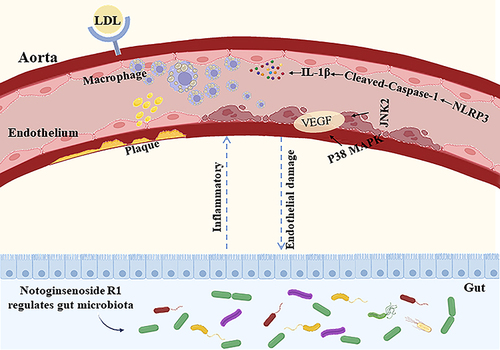
Abstract
Aim
Natural medicines possess significant research and application value in the field of atherosclerosis (AS) treatment. The study was performed to investigate the impacts of a natural drug component, notoginsenoside R1, on the development of atherosclerosis (AS) and the potential mechanisms.
Methods
Rats induced with AS by a high-fat-diet and vitamin D3 were treated with notoginsenoside R1 for six weeks. The ameliorative effect of NR1 on AS rats was assessed by detecting pathological changes in the abdominal aorta, biochemical indices in serum and protein expression in the abdominal aorta, as well as by analysing the gut microbiota.
Results
The NR1 group exhibited a noticeable reduction in plaque pathology. Notoginsenoside R1 can significantly improve serum lipid profiles, encompassing TG, TC, LDL, ox-LDL, and HDL. Simultaneously, IL-6, IL-33, TNF-α, and IL-1β levels are decreased by notoginsenoside R1 in lowering inflammatory elements. Notoginsenoside R1 can suppress the secretion of VCAM-1 and ICAM-1, as well as enhance the levels of plasma NO and eNOS. Furthermore, notoginsenoside R1 inhibits the NLRP3/Cleaved Caspase-1/IL-1β inflammatory pathway and reduces the expression of the JNK2/P38 MAPK/VEGF endothelial damage pathway. Fecal analysis showed that notoginsenoside R1 remodeled the gut microbiota of AS rats by decreasing the count of pathogenic bacteria (such as Firmicutes and Proteobacteria) and increasing the quantity of probiotic bacteria (such as Bacteroidetes).
Conclusion
Notoginsenoside R1, due to its unique anti-inflammatory properties, may potentially prevent the progression of atherosclerosis. This mechanism helps protect the vascular endothelium from damage, while also regulating the imbalance of intestinal microbiota, thereby maintaining the overall health of the body.
Introduction
In China and worldwide, cardiovascular diseases (CVDs) are primary cause of fatalities, impairments, and a significant health burden. Atherosclerosis (AS), a chronic condition characterized by the buildup of lipids and fibrous substances in large arteries, plays a significant role in the onset and progression of CVDs.Citation1 Thus, preventing the development and advancement of AS could potentially reduce the mortality rate due to CVDs.
Chronic inflammation theory, lipid infiltration theory, and endothelial injury theory have occupied important positionsCitation2 in the study of the pathophysiological mechanisms of AS. It is subject to numerous factors, incorporating pathological mechanisms like inflammation, oxidative stress, apoptosis, and lipid buildup.Citation3 The features of AS primarily involve the participation of immunity and inflammation. Prolonged development of inflammation in the arterial intima leads to pathological changes in the aorta and triggers endothelial damage.Citation4 This ultimately results in the formation of plaques and increases plaque instability.Citation5 The development of AS is thought to coincide with flow-mediated inflammatory alterations in endothelial cells (ECs). Possible mechanisms of inflammation include cellular senescence, NLRP3 inflammasome activation, oxidative stress, increased intestinal permeability, and lifestyle and dietary effects on microbial composition.Citation6,Citation7 Meanwhile, endothelial dysfunction also promotes rupture of atherosclerotic plaques.Citation8
Furthermore, growing research suggests that changes in the in the composition and function of intestinal microbiota can lead to disruptions in lipid metabolism and intestinal inflammation, which ultimately contribute to the initiation and advancement of AS.Citation9 Disturbances in intestinal microbiota and over colonization by harmful bacteria can damage the intestinal mucosa, disrupting its integrity and leading to greater permeability, which triggers an abnormal immune response, causing inflammation in exacerbating the development of AS.Citation10 Thus, the prevention of vascular damage by modulating inflammation and endothelial injury has the potential to ameliorate AS. Simultaneously, maintaining the integrity of the intestinal barrier and regulating the homeostasis of the intestinal microbiota are new strategies for the prevention of AS.
Notoginsenoside R1 (NR1) is an herbal monomer isolated from Panax notoginseng (Burk.) F. H. Chen,Citation11 which has the efficacy of relieving pain, addressing blood stasis and halting hemorrhaging. Notoginsenoside R1 has been found applications for the avoidance and management of heart ischemic,Citation12 diabetes,Citation13 and cerebrovascular disease.Citation14 Emerging data show that notoginsenoside R1 can ameliorate abnormal lipid metabolism in myocardial ischemic rats by promoting the activation of the Akt/mTOR pathway.Citation15 In addition, it was found that monomeric notoginsenoside R1 could inhibit the body’s oxidative stress and reduce the generation of cytokines with inflammatory properties by inflammatory response.Citation16 However, it is known that the effect of notoginsenoside R1 on AS is ambiguous. Accordingly, in the ongoing research, we aimed to examine the impact of notoginsenoside R1 on impeding the progression of inflammation to protect against endothelial damage, and in modulating gut microbiota function to protect against AS. We studied atherosclerotic rats established on a high-fat-diet (HFD) combined with vitamin D3 (VD3), and verified the anti-atherosclerotic effect of notoginsenoside R1 by analyzing intestinal microbiota enrichment and endothelial damage results. Studying of the mechanism of action of notoginsenoside R1 in ameliorating AS may provide new options for the treatment of AS.
Materials and Methods
Notoginsenoside R1
Notoginsenoside R1 purchased from Shanghai yuanye Bio-Technology Co., Ltd (Shanghai, China). The purity of notoginsenoside R1 was ≥98%. It was dissolved in phosphate buffer saline (PBS) and stored at −4 °C until it was administered to the rats.
Animal Experiments
To determine the appropriate sample size for animal experiments, the Resource Equation Method was employed.Citation17 Based on the formula, which calculates the number of subjects per group as n = 10/k + 1 (k = number of groups, n = number of subjects per group), it was determined that the minimum number of rats in each group should be five, resulting in a total minimum sample size of 15 rats. To gather sufficient experimental data and ensure the robustness of the results, a total of 24 rats were ultimately utilized for the experiments. Twenty-four male Sprague-Dawley rats (180–200 g, 8-week-old) were purchased and accommodated in a room for 1-week adaptation period for follow-up experiments (temperature: 22 ± 1°C; humidity: 50 ± 1%; light/dark cycle: 12 h/12 h). The experiment adhered to the Animal Care Committee guidelines of Jilin Academy of Agricultural Sciences (Ethical reference number: 57/2021). The rats were randomly assigned to experimental groups (n=8) (i) control; (ii) model and (iii) NR1. The model and NR1 group were intraperitoneally maintained to be fed a high-fat-diet (HFD) (the composition consists of basic feed (62%), cholesterol (2.5%), lard (10%), egg yolk (5%), sodium cholate hydrate (0.5%), and sucrose (20%)) and injected intraperitoneally with VD3 (700,000 U/kg/bw) on days 3, 5, and 7. A normal diet was provided to the control group. The model and control groups received PBS solution. NR1 group were administered 25 mg/kg bw notoginsenoside R1 monomer by gavage per day for six weeks. After fasting for 12 h, the rats were injected intraperitoneally with 2 mL/kg of 5% pentobarbital sodium and euthanized after blood collection through heart puncture. Their blood was then collected and preserved.
Biochemistry Analysis
The serum was obtained by centrifuging the blood. Lipid indicators and adhesion molecules were analyzed with commercial diagnostic kits including triglyceride (TG), total cholesterol (TC) (Suzhou Michy Biomedical Technology Co., Ltd., Suzhou, China) and nitric oxide (NO) (JL-T1271; Jianglai biology, Shanghai, China). Lipid, adhesion molecules and inflammation indicators were analyzed with the kits of commercial enzyme-linked immunosorbent assay (ELISA) including, low-density lipoprotein (LDL), high-density lipoprotein (HDL), oxidized low density lipoprotein (ox-LDL), vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecule-1 (ICAM-1), interleukin-6 (IL-6), interleukin-33 (IL-33), interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α) (Jiangsu Meibiao Biotechnology Co. Ltd., Jiangsu, China) and endothelial nitric oxide synthase (eNOS) (JL21190; Jianglai biology, Shanghai, China).
Oil Red Staining of the Aorta
The aorta of the entire rat was extracted and adjacent connective tissue was carefully eliminated. The abdominal aorta tissues were placed in a 10% neutral formaldehyde solution for immersion. After a 48 h fixation, the abdominal aorta tissues underwent freezing and were then sectioned into cryosections measuring 5 μm in thickness using a microtome. The abdominal aorta cryosections were subjected to Oil Red O staining. The histopathological sections obtained were examined using an optical microscope (Olympus, Tokyo, Japan) for the examination of the atherosclerotic lesions in the aortic.
Western Blotting
Aorta protein extracts were prepared by utilizing cell lysis buffer (Solarbio, China); the supernatant was gathered following centrifugation. The BCA Protein Assay Kit (Coolaber, China) was employed to assess the protein levels. The protein samples underwent electrophoresis and were then transferred to membranes. The membranes were initially blocked, followed by an overnight incubation with specific primary antibodies targeting NLRP3 (Solarbio, China), Cleaved Caspase-1, IL-1β, JNK2, P38 MAPK, VEGF (Cell Signaling Technology) and β-Actin (Bioss, Beijing, China). The membranes were subjected to appropriate secondary antibodies conjugated with HRP for a duration of 1 hour. Signal detection was performed using ECL (UElandy, Suzhou, China).
16S rRNA Gene High-Throughput Sequencing to Detect Intestinal Microbiota
Rat fecal samples were collected, promptly quickly frozen with liquid nitrogen and maintained at −80°C for later examination. Total DNA was isolated and refined from fecal material using a QIAamp Fast DNA Fecal Mini Kit (QIAGEN, Germany). The primers designed are F: ACTCCTACGGGAGGCAGCA R: GGACTACHVGGGTWTCTAAT, targeting the bacterial 16S rRNA (V3-V4) region for PCR amplification. Subsequently, The PCR products obtained were then sequenced on the Illumina MiSeq platform, and sequences with a high degree of homology at 97% were classified into operational taxonomic units (OTU). The analysis of gut microbiota diversity was conducted using the QIIME tool (v 1.9).
Statistical Analysis
The mean ± standard deviation (SD) is used to display the data. For datasets with n ≥ 5, we employ the Q-Q plot to assess the normality of the data and Levene’s test to evaluate the homogeneity of variance. If the results indicate that the data conform to a normal distribution and exhibit equal variation, we proceed to analyze the significance of differences among multiple groups using the one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test for further intergroup comparisons. With the utilization of GraphPad Prism 5 software and SPSS 20 software, the data was aggregated, and box-and-whisker plots were generated. At a significance level of p < 0.05, statistical significance was established.
Results
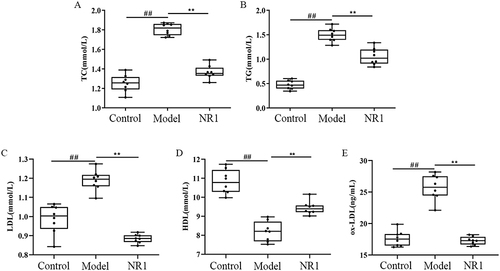
NR1 Regulates Serum Lipid Levels in AS Rats
We conducted experimental assessments of alterations in the levels of serum lipid in AS rats (). By gavage for six weeks with notoginsenoside R1, the results showed a reduced serum TG and TC levels by 24.31% and 29.53% (p < 0.001, p < 0.001) in the NR1 group, respectively. The level of HDL increased by 16.12% (p <0.001), the LDL level was reduced by 26.05% (p < 0.001) and the ox-LDL level was downregulated by 32.88% than model group (p < 0.001).
NR1 Ameliorates Pathological Morphological Features of Aorta in AS Rats
The extent of lipid accumulation was disclosed through Oil Red O staining. in AS lesions in rats treated with notoginsenoside R1 was evaluated by observing sections of the rats’ aortic root (). The aortic tissue’s pathological morphology was assessed using optical microscopy. The results of staining in the pathological sections revealed that in comparison to the control group, in the model group, a substantial quantity of plaque was observed within the aortic root, and there’s more lipid accumulation. It indicated that administration of HFD and VD3 to AS rats could successfully replicate AS model. After treatment with notoginsenoside R1, the lesion area of the aortic root plaque was lower than that in the model group.
Figure 2 Impact of notoginsenoside R1 administration on aortic histopathological features in rats (200×). The abdominal aorta was extracted promptly upon rat euthanasia. We conducted Oil Red O staining and H&E staining to visualize plaque size and lipid content.
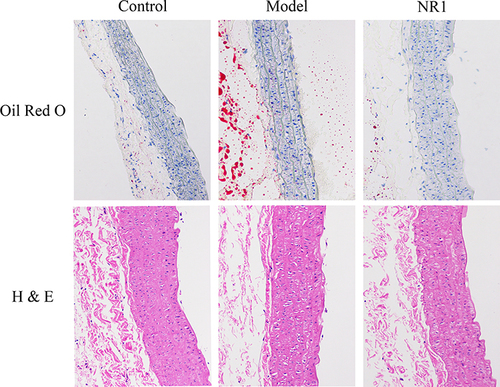
The H&E staining revealed the plaque area at the abdominal aortic root (). The results indicated that the lipid distribution and calcium salt distribution were uniform in the control group. In contrast, the abdominal aortic structure in the model group was more disordered, with significantly increased lipid deposition and a doubled amount of calcium salt. Conversely, in the NR1 group, there was a significant decrease in lipid deposition and a reduction in calcium salt quantity, with the abdominal aortic morphology approaching that of the control group. This suggests that supplementation with notoginsenoside R1 can reduce the plaque area at the abdominal aortic root in atherosclerosis.
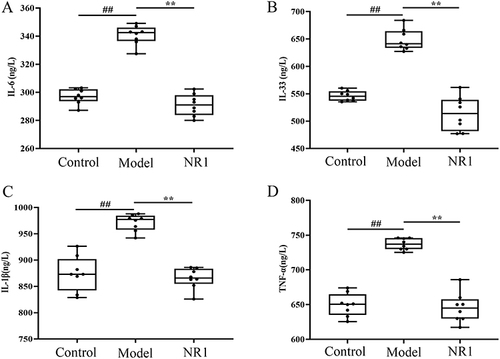
NR1 Inhibits the Production of Inflammatory Cytokines in AS Rats
The levels of IL-6, IL-33, IL-1β, and TNF-α in the serum were quantified to evaluate the effect of notoginsenoside R1 on inflammatory cytokine production in AS rats (). Compared to the control group, all four inflammatory factors exhibited significantly heightened expression within the model group under investigation. Whereas this was markedly inversed after notoginsenoside R1 administration. The factors of IL-6, IL-33, TNF-α and IL-1β all experienced varying degrees of diminishing, with drops of 14.60%, 20.71%, 10.92% and 12.43%, respectively (p < 0.001).
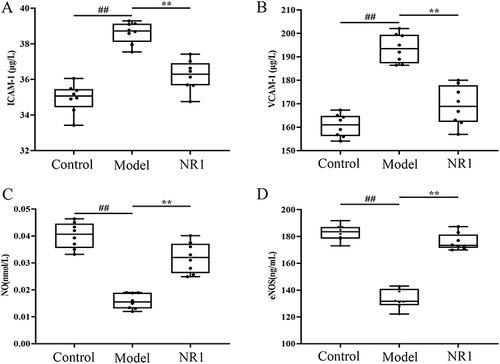
NR1 Exerts an Impact on the Serum Adhesion Molecules Expression in AS Rats
To explore the suppressive impact of notoginsenoside R1 on cell adhesion in atherosclerotic rats, we measured the concentrations of cell adhesion molecule within the aorta. The results are shown in , the levels of plasma ICAM-1 and VCAM-1 were significantly enhanced, in model group compared to control-treated rats. The intervention of notoginsenoside R1 has demonstrated significant effects (p < 0.001). The expression of the two elements, NO and eNOS, showed opposite results. There is a decline in the expression of NO and eNOS in atherosclerotic rats, while their expression grew by 103.11% and 31.78%, respectively, after ingesting notoginsenoside R1 (p < 0.001).
Figure 4 Impacts of notoginsenoside R1 on the adhesion molecule levels: (A)ICAM-1, (B)VCAM-1, (C)NO and (D)eNOS in AS rats. Results are expressed as the mean ± SD. ##p < 0.01 versus control group; **p < 0.01 versus model group (n = 8).
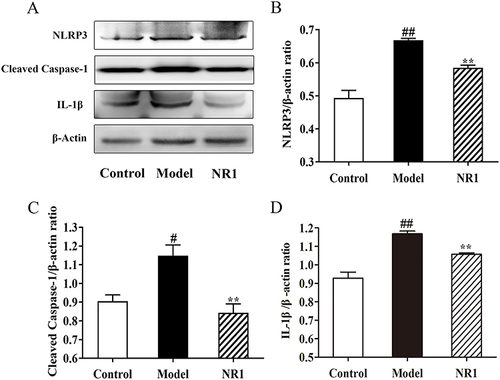
NR1 Suppresses Inflammation Occurs in AS Rats
As endothelial cell apoptosis is a significant factor in AS progression, our investigation aimed to determine if supplementing with notoginsenoside R1 could mitigate the apoptotic response triggered by AS rats ( and S1). To explore the mechanism of action of notoginsenoside R1 in AS rats, we detected inflammation-related proteins by analyzing the expression levels of NLRP3, Cleaved Caspase-1 and IL-1β. We found that HFD combined with VD3 significantly promoted the expression of NLRP3 (p <0.001), IL-1β (p <0.001) and Cleaved Caspase-1 (p = 0.026), and proteins in aortic tissues of SD rats. Notoginsenoside R1 inhibited the expression of NLRP3 (p = 0.016), Cleaved Caspase-1 (p = 0.009) and IL-1β (p = 0.024).
Figure 5 Notoginsenoside R1 inhibits activation and expression of NLRP3/Cleaved Caspase-1/IL-1β pathway in AS rats. (A) Expression levels of related protein expression were determined by the Western blot. Quantitative analysis of the expression of (B) NLRP3, (C) Cleaved Caspase-1, and (D) IL-1β, and β-Actin served as the internal control for protein analysis. All the values are represented by the means ± SD. ## p < 0.01 and # p < 0.05 versus control group; ** p < 0.01 and * p < 0.05 versus model group.
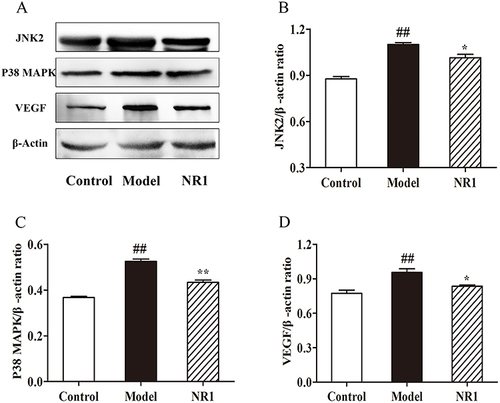
NR1 Can Hinder Vascular Damage in AS Rats
Arterial endothelial damage is the main cause of the development of AS. Hence, modulation of arterial endothelium-related pathways is essential for alleviating AS. Western blot analysis ( and S2) revealed that in vascular tissue, HFD combined with VD3 triggered a substantial elevation with the expression in JNK2 (p <0.001), P38 MAPK (p <0.001), and VEGF (p = 0.005). Nevertheless, after notoginsenoside R1 intervention, the expression of JNK2 (p = 0.028) and VEGF was inhibited (p = 0.031) and the expression of P38 MAPK was significantly down-regulated (p = 0.001). Hence, we can speculate that notoginsenoside R1 may exert its protective effect on endothelial injury by inhibiting the expression of JNK2 pathway members.
Figure 6 Notoginsenoside R1 modulates the expression of proteins related to endothelial factors. (A) Expression levels of related protein expression were ascertained by the Western blot. Quantitative analysis of the levels of (B) JNK2, (C) P38 MAPK, and (D) VEGF expression, and β-Actin was used the internal control of proteins. All data points are expressed by the means ± SD. ## p < 0.01 and # p < 0.05 versus control group; ** p < 0.01 and * p < 0.05 versus model group.
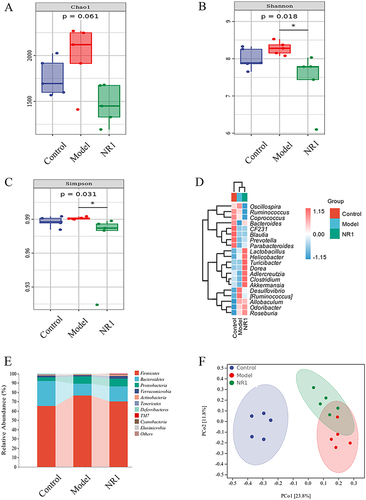
NR1 Improves Gut Microbiota Dysbiosis in AS Rats
We elucidated the impact of Notoginsenoside R1 intervention on the abundance of the gut microbiota using 16S rRNA sequencing (). In the alpha diversity analysis (), Chao 1 (p = 0.061), Shannon (p = 0.018) and Simpson (p = 0.031) indexes of the NR1 group were observably decreased compared with the model group. At the genus level (), notoginsenoside R1 intervention led to a reduction in the relative abundance of Desulfovibrio and Ruminococcus, and markedly increased the Akkermansia, Bacteroides, Lactobacillus, Prevotella, and Clostridium levels. Hence, the administration of Notoginsenoside R1 reinstated the prevalence of beneficial bacteria and diminished the presence of deleterious bacteria. At the phylum level (), the relative abundance of deleterious bacteria in AS rats, including Firmicutes and Proteobacteria, exhibited an increase, whereas the relative abundance of beneficial bacteria, such as Bacteroidetes, demonstrated a decrease. Of note, the administration of notoginsenoside R1 resulted in a substantial rise in the abundance of Bacteroidetes and a concurrent reduction in the abundance of Firmicutes and Proteobacteria. Moreover, in the PCoA analysis (), We identified a substantial disparity in distance among the model and control groups, while the NR1 group closely resembled the normal group. The results showed that notoginsenoside R1 treatment normalized the gut microbial diversity.
Figure 7 Notoginsenoside R1-mediated changes of gut microbiota in HFD combined with VD3-induced AS rats (n = 5). (A) Chao 1 indexes. (B) Shannon indexes. (C) Simpson indexes. (D) Heat map analysis of species composition. (E) Species compositions at the phylum level and (F) Principal coordinate analysis (PCoA) of all samples by weighted UniFrac distance.
Discussion
The distinctive pathogenesis of AS involves the accumulation of lipids and the rapid escalation of inflammatory responses.Citation18 The process of modified and absorbed retained LDL leads to continuous growth of fatty infiltrates rich in inflammatory leukocytes, which macroscopically manifests as plaques.Citation5 It has been shown that geniposide in combination with notoginsenoside R1 can inhibit AS plaque formation by improving lipid levels.Citation19 This implies that notoginsenoside R1 can play an anti-lipid accumulation role by reducing the lipid content in AS rats. This was also demonstrated in our current findings. We found that oral administration of notoginsenoside R1 significantly reduced the lipid levels, including TG, TC, HDL, LDL and ox-LDL, and the formation of atherosclerotic plaques was inhibited induced by HFD combined with VD3 in AS rats. And, LDL loading promotes the entry of ox-LDL into macrophages, triggering inflammation in the arterial wall, which in turn causes pro-inflammatory signaling.Citation20,Citation21 Hence, we first evaluated metrics related to inflammatory factors. The results indicate that supplementation of notoginsenoside R1 markedly weakened the amounts of inflammatory factors IL-6, IL-33, IL-1β and TNF-α. It demonstrates that the addition of notoginsenoside R1 has a notable impact on the inflammatory state. Xiao’s findings also illustrate that notoginsenoside R1 can attenuate inflammation in by inhibiting the elevation of factors such as TNF-α, IL-1β, and IL-6.Citation22 In addition, cholesterol loading also activates the inflammatory vesicle NLRP3.Citation23 Activation of the NLRP3 inflammasome facilitates the cleavage of caspase-1 and the production of IL-1β, processes that play pivotal roles in various diseases, including atherosclerosis and nonalcoholic steatohepatitis.Citation24 Animal experiments have confirmed that the Huanglian-Wuzhuyu herb pair significantly protects against NASH by inhibiting the NLRP3 inflammasome, thereby reducing inflammation and hepatic steatosis.Citation25 In the present study, the NLRP3/Cleaved Caspase-1/IL-1β pathway was activated in AS rats. In contrast, notoginsenoside R1 treatment downregulates the protein expression of NLRP3, which affects the inhibition of Cleaved Caspase-1 and IL-1β expression, ultimately exerting an anti-inflammatory effect. By diminishing the activity of Cleaved Caspase-1, NLRP3, and IL-1β in diabetic nephropathy, notoginsenoside R1 has also been proven to suppress the cellular inflammatory response.Citation26
Besides, vascular inflammation in AS leads to endothelial dysfunction and plaque formation.Citation27 Simultaneously, evidences have indicated that cells within the vascular wall are capable of releasing inflammatory mediators, establishing the foundation for the development of atherosclerotic lesions.Citation28 Notoginsenoside R1 has been proven to decrease ox-LDL-mediated apoptosis and adhesion-related molecule release in human umbilical vein endothelial cells (HUVECs).Citation29 Drawing from the findings of our experiments, supplementing notoginsenoside R1 has the ability to effectively suppress the MAPK signaling pathway. As a result, stimulates the production of NO. This contributes to the reduction of expression levels of VEGF, as well as endothelial cell adhesion molecules ICAM-1 and VCAM-1. There have also been studies showing that notoginsenoside R1 can inhibit the production of inflammatory cytokines induced by ox-LDL, thereby impeding the initiation of NF-κB and MAPK pathways,Citation30 exerting anti-inflammatory effects. Overall, this process ultimately aids in lowering the risk of AS development.
In recent years, growing body of research suggests that alterations in gut microbiota are linked to various disease states, including cardiovascular diseases (CVDs).Citation31 Reports have shown that alterations in the diversity and abundance of gut microbial species can heighten the risk of AS development.Citation32 It has been demonstrated that Panax notoginseng saponins (PNS) reduced the diversity of intestinal microorganisms in mice fed on high-fat diets and augmented the abundance of beneficial bacteria, including Akkermansia.Citation33 This is consistent with our report. In the present study, from various indices of alpha diversity, it is apparent that the microbial diversity in the intestines of the model group rats is richer than that in the normal group rats. This indicates an imbalance in the intestinal microbiota of atherosclerotic rats. In contrast, the intestinal microbial diversity in the NR1 intervention group rats is the lowest, which may be due to the saponin components reducing bacterial diversity indices. Supplementing with Akkermansia has the potential to alleviate metabolic disorders like elevated lipid levels and insulin resistance in mice and humans with diet-induced obesity.Citation34 Akkermansia can improve gut barrier function.Citation35 At the phylum level, notoginsenoside R1 remodeled the gut microbiota of AS rats by decreasing the count of pathogenic bacteria (such as Firmicutes and Proteobacteria) and increasing the quantity of probiotic bacteria (such as Bacteroidetes). At the genus level, HFD combined with VD3 reduced the relative richness of Akkermansia, Bacteroides and Lactobacillus. It augmented the relative richness of Desulfovibrio and Ruminococcus. However, notoginsenoside R1 treatment dramatically reversed the relative richness of these genera in AS rats. Studies have proven that fermented ginseng upregulates probiotics such as Lactobacillus and Akkermansia, thereby reducing inflammation levels.Citation36 In conclusion, these findings indicate that oral ingestion of notoginsenoside R1 influences the composition of the intestinal microbiota and is beneficial in ameliorating the dysbiosis induced by HFD combined with VD3.
Conclusions
The present study preliminarily suggests that notoginsenoside R1 can lower blood lipid levels, diminish the formation of atherosclerotic plaques, and reveal the protective effect of NR1 on endothelial cell injury and inflammatory responses. Concurrently, it is found that NR1 can improve the development of AS by regulating the balance of intestinal microbiota. Collectively, notoginsenoside R1 can improve AS by regulating the interaction between the intestinal and vascular, providing strong support for further research and application of notoginsenoside R1.
Ethics Approval and Consent to Participate
The ethical approval of this study was provided by the the Animal Care Committee guidelines of Jilin Academy of Agricultural Sciences (Ethical reference number: 57/2021).
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was financially supported by Jilin Province Science and Technology Development Program (20240601012RC).
Data Sharing Statement
The data used to support the findings of this study are included in the article.
References
- Li F, Zhang T, He Y, et al. Inflammation inhibition and gut microbiota regulation by TSG to combat atherosclerosis in ApoE-/-mice. J Ethnopharmacol. 2020;247:112232. doi:10.1016/j.jep.2019.112232
- Xu S, Ilyas I, Little PJ, et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: from Mechanism to Pharmacotherapies. Pharmacol Rev. 2021;73(3):924–967. doi:10.1124/pharmrev.120.000096
- Ma L, Zhao Z, Zhao Y, Gao Y, Zhao L, Li S. Weizmannia coagulans JA845 improves atherosclerosis induced by vitamin D3 and high-fat diet in rats through modulating lipid metabolism, oxidative stress, and endothelial vascular injury. J Appl Microbiol. 2023;134(8):lxad165. doi:10.1093/jambio/lxad165
- Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting Early Atherosclerosis: a Focus on Oxidative Stress and Inflammation. Oxid Med Cell Longev. 2019;2019:8563845. doi:10.1155/2019/8563845
- Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
- Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res. 2018;122(12):1722–1740. doi:10.1161/CIRCRESAHA.118.311362
- Brandsma E, Kloosterhuis NJ, Koster M, et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ Res. 2019;124(1):94–100. doi:10.1161/CIRCRESAHA.118.313234
- Sedding DG, Boyle EC, Demandt JAF, et al. Vasa Vasorum Angiogenesis: key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front Immunol. 2018;9:706. doi:10.3389/fimmu.2018.00706
- Navab-Moghadam F, Sedighi M, Khamseh ME, et al. The association of type II diabetes with gut microbiota composition. Microb Pathog. 2017;110:630–636. doi:10.1016/j.micpath.2017.07.034
- Schietroma M, Pessia B, Carlei F, Mariani P, Sista F, Amicucci G. Intestinal permeability and systemic endotoxemia in patients with acute pancreatitis. Ann Ital Chir. 2016;87:138–144.
- Wang T, Guo R, Zhou G, et al. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J Ethnopharmacol. 2016;188:234–258. doi:10.1016/j.jep.2016.05.005
- Ge ZR, Xu MC, Huang YU, Zhang CJ, Lin JE, Ruan CW. Cardioprotective effect of notoginsenoside R1 in a rabbit lung remote ischemic postconditioning model via activation of the TGF-β1/TAK1 signaling pathway. Exp Ther Med. 2016;11(6):2341–2348. doi:10.3892/etm.2016.3222
- Zhai Y, Meng X, Luo Y, et al. Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget. 2018;9(10):9344–9363. doi:10.18632/oncotarget.24295
- Guo S, Xi X, Li J. Notoginsenoside R1: a systematic review of its pharmacological properties. Pharmazie. 2019;74(11):641–647. doi:10.1691/ph.2019.9534
- Lei W, Yan Y, Ma Y, et al. Notoginsenoside R1 Regulates Ischemic Myocardial Lipid Metabolism by Activating the AKT/mTOR Signaling Pathway. Front Pharmacol. 2022;13:905092. doi:10.3389/fphar.2022.905092
- Shi X, Yu W, Liu L, et al. Panax notoginseng saponins administration modulates pro- /anti-inflammatory factor expression and improves neurologic outcome following permanent MCAO in rats. Metab Brain Dis. 2017;32(1):221–233. doi:10.1007/s11011-016-9901-3
- Arifin WN, Zahiruddin WM. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays J Med Sci. 2017;24(5):101–105. doi:10.21315/mjms2017.24.5.11
- Kim M, Sahu A, Hwang Y, et al. Targeted delivery of anti-inflammatory cytokine by nanocarrier reduces atherosclerosis in Apo E-/- mice. Biomaterials. 2020;226:119550. doi:10.1016/j.biomaterials.2019.119550
- Liu X, Xu Y, Cheng S, et al. Geniposide Combined With Notoginsenoside R1 Attenuates Inflammation and Apoptosis in Atherosclerosis via the AMPK/mTOR/Nrf2 Signaling Pathway. Front Pharmacol. 2021;12:687394. doi:10.3389/fphar.2021.687394
- van der Valk FM, Bekkering S, Kroon J, et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation. 2016;134(8):611–624. doi:10.1161/CIRCULATIONAHA.116.020838
- Mundi S, Massaro M, Scoditti E, et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res. 2018;114(1):35–52. doi:10.1093/cvr/cvx226
- Xiao J, Zhu T, Yin YZ, Sun B. Notoginsenoside R1, a unique constituent of Panax notoginseng, blinds proinflammatory monocytes to protect against cardiac hypertrophy in ApoE-/- mice. Eur J Pharmacol. 2018;833:441–450. doi:10.1016/j.ejphar.2018.07.004
- Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi:10.1038/nature08938
- Liu D, Zeng X, Li X, Mehta JL, Wang X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol. 2017;113(1):5. doi:10.1007/s00395-017-0663-9
- Zhang X, Gao R, Zhou Z, et al. Uncovering the mechanism of Huanglian-Wuzhuyu herb pair in treating nonalcoholic steatohepatitis based on network pharmacology and experimental validation. J Ethnopharmacol. 2022;296:115405. doi:10.1016/j.jep.2022.115405
- Zhang C, Yuan R, Li S, et al. Notoginsenoside R1 Protects against Diabetic Nephropathy through TXNIP-NLRP3 Signaling Pathway. Clin Compl Med Pharmacol. 2023;3(4):100100. doi:10.1016/j.ccmp.2023.100100
- Horio E, Kadomatsu T, Miyata K, et al. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2014;34(4):790–800. doi:10.1161/ATVBAHA.113.303116
- Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol. 2016;84:1–7. doi:10.1016/j.vph.2016.05.013
- Fu C, Yin D, Nie H, Sun D. Notoginsenoside R1 Protects HUVEC Against Oxidized Low Density Lipoprotein (Ox-LDL)-Induced Atherogenic Response via Down-Regulating miR-132. Cell Physiol Biochem. 2018;51(4):1739–1750. doi:10.1159/000495677
- Su P, Du S, Li H, Li Z, Xin W, Zhang W. Notoginsenoside R1 inhibits oxidized low-density lipoprotein induced inflammatory cytokines production in human endothelial EA.hy926 cells. Eur J Pharmacol. 2016;770:9–15. doi:10.1016/j.ejphar.2015.11.040
- Witkowski M, Weeks TL, Hazen SL. Gut Microbiota and Cardiovascular Disease. Circ Res. 2020;127(4):553–570. doi:10.1161/CIRCRESAHA.120.316242
- Pieczynska MD, Yang Y, Petrykowski S, Horbanczuk OK, Atanasov AG, Horbanczuk JO. Gut Microbiota and Its Metabolites in Atherosclerosis Development. Molecules. 2020;25(3):594. doi:10.3390/molecules25030594
- Xu Y, Wang N, Tan HY, et al. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics. 2020;10(24):11302–11323. doi:10.7150/thno.47746
- Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi:10.1073/pnas.1219451110
- Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation. 2016;133(24):2434–2446. doi:10.1161/CIRCULATIONAHA.115.019645
- Fan J, Wang Y, You Y, et al. Fermented ginseng improved alcohol liver injury in association with changes in the gut microbiota of mice. Food Funct. 2019;10(9):5566–5573. doi:10.1039/c9fo01415b