Kong X, Zhou H, Tian J. μ-Opioid agonists for preventing emergence agitation under sevoflurane anesthesia in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2016;26(2):139–150. doi:10.1111/pan.12815
- Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007;62(12):1266–1280. doi:10.1111/j.1365-2044.2007.05221.x
- Ji K, Gong X, Luan T, Gao X, Zang B. Pain management of nalbuphine and sufentanil in patients admitted intensive care unit of different ages. BMC Emerg Med. 2022;22(1):50. doi:10.1186/s12873-022-00592-x
- Chen F, Wang CY, Zhang J, et al. Comparison of postoperative analgesic effects between nalbuphine and fentanyl in children undergoing adenotonsillectomy: a prospective, randomized, double-blind, multicenter study. Front Pharmacol. 2020;11:597550. doi:10.3389/fphar.2020.597550
- Deng C, Wang X, Zhu Q, Kang Y, Yang J, Wang H. Comparison of nalbuphine and sufentanil for colonoscopy: a randomized controlled trial. PLoS One. 2017;12(12):e0188901. doi:10.1371/journal.pone.0188901
- Leister N, Trieschmann U, Yücetepe S, et al. Nalbuphine as analgesic in preschool children undergoing ophthalmic surgery and the occurrence of emergence delirium. Br J Ophthalmol. 2022. doi:10.1136/bjo-2022-321575
- Dalens BJ, Pinard AM, Létourneau DR, Albert NT, Truchon RJ. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth Analg. 2006;102(4):1056–1061. doi:10.1213/01.ane.0000200282.38041.1f
- He J, Zhang L, Tao T, et al. Nalbuphine reduces the incidence of emergence agitation in children undergoing Adenotonsillectomy: a prospective, randomized, double-blind, multicenter study. J Clin Anesth. 2023;85:111044. doi:10.1016/j.jclinane.2022.111044
- Cho EJ, Yoon SZ, Cho JE, Lee HW. Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology. 2014;120(6):1354–1361. doi:10.1097/ALN.0000000000000181
- Pedersen LK, Rahbek O, Nikolajsen L, Møller-Madsen B. The revised FLACC score: reliability and validation for pain assessment in children with cerebral palsy. Scand J Pain. 2015;9(1):57–61. doi:10.1016/j.sjpain.2015.06.007
- Fallah R, Yadegari Y, Behdad S, Akhavan Karbasi S. Melatonin and intravenous midazolam administered orally in drug induced sleep electroencephalography of children: randomized clinical trial of efficacy. Arch Iran Med. 2014;17(11):741–745.
- Chu L, Wang Y, Wang S, Su S, Guo Z, Wang G. Intranasal Dexmedetomidine Accompanied by Cartoon Video Preoperation for Reducing Emergence Delirium in Children Undergoing Strabismus Surgery: a Prospective Randomized Trial. Front Surg. 2021;8:754591. doi:10.3389/fsurg.2021.754591
- Lei DX, Wu CJ, Wu ZY, Wang LY, Zhao Q, She YJ. Efficacy of different doses of intranasal dexmedetomidine in preventing emergence agitation in children with inhalational anaesthesia: a prospective randomised trial. Eur J Anaesthesiol. 2022;39(11):858–867. doi:10.1097/EJA.0000000000001743
- Akbulut UE, Cakir M. Efficacy and safety of low dose ketamine and midazolam combination for diagnostic upper gastrointestinal endoscopy in children. Pediatr Gastroenterol Hepatol Nutr. 2015;18(3):160–167. doi:10.5223/pghn.2015.18.3.160
- Rusy LM, Hoffman GM, Weisman SJ. Electroacupuncture prophylaxis of postoperative nausea and vomiting following pediatric tonsillectomy with or without adenoidectomy. Anesthesiology. 2002;96(2):300–305. doi:10.1097/00000542-200202000-00013
- Aono J, Ueda W, Mamiya K, Takimoto E, Manabe M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology. 1997;87(6):1298–1300. doi:10.1097/00000542-199712000-00006
- Abdulatif M, Ahmed A, Mukhtar A, Badawy S. The effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesia. Anaesthesia. 2013;68(10):1045–1052. doi:10.1111/anae.12380
- Darlong V, Khanna P, Baidya DK, et al. Perioperative complications of cochlear implant surgery in children. J Anesth. 2015;29(1):126–130. doi:10.1007/s00540-014-1878-7
- Menser C, Smith H. Emergence agitation and delirium: considerations for epidemiology and routine monitoring in pediatric patients. Local Reg Anesth. 2020;13:73–83. doi:10.2147/LRA.S181459
- Kim HC, Kim E, Jeon YT, et al. Postanaesthetic emergence agitation in adult patients after general anaesthesia for urological surgery. J Int Med Res. 2015;43(2):226–235. doi:10.1177/0300060514562489
- Liaqat N, Dar SH. Comparison of single-dose nalbuphine versus tramadol for postoperative pain management in children: a randomized, controlled trial. Korean J Anesthesiol. 2017;70(2):184–187. doi:10.4097/kjae.2017.70.2.184
- Birman CS, Gibson WP, Elliott EJ. Pediatric cochlear implantation: associated with minimal postoperative pain and dizziness. Otol Neurotol. 2015;36(2):220–222. doi:10.1097/MAO.0000000000000569
- Brenner L, Kettner SC, Marhofer P, et al. Caudal anaesthesia under sedation: a prospective analysis of 512 infants and children. Br J Anaesth. 2010;104(6):751–755. doi:10.1093/bja/aeq082
- Gomes VH, Marques JLR, Janiques Borré LDS, de Cerqueira Teixeira JG, da Silva MFA. Comparison of the sedative effects of three nalbuphine doses, alone or combined with acepromazine, in dogs. Am J Vet Res. 2022;83(7). doi:10.2460/ajvr.21.12.0214
- Gomes VH, Barcellos MC, Lima VC, de Moura RA, de Freitas JB, da Silva MF. Effect of three doses of nalbuphine on reversal of sedation and cardiopulmonary effects of morphine-acepromazine in healthy dogs. Vet Anaesth Analg. 2019;46(4):429–434. doi:10.1016/j.vaa.2019.03.001
- Jannuzzi RG. Nalbuphine for treatment of opioid-induced pruritus: a systematic review of literature. Clin J Pain. 2016;32(1):87–93. doi:10.1097/AJP.0000000000000211