Abstract
Purpose
The aim of the present study was to investigate the immune response induced by Mycobacterium marinum infection in vitro and the potential of M. marinum as an immunotherapy for M. tuberculosis infection.
Methods
The potential human immune response to certain bacillus infections was investigated in an immune cell-bacillus coculture system in vitro. As a potential novel immunotherapy, M. marinum was studied and compared with two other bacilli, Bacillus Calmette-Guérin (BCG) and live attenuated M. tuberculosis. We examined the changes in both the bacilli and immune cells, especially the time course of the viability of mycobacteria in the coculture system and host immune responses including multinuclear giant cell formation by Wright-Giemsa modified staining, macrophage polarization by cell surface antigen expression, and cytokines/chemokine production by both mRNA expression and protein secretion.
Results
The M. marinum stimulated coculture group showed more expression of CD209, CD68, CD80, and CD86 than the BCG and M. tuberculosis (an attenuated strain, H37Ra) groups, although the differences were not statistically significant. Moreover, the M. marinum group expressed more interleukin (IL)-1B and IL-12p40 on day 3 (IL-1B: P = 0.003 and 0.004, respectively; IL-12p40: P = 0.001 and 0.011, respectively), a higher level of CXCL10 on day 1 (P = 0.006 and 0.026, respectively), and higher levels of chemokine (C-X-C motif) ligand (CXCL) 8 and chemokine (C motif) ligand (XCL) 1 on day 3 (CXCL8: P = 0.012 and 0.014, respectively; XCL1: P = 0.000 and 0.000, respectively). The M. marinum stimulated coculture group also secreted more tumor necrosis factor (TNF)-α, IL-1β, and IL-10 on day 1 (TNF-α: P = 0.000 and 0.000, respectively; IL-1β: P = 0.000 and 0.000, respectively; IL-10: P = 0.002 and 0.019, respectively) and day 3 (TNF-α: P = 0.000 and 0.000, respectively; IL-1β: P = 0.000 and 0.001, respectively; IL-10: P = 0.000 and 0.000, respectively). In addition, the colony-forming units (an index of viability) of M. marinum in the M. marinum stimulated coculture group was significantly less than that of BCG and H37Ra in their corresponding bacillus stimulated groups (P = 0.037 and 0.013, respectively).
Conclusion
Our results indicated that M. marinum could be a potentially safe and effective immunotherapy.
Introduction
Immunotherapy, via modulation of the host immune response to infection, tumor, and autoantigen in autoimmune diseases, has been increasingly applied in treatment of many diseases, especially in combination with other therapeutic approaches. Tuberculosis (TB) is a global infectious disease caused by Mycobacterium tuberculosis (Mtb) infection and is usually treated with a combination of different antibiotics for more than 6 months. However, the recent appearance of drug resistant Mtb strains has led to treatment failure and increased incidence of TB worldwide.Citation1 Nowadays, immunotherapy is the focus of research for new effective treatment of TB. Cytokines and immune cells have been explored as potential immunotherapies with limited or disappointing efficacy.Citation2 Meanwhile, studies have shown that the prophylactic and therapeutic vaccines, including heat-killed and live attenuated mycobacteria, and DNA/proteins of mycobacterial origin, appear to be more safe and effective.Citation2–Citation4 These vaccines have been used both as adjuvants for immunization and immunotherapies for several diseases such as Mtb infection and bladder cancer;Citation5–Citation9 however, all of these vaccines were known to control, but not to eradicate, Mtb infection.Citation10,Citation11 Theoretically speaking, mycobacterial priming vaccines of an improved whole organism could potentially induce a more effcient immune response than other vaccines such as those from bacterial components.Citation12 We chose to focus our study on M. marinum (Mm) considering its reported safety that (1) most exposures in humans do not result in infectious disease and (2) the infected individual only shows several skin lesions at local sites without dispersal to the entire body or severe systemic responses.Citation13 Thus Mm is possibly a safe whole organism vaccine that can potentially be used as immunotherapy to treat TB infection.
Host immunity against mycobacterial infections involves complex interactions between the bacterium and various components of the host immune system including immune cells, cytokines, and chemokines. Macrophages are the most important immune cells against mycobacterial infections. Macrophages can phagocytose the bacilli at the local sites but are unable to eradicate them.Citation14,Citation15 Studies have shown that macrophages can polarize into two subtypes (M1 and M2) in response to infections, autoimmune diseases, and tumors.Citation16,Citation17 However, the function of its polarization in mycobacterial infection remains unclear. In addition to the immune cells, several cytokines such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-12 play critical roles in immune reactions against mycobacterial infections.Citation18 Moreover, chemokines such as chemokine (C-C motif) ligand (CCL) 3 and 5, chemokine (C-X-C motif) ligand (CXCL) 2, 9, and 10, and chemokine (C motif) ligand (XCL) 1 were reported to be involved in the type 1 response to mycobacterial infection and the recruitment of monocytes in local sites to constitute granuloma formation during mycobacterial infection.Citation19 Although many studies used animal models to study mycobacterial infection, extrapolation of results obtained in animals (eg, mice) to humans was usually difficult because the immune response could be drastically different in various species. In vitro studies using human cells do not have this issue and are useful tools for studying human immunity.Citation20 Various human immune cells such as human monocytes, macrophages, and peripheral blood mononuclear cells (PBMCs) have been examined in the study of infections caused by various bacteria such as Mtb, Bacillus Calmette-Guérin (BCG), M. avium, and Mm.Citation20,Citation21 In mycobacterial infection, local macrophages that engulf the bacilli are the most important cells in the innate immune response which induce the recruitment of monocytes/macrophages and lymphocytes to local sites to constitute granuloma formation and stimulate adaptive immunity.Citation14
In the present study, we used a macrophage, PBMC, and mycobacterium coculture system as a tool to study the immune response to mycobacterial infections in vitro. We examined various components of the immune response including immune cells, cytokines, and chemokines stimulated by Mm (TMC 1218), BCG (TMC 1010), and Mtb (H37Ra). We found that Mm (TMC 1218) induced more significant Th1 and Th2 cell immunity with better safety compared with BCG and Mtb. Our results suggested that Mm had potential value as an immunotherapy for TB patients.
Material and methods
Immune cells and bacteria
Peripheral blood was obtained from healthy Chinese donors with informed consent. In the People’s Republic of China, BCG vaccination is compulsory for children (0–4 years). PBMCs were isolated using gradient centrifugation on Ficoll-Paque PREMIUM (GE Healthcare, Piscataway, NJ, USA), and monocytes were further enriched by the adherence method in AIM-V medium (Invitrogen, Grand Island, N Y, USA). Macrophages were differentiated from monocytes using GM-CSF (Peprotech, Rocky Hill, NJ, USA) as previously describedCitation22 and cultured for 5 days in RPMI 1640 medium and 20% fetal calf serum (HyClone, Waltham, MA, USA) at 37°C.
Mm (TMC 1218), BCG (TMC 1010), and Mtb (H37Ra) were cultured on modified were cultured on modified Lowenstein-Jensen Medium Base. All bacteria were collected in Middlebrook 7H9 Broth (BD Difco, Sparks, MD, USA) and mixed with syringe needles. The bacteria were cultured with a serial dilution on modified Lowenstein-Jensen medium and the viability was monitored by counting the colony-forming units (CFU) on the base.
Coculture of macrophages, PBMCs, and bacteria
Macrophages differentiated from monocytes were transferred into 24 well tissue culture plates (Corning, Tewksbury MA, USA) (1 × 10Citation5 cells per well). Freshly prepared Mm, BCG, and H37Ra were subsequently added to each well with a multiplicity of infection (MOI) of 0.5 based on trial results with different MOIs. After 24 hours, autologous PBMCs were added into macrophages infected with bacteria (M+P+B) at a ratio of 5:1. Meanwhile, macrophages infected with different bacteria without PBMCs (M+B) and macrophages cocultured with PBMCs without bacteria (M+P) were included as controls. The cells were cultured for periods from 24 hours to 10 days at optimal growth temperatures of different bacilli (35°C for Mm and 37°C for the other two bacilli) with medium changed every other day.
Microscopy and cell examination
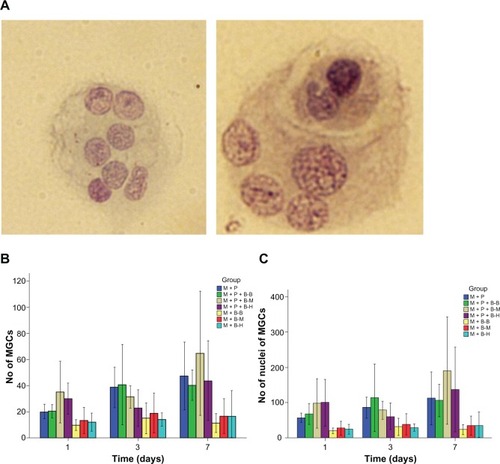
The cocultured cells were observed under an inverted microscope (Nikon, Chiyoda-ku, Tokyo, Japan). Photographs were taken with a Nikon capture system. Cells were stained with Wright-Giemsa (W-G) modified staining (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions after 1, 3, and 7 days cultivation, and multinuclear giant cells (MGCs) with different numbers of nuclei were observed and counted using a light microscope up to a fixed total number of cells.
Analysis of cell surface antigens by flow cytometry
Cells were collected after 1 and 7 days cultivation and the expression of cell surface antigens on macrophages, especially those associated with macrophage polarization, was analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Dead cells stained positive with 7-amino actinomycin D stain were excluded from the analysis. The monoclonal antibodies (mAbs) used in the antigen analysis included fluorescein isothiocyanate-conjugated mAb against CD68 (Y1/82A), phycoerythrin-conjugated mAb against CD163 (GHI/61) and CD80 (L307.4), and PerCP-CyTM 5.5-conjugated mAb against CD209 (DCN46), CD86 (FUN-1), and CD14 (M5E2) from BD Biosciences. The percentages in specific quadrants indicated the ratio of the cells stained positively with the variable antigens.
Real time polymerase chain reaction (RT-PCR) and ELISA assays
The expression of different cytokines and chemokines was detected by RT-PCR. RNAs were extracted using RNeasy Mini kit (QIAGEN, Düsseldorf, NW, Germany) and reverse transcribed by a reverse transcription system (Promega, Madison, WI, USA). RT-PCR was performed using SYBR Green PCR Master Mix (Promega) according to the manufacturer’s instructions. The mRNA expression was determined on the ABI 7300 instrument with the ABI software (Applied Bio-systems, Grand Island NY, USA). Various cytokines in the culture supernatants including IFN-γ, TNF-α, IL1-β, IL-4, IL-10, and IL-12p70 were quantified using human double-antibody sandwich indirect ELISA (enzyme-linked immunosorbent assay) kits (NeoBioscience, Shenzhen, Guangdong, People’s Republic of China). The quantities of cytokines were presented in terms of protein concentration (pg/mL).
Determination of bacterial viability
The three bacteria were recovered from the macrophages with 0.1 M sodium hydroxide and their viability was determined by culturing on Middlebrook 7H10 Agar (BD Bioscience). Mm was cultured at 32°C, and BCG and H37Ra at 37°C.
Statistical analysis
All experiments were carried out in triplicate. The relative mRNA expression of target genes was calculated with the 2−∆∆Ct method. Data were analyzed using SPSS 13.0 (SPSS Inc, Chicago, IL, USA). The statistical significance of the data was analyzed using univariate ANOVA and the general linear model. Error bars represent the standard deviations of the triplicate values.
Results
Granuloma-like aggregate formation
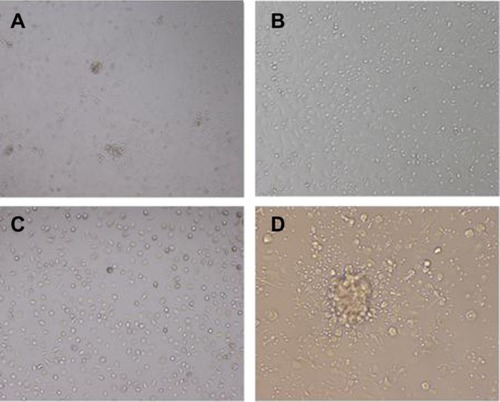
When macrophages infected by Mm, BCG, or H37Ra were cocultured with autologous PBMCs (M+P+B−M, M+P+B−B, and M+P+B−H) in a 24 well tissue culture plate, some cells aggregated to form granuloma-like aggregates on day 7 as shown in . No aggregates were observed in the M+P and M+B control groups at this stage (). The cellular structure of the granuloma-like aggregates resembled that observed in histopathologic specimens of the tuberculoid leprosy lesion in which lymphocytes were on the outskirts surrounding the cellular aggregates (). The sizes of cellular aggregates in the three M+P+B groups showed no visible differences under the microscope.
Figure 1 Granuloma-like aggregates formed by cocultivation of three different bacilli and two kinds of immune cells.
MGC formation
The cells were collected after 1, 3, and 7 days cocultivation, plated on glass slides, and stained with W–G modified staining for examination under the microscope. It was observed that several cells aggregated and fused in the M+P+B groups (). A significant difference was observed between the M+P+B−M and M+B−M groups (P = 0.039). While most MGCs formed in the three M+P+B groups had two nuclei, the MGCs in the M+P+B−M group on average had more nuclei than those in the M+B−M group (P = 0.023) (). On day 7, there were more MGCs observed in the M+P+B−M group than in the M+P+B−B and M+P+B−H groups, and the MGCs in the M+P+B−M group showed a higher number of nuclei as well, although the differences were not statistically significant (P = 0.266 and 0.334 for the number of MGCs, respectively; P = 0.230 and 0.444 for the number of nuclei per MGC, respectively) ().
Figure 2 Characterization of multinuclear giant cells (MgCs) formed after stimulation with different bacilli.
Cell surface antigen expression
The expression of macrophage/monocyte cell surface antigens was investigated on days 1 and 7. Seven antigens were detected in the study, which were grouped into three types: monocyte (CD14), M1 type (CD68, 80, and 86), and M2 type (163, 206, and 209). On day 1, no significant differences were detected in the expression of most antigens between any two groups. Cells in the M+P+B (−B, −M, and −H) groups seemed to express more CD86 on day 1 and less CD 163 on day 7 than cells in the M+P group (CD86: P = 0.041, 0.029, and 0.041, respectively; CD163: P = 0.003, 0.000, and 0.001, respectively) (). However, on day 7, cells in the M+B (−B, −M, and −H) groups expressed less CD209 and CD80 than cells in both the M+P+B (−B, −M, and −H) groups (CD209: P = 0.037, 0.007 and 0.042, respectively; CD80: P = 0.002, 0.002 and 0.006, respectively) and M+P groups (CD209: P = 0.001, 0.000 and 0.000, respectively; CD80: P = 0.000, 0.000 and 0.000, respectively). On day 7, cells in the M+P+B−M group showed more CD206, CD209, CD68, CD80, and CD86 expression than cells in the M+P+B−B and M+P+B−H groups (). However, no significant differences in the antigens were observed between any two of the M+P+B groups.
Table 1 The percentage of antigen (CD163, CD206, CD209, CD68, CD80, and CD86) positive cells in various groups on day 1 and day 7 (%)
mRNA expression of cytokines and chemokines
The cellular mRNA expression of the cytokines TNF-α, IFN-γ, IL-12p40, and IL-1B was detected by RT-PCR. The TNF-α expression in the M+P+B groups peaked on day 3 with a level higher in the M+P+B−M group than in the M+B−M group (P = 0.017). The IFN-γ expression in the M+P+B groups also peaked on day 3 with a much higher expression in the M+P+B-M group than in the M+P and M+B−M groups (P = 0.000 and 0.000, respectively). The M+P+B−H group also had a higher IFN-γ expression than the M+P and the M+B−H groups (P = 0.000 and 0.000, respectively). Among the three M+P+B groups, the M+P+B−M and the M+P+B−H groups exhibited higher IFN-γ expression than the M+P+B−B group (P = 0.000 and 0.000, respectively). On day 3 and 7, the M+P+B−M group expressed higher levels of IL-12p40 than the M+P+B−B and M+P+B−H groups (day 3: P = 0.001 and 0.011, respectively; day 7: P = 0.001 and 0.000, respectively). The IL-1B expression was highest on day 1 in the M+P+B groups, and higher IL-1B expression was observed in the M+P+B−M group when compared with the M+P, M+B−M, M+P+B−B, and M+P+B−H groups on day 3 (P = 0.021, 0.000, 0.003, and 0.004, respectively) ( and ).
Table 2 The relative mRNA expression kinetics of different cytokines on day 1, 3, and 7
Figure 3 The mRNA expression kinetics of cytokines and chemokines was detected by RT-PCR.
Abbreviations: CXCL, chemokine (C-X-C motif) ligand; IL, interleukin; TNF, tumor necrosis factor; XCL, chemokine (C motif) ligand; RT-PCR, real time polymerase chain reaction.
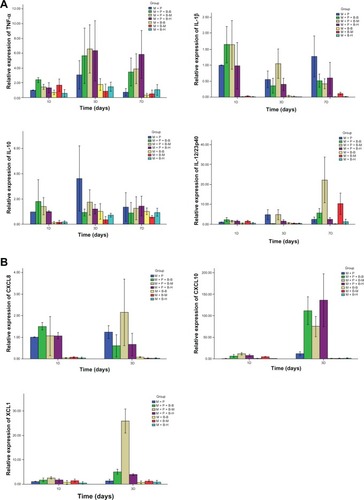
Expression of chemokines was investigated by detecting their mRNA levels on day 1 and 3. On day 1, cells in the M+P+B−M group showed higher levels of CCL3, CCL5, CXCL2, CXCL8, CXCL9, CXCL10, and XCL1 than cells in the M+B−M group (P = 0.031, 0.005, 0.000, 0.004, 0.002, 0.000, and 0.036, respectively). On day 3, cells in the M+P+B−M group expressed higher levels of CCL5, CXCL2, CXCL8, CXCL10, and XCL1 than cells in the M+B−M group (P = 0.023, 0.012, 0.001, 0.005, and 0.000, respectively). Cells in the M+P+B−M group demonstrated higher levels of CCL5, CXCL2, and CXCL9 on day 1 (P = 0.035, 0.002, and 0.000, respectively), and CXCL10 and XCL1 on day 1 and 3 than cells in the M+P group (day 1: P = 0.000 and 0.007, respectively; day 3: P = 0.014 and 0.000, respectively). On day 1, cells in the M+P+B−M and M+P+B−B groups expressed higher CXCL2 levels than cells in the M+P+B−H group (P = 0.002 and 0.001, respectively). When compared with cells in the M+P+B−B and M+P+B−H groups, cells in the M+P+B−M group expressed a higher level of CXCL10 on day 1 and higher levels of CXCL8 and XCL1 but a lower level of CXCL9 on day 3 (CXCL10: P = 0.006 and 0.026, respectively; CXCL8: P = 0.012 and 0.014, respectively; XCL1: P = 0.000 and 0.000, respectively; CXCL9: P = 0.005 and 0.028, respectively) ( and ).
Table 3 The relative mRNA expression kinetics of different chemokines on day 1 and day 3
Cytokines in the culture supernatants
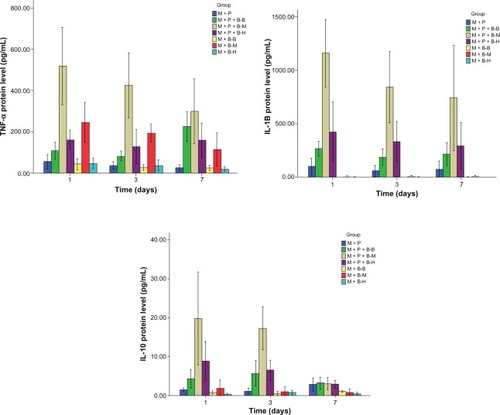
Cytokines were also detected by ELISA in the supernatants of various cell cultures on days 1, 3, and 7. In the M+P+B groups, TNF-α and IL-1β secretion peaked on day 1, IFN-γ was higher on days 3 and 7, whereas IL-10 showed higher levels on day 1 and 3 than on day 7. The culture supernatants from the M+P+B−M group contained higher levels of TNF-α, IL-1 β, and IL-10 than those from the M+P, M+B−M, M+P+B−B, and M+P+B−H groups on days 1 and 3 (day 1, TNF-α: P = 0.000, 0.001, 0.000, and 0.000, respectively; IL-1β: P = 0.000, 0.000, 0.000, and 0.000, respectively; IL-10: P = 0.001, 0.001, 0.002, and 0.019, respectively; day 3, TNF-α: P = 0.000, 0.001, 0.000, and 0.000, respectively; IL-1β: P = 0.000, 0.000, 0.000, and 0.001, respectively; IL-10: P = 0.000, 0.000, 0.000, and 0.000, respectively). On day 1, a higher level of IFN-γ was observed in the culture supernatant from the M+P+B−M group than those from the M+P and M+B−M groups (P = 0.012 and 0.039, respectively). No significant differences in IFN-γ secretion were found between the M+P+B−M and the M+P+B−H group on day 1 and 3. However, higher IFN-γ secretion was observed in the M+P+B−H group when compared with the M+P+B−B group (P = 0.048 and 0.012, respectively) ( and ). The amount of IL-12/23 was surprisingly low in the supernatants of all groups (IL-12 < 1.5 pg/mL, IL-23 < 4 pg/mL); nonetheless, IL-12/23 levels were slightly higher in the culture supernatant from the M+P+B−M group (data not shown). The IL-4 levels in the culture supernatants were below the detection level of ELISA (data not shown).
Table 4 The protein level of tumor necrosis factor-α, interferon-γ, interleukin (IL)-1β, and IL-10 on day 1, 3, and 7 (pg/mL)
Figure 4 The levels of cytokines secreted in the culture supernatant were detected by ELISA on day 1, 3, and 7.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor; ELISA, enzyme-linked immunosorbent assay.
Viability of bacilli in granuloma-like aggregates
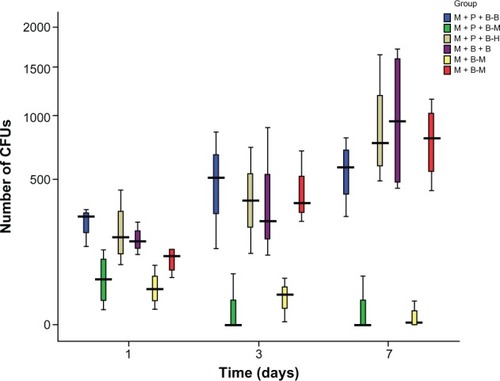
We determined the viability of Mm, BCG, and H37Ra released from phagocytes on days 1, 3, and 7 by counting the CFUs on the 7H10 medium. The number of CFUs of Mm in the M+P+B−M group was less than that of BCG in the M+P+B−B group and H37Ra in the M+P+B−H group (P = 0.037 and 0.013, respectively). The viability of Mm from the M+P+B−M group dropped more significantly when compared with that of BCG and H37Ra from the other two groups. Surprisingly, the viability of BCG and H37Ra gradually increased with cocul-tivation time following an almost identical ascending trend. No significant difference in the viability of Mm was observed between the M+P+B−M and M+B−M groups ().
Figure 5 The viability of bacilli in various groups on day 1, 3, and 7. The number of colony-forming units on the agar was counted.
Abbreviation: CFU, colony-forming unit.
Discussion
Mm is the most common nontuberculous mycobacterium, causing skin infection with no vector in humans. Most Mm exposure in humans does not cause infectious diseases.Despite causing various lesions on the skin of extremities, Mm infections rarely induce a severe systemic response. No fatal cases have thus far been reported.Citation13 These observations indicate that Mm is able to induce a sufficient host immune response while being relatively safe in humans, endowing it potential value as an agent for immunotherapy. All current immunotherapies for tuberculosis, while being able to reduce the bacterial load, fail to eradicate the bacteria. New and more effective therapies are needed for better therapeutic outcome.Research on Mm and other potential vaccines may lead to the discovery of more effective immunotherapies for better disease control.
In the present study, we used an in vitro system of macrophage, PBMC, and mycobacterium cocultivation as a tool to study the immune response to infections caused by Mm in comparison with BCG and H37Ra. The safety of the bacilli was also tested in these in vitro systems by measuring bacterial viability during cocultivation. We found that Mm was the safest among the three bacilli, exhibiting the lowest viability after cocultivation with human immune cells. The viability of bacilli in the host in the presence of host immune cells determines the severity of infection. Culturing results in our study demonstrated that Mm from the Mm-stimulated M+P+B−M and M+B−M groups exhibited much lower CFUs than BCG and H37Ra from their corresponding bacillus-stimulated groups. These differences were statistically significant on day 1, 3, and 7. The CFUs of Mm declined throughout the cocultivation period and approached zero on day 7, whereas those of the other two bacilli gradually increased. Our results indicated that Mm infection could be well controlled in our in vitro model while BCG and H37Ra could not.
Mm induced macrophage polarization to both M1 and M2 phenotypes. Monocytes/macrophages can be polarized into two subsets, the classical (M1) and the alternative (M2) one, in response to infections, solid tumors, and autoantigens in autoimmune diseases.Citation23 Previous studies have indicated that M1 exhibits CD80 and CD86 on the cell surface, whereas M2 expresses CD163, CD206, and CD209 which are diverse among various subtypes of the M2 phenotype.Citation24 However, unique or restrictive markers for identification of different subsets of macrophages have not been fully determined. In the present study, we investigated the polarization of macrophages by measuring several CD molecules on the cell surface. Previous studies validated the function of the M1 subtype against various infections,Citation14,Citation16,Citation23,Citation25 which remained unconfirmed against Mtb infection. In our study, the M1 and M2 macrophages seemed to coexist with a fine balance in the bacillus-stimulated and control groups in the early phase of infection. As the infection progressed, the expression of M2 type antigens increased at a slower rate while the M1 type increased at a faster rate in the M+P+B groups when compared with the M+P group, suggesting that mycobacterial infection gradually stimulated the M1 and inhibited the M2 polarization of macrophages. Notably, Mm induced higher expression of CD80, CD86, CD206, and CD209 than the other two bacilli, suggesting that Mm promoted both M1 and M2 programming more effectively.
Since M1 and M2 macrophage polarization has been found to be analogous to Th1 and Th2 division of CD4+ T cells in a previous study,Citation17 our findings suggested that Mm could potentially be more effective in inducing both Th1- and Th2-type immune reactions than the other two bacilli. The lack of statistical difference might be attributed to the limited samples in the study.
Mm also induced higher production of TNF-α, IL1β, IL-10, and higher IL-12p40 expression than the other two bacilli in the M+P+B groups. TNF-α was found to be associated with host resistance to various species of mycobacteria such as Mtb, M. bovis, and Mm, and TNF-α deficiency caused defects in phagocyte activation, chemokine expression, and influx of various cells into the granuloma.Citation17,Citation21 TNF-α was also found to be associated with long-term maintenance of granuloma and control of bacterial growth,Citation26,Citation27 and has been used as a marker of immune response against mycobacterial infections.Citation28 Among the three M+P+B groups, the M+P+B−M group showed highest TNF-α secretion throughout the cocultivation experiment. In addition, the M+P+B−M group also had the highest TNF-α mRNA expression on day 3 among the three M+P+B groups. The observation that the M+P+B−M group, which had the highest mRNA expression and protein secretion of TNF-α, showed the lowest bacillus viability suggested that TNF-α played a significant role in controlling bacillus infection in our in vitro model.
The IL-1 family of cytokines, especially IL-1ß, is also required for host resistance against mycobacterial infection. IL-1B deficient mice demonstrated acute susceptibility to Mtb.Citation29 In the present study, the M+P+B−M group also showed higher IL-1B mRNA expression and protein secretion than the M+P+B−B and M+P+B−H groups. Similar results were observed in levels of IL-12, another pro-inflammatory and Th1-type cytokine induced by mycobacterial stimulation. IL-12p40 is rapidly induced after Mtb, or its components are ligated with Toll-like receptor TLR2 and TLR9 in vitro.Citation18,Citation30 In this study, the IL-12p40 mRNA expression was higher in the M+P+B−M group on days 3 and 7 than in the other two M+P+B groups (P < 0.05). However, the level of secreted IL-12p70 was surprisingly low in the supernatants. The IL12p40 expression was higher when compared with the low secretion level of IL-12p70. Our results confirmed the bioactivity of IL-12p40 rather than IL-12p70, and previous studies indicated both the existence of free IL-12p40 sub-unit and formation of IL-12p40 homodimers (known as IL-12p80) in mycobacterial infection,Citation31,Citation32 which in this study needed further investigation.
The three aforementioned cytokines (TNF-α, IL-1β, and IL-12) are known to be associated with the Th1 immune response against various mycobacteria. Our cytokine expression results indicated that Mm induced a stronger Th1 immune response than BCG and H37Ra in our in vitro model. Mm unexpectedly induced increased production of IL-10. IL-10 is a typical anti-inflammatory and Th2 cytokine that modulates the immune response to mycobacteria. Elevated IL-10 was observed in TB patients and and TB-infected mice.Citation33,Citation34 IL-10 was also reported to reduce the expression of both TNF-α and IL-12/23 p40.Citation33,Citation34 In the present study, although the mRNA expression of IL-10 was lower in the M+P+B groups than in the M+P group on day 3, IL-10 secretion appeared to be easily detectable on day 1 and 3 in both the M+P+B and M+P groups, and cells in the M+P+B groups secreted more IL-10 than cells in the M+P group at the early stage of infection. Among the three M+P+B groups, cells in the M+P+B−M group secreted much more IL-10 than cells in the M+P+B−B and M+P+B−H groups. Taken together, our results have shown that mycobacterial infection induced stronger Th1 and Th2 type immune responses at the early stage of infection, highlighting the importance of balance between pro- and anti-inflammatory responses. Other studies have also stressed the fine balance between TNF-α and IL-10 (the TNF-α/IL-10 ratio) in determining the protective effect against TB.Citation18 The balance between TNF-α and IL-10 was maintained in the M+P+B−M group with higher levels of both cytokines induced by Mm.
Mm also caused higher expression of chemokines including CXCL8, CXCL10, and XCL1. Chemokines present at appropriate levels may prevent cells from migrating out of the granuloma, and hence, contribute to the maintenance of granuloma.Citation35 They have been demonstrated to be associated with mycobacterial infections. CXCL8 can induce neutrophil chemotaxis in TB patients, and exogenous CXCL8 reduced Mtb survival, while its inhibition caused Mtb proliferation.Citation36 In leprosy patients, mycobacterium-induced CXCL8 was 2 to 3 fold lower than in TB patients.Citation37 Increased chemokine levels of CCL3, 4, 5, and 12 and CXCL9 and 10 were also found in Mtb infected mice.Citation35 XCL1 was reported to be involved in chronic infection of Mtb and suppressed the induction of IFN-γ,Citation38 which might partly explain the lack of high levels of IFN-γ in the M+P+B−M group. Chiu et al proposed the hypothesis that expression patterns of chemokines were associated with upstream cytokine signaling.Citation39 They found that transcripts of CXCL10 and XCL1 were largely enhanced by Th1-related cytokines, which is consistent with our hypothesis that Mm might induce a more significant Th1 response.
Conclusion
In the present study, Mm was found to induce higher production of important cytokines (TNF-α, IL1β, and IL-10) and chemok-ines (CXCL8, CXCL10, and XCL1), suggesting that Mm may significantly induce both Th1 and Th2 cellular immunity. In contrast to BCG and H37Ra, Mm completely lost its viability in the cocultivation system with the immune cells, demonstrating the effective and superior immune response induced by Mm. Our findings also suggested that human exposure to Mm would be more tolerable than exposure to the same amount of BCG and H37Ra, implying that Mm could be superior to BCG and H37Ra with regard to the immunotherapy associated safety profile.Citation11,Citation12 The fact that Mm infections rarely lead to severe systemic involvement indicates that the immune system of a healthy human is able to provide sufficient defense against this specific pathogen. The safety of Mm can be attributed to its unique characteristics such as the proteins of the proline– glutamic acid (PE) and the proline–proline–glutamic acid (PPE) families mentioned in the previous studies.Citation40 However, further studies on the pathogenesis of Mm needs to be conducted to verify and extend our findings. The possible roles that various components of the bacillus play in inducing an immune response are not clear and require investigation. Our future research is to focus on the study of pathogenesis of Mm.
Acknowledgments
This work was funded by Grant No 30972651 from the National Natural Science Foundation of China, Grant No 2010-2012-125 from the fund for Key Clinical Program of the Ministry of Health of China, and Grant No 2011-1002-030 from Graduate Innovation Foundation of Peking Union Medical College.
Disclosure
The authors report no conflict of interest in this work.
References
- Glaziou P Floyd K Raviglione M Global burden and epidemiology of tuberculosis Clin Chest Med 2009 30 4 621 636 19925958
- Bourinbaiar AS Mezentseva M V Butov DA Immune approaches in tuberculosis therapy: a brief overview Expert Rev Anti Infect Ther 2012 10 3 381 389 22397570
- Orme IM Development of new vaccines and drugs for TB: limitations and potential strategic errors Future Microbiol 2011 6 2 161 177 21366417
- Elsabahy M Wooley KL Design of polymeric nanoparticles for biomedical delivery applications Chem Soc Rev 2012 41 7 2545 2561 22334259
- Gupta A Ahmad FJ Ahmad F Efficacy of Mycobacterium indicus pranii immunotherapy as an adjunct to chemotherapy for tuberculosis and underlying immune responses in the lung PLoS One 2012 7 7 e39215 22844392
- Mayosi BM Ntsekhe M Bosch J Rationale and design of the Investigation of the Management of Pericarditis (IMPI) trial: a 2 × 2 factorial randomized double-blind multicenter trial of adjunctive prednisolone and Mycobacterium w immunotherapy in tuberculous pericarditis Am Heart J 2013 165 2 109 115 23351812
- Kawai K Miyazaki J Joraku A Nishiyama H Akaza H Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine Cancer Sci 2013 104 1 22 27 23181987
- Rich FJ Kuhn S Hyde EJ Harper JL Ronchese F Kirman JR Induction of T cell responses and recruitment of an inflammatory dendritic cell subset following tumor immunotherapy with Mycobacterium smegmatis Cancer Immunol Immunother 2012 61 12 2333 2342 22714285
- Fávaro WJ Nunes OS Seiva FR Effects of P-MAPA immunomodulator on toll-like receptors and p53: potential therapeutic strategies for infectious diseases and cancer Infect Agent Cancer 2012 7 1 14 22709446
- Orme IM The Achilles heel of BCG Tuberculosis (Edinb) 2010 90 6 329 332 20659816
- Kaufmann SH Fact and fiction in tuberculosis vaccine research: 10 years later Lancet Infect Dis 2011 11 8 633 640 21798463
- Martin C The dream of a vaccine against tuberculosis; new vaccines improving or replacing BCG? Eur Respir J 2005 26 1 162 167 15994403
- Slany M Jezek P Fiserova V Mycobacterium marinum infections in humans and tracing of its possible environmental sources Can J Microbiol 2012 58 1 39 44 22182182
- Gordon S The macrophage: past, present and future Eur J Immunol 2007 37 Suppl 1 S9 S17 17972350
- Peyron P Vaubourgeix J Poquet Y Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence PloS Pathog 2008 4 11 e1000204 19002241
- Martinez FO Sica A Mantovani A Locati M Macrophage activation and polarization Front Biosci 2008 13 453 461 17981560
- Mills CD Kincaid K Alt JM Heilman MJ Hill AM M-1/M-2 macrophages and the Th1/Th2 paradigm J Immunol 2000 164 12 6166 6173 10843666
- Cooper AM Mayer-Barber KD Sher A Role of innate cytokines in mycobacterial infection Mucosal Immunol 2011 4 3 252 260 21430655
- Zhu X W Friedland JS Multinucleate giant cells and the control of chemokine secretion in response to Mycobacterium tuberculosis Clin Immunol 2006 120 1 10 20 16504587
- McElvania Tekippe E Allen IC Hulseberg PD Granuloma formation and host defense in chronic mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1 PloS One 2010 5 8 e12320 20808838
- Price NM Gilman RH Uddin J Recavarren S Friedland JS Unopposed matrix metalloproteinase-9 expression in human tuberculous granuloma and the role of TNF-α-dependent monocyte networks J Immunol 2003 171 10 5579 5586 14607966
- Makino M Baba M A cryopreservation method of human peripheral blood mononuclear cells for effcient production of dendritic cells Scand J Immunol 1997 45 6 618 622 9201301
- Cassetta L Cassol E Poli G Macrophage polarization in health and disease Scientific World Journal 2011 11 2391 2402 22194670
- Krausgruber T Blazek K Smallie T IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses Nat Immunol 2011 12 3 231 238 21240265
- Gordon S Taylor PR Monocyte and macrophage heterogeneity Nat Rev Immunol 2005 5 12 953 964 16322748
- Saunders BM Tran S Ruuls S Sedgwick JD Briscoe H Britton WJ Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection J Immunol 2005 174 8 4852 4859 15814712
- Olleros ML Guler R Vesin D Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-guerin and Mycobacterium tuberculosis infections Am J Pathol 2005 166 4 1109 1120 15793291
- Manzanillo PS Shiloh MU Portnoy DA Cox JS Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages Cell Host Microbe 2012 11 5 469 480 22607800
- Mayer-Barber KD Barber DL Shenderov K Cutting edge: caspase-1 independent IL-1{beta} production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo J Immunol 2010 184 7 3326 3330 20200276
- Bafica A Scanga CA Feng CG Leifer C Cheever A Sher A TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis J Exp Med 2007 202 12 1715 1724 16365150
- Gerosa F Baldani-Guerra B Lyakh LA Differential regulation of interleukin 12 and interleukin production in human dendritic cells J Exp Med 2008 205 6 1447 1461 18490488
- Hölscher C Atkinson RA Arendse B A protective and agonistic function of IL-12p40 in mycobacterial infection J Immunol 2001 167 12 6957 6966 11739515
- Awomoyi AA Marchant A Howson JM McAdam KP Blackwell JM Newport MJ Interleukin-10, polymorphism in SLC11A1 (formerly NRAMP1), and susceptibility to tuberculosis J Infect Dis 2002 186 12 1808 1814 12447767
- Beamer GL Flaherty DK Assogba BD Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice J Immunol 2008 181 8 5545 5550 18832712
- Algood HM Lin PL Flynn JL Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis Clin Infect Dis 2005 41 Suppl 3 S189 S193 15983898
- O’Kane CM Boyle JJ Horncastle DE Elkington PT Friedland JS Monocyte-dependent fibroblast CXCL8 secretion occurs in tuberculosis and limits survival of mycobacteria within macrophages J Immunol 2007 178 6 3767 3776 17339475
- Hasan Z Jamil B Zaidi I Zafar S Khan AA Hussain R Elevated serum CCL2 concomitant with a reduced mycobacterium-induced response leads to disease dissemination in leprosy Scand J Immunol 2006 63 3 241 247 16499578
- Ordway D Higgins DM Sanchez-Campillo J XCL1 (lymphotactin) chemokine produced by activated CD8 T cells during the chronic stage of infection with Mycobacterium tuberculosis negatively affects production of IFN-gamma by CD4 T cells and participates in granuloma stability J Leukoc Biol 2007 82 5 1221 1229 17699612
- Chiu BC Freeman CM Stolberg VR Cytokine-chemokine networks in experimental mycobacterial and schistosomal pulmonary granuloma formation Am J Respir Cell Mol Biol 2003 29 1 106 116 12600821
- Wang H Dong D Tang S Chen X Gao Q PPE38 of Mycobacterium marinum triggers the cross-talk of multiple pathways involved in the host response, as revealed by subcellular quantitative proteomics J Proteome Res 2013 12 5 2055 2066 23514422