Abstract
Introduction
Nerve injury is a serious complication of percutaneous endoscopic transforaminal lumbar discectomy due to nerve root contact. The maximum tolerable concentration (MTC) of ropivacaine concentration for epidural anaesthesia, is defined as the concentration that minimises pain while preserving the sensation of the nerve roots. This distinct advantage allows the patient to provide feedback to the surgeon when the nerve roots are contacted.
Methods
We used a biased-coin design to determine the MTC, which was estimated by the 10% effective concentration (EC10), ie, the concentration at which 10% of patients lost sensation in the nerve roots. The determinant for positive response was lack of sensory feedback upon contact with the nerve root, and the feedback from occurrence of sensations in the innervation area upon contact with the nerve root was defined as a negative response. Primary outcome was the response from contact nerve root. Secondary outcomes were the type and number of statements of negative response and each patient’s pain score during surgery.
Results
Fifty-four patients were included in this study. The EC10 was 0.434% (95% CI: 0.410%, 0.440%) using isotonic regression in comparison with 0.431% (95% CI: 0.399%, 0.444%) using probit regression. Three type statements of negative response were reported including “tactile sensation”, radiculalgia, and numbness.
Conclusion
The MTC of ropivacaine used for epidural anaesthesia was 0.434% to avoid nerve injury in percutaneous endoscopic transforaminal lumbar discectomy.
Introduction
Nerve injury is a complication of percutaneous endoscopic transforaminal lumbar discectomy (PELD) that results in a severe burden of disease. A previous study showed that the incidence was as high as 1.2% (5/426).Citation1 Local anaesthesia is recommended for PELD to avoid nerve injury because surgeons can predict nerve root injury according to feedback from the patients.Citation2,Citation3 However, local anaesthesia could not relieve pain completely. Therefore, general anaesthesia was recommended by some surgeons to obtain perfect analgesic efficacy.Citation4 However, the loss of consciousness means that there is no feedback from nerve root contact. General anaesthesia was associated with a higher prevalence of nerve injury than local anaesthesia.Citation5 In one study, the incidence of postoperative complications related to nerve injury was 14.6% with general anaesthesia and 8.9% with epidural anaesthesia.Citation6 A good option to solve this problem is intraoperative neurophysiological monitoring.Citation7 Nevertheless, a systematic review showed that intraoperative neurophysiological monitoring did not reduce the incidence of neurological events.Citation8 Therefore, using intraoperative neurophysiological monitoring in general anaesthesia is more meaningful in complex operations, such as resection of spinal cord tumours.
Anaesthesiologists have been searching for a better method that provides both feedback and sufficient analgesic efficacy to replace local anaesthesia. In recent years, some researchers have explored epidural anaesthesia with low concentrations of ropivacaine to achieve this goal.Citation9 Ropivacaine has the property of blocking sensory signals while preserving motor function. The concentration of 0.25%~0.375% had better analgesic efficacy in epidural anaesthesia than in local anaesthesia,Citation2,Citation6,Citation9,Citation10 and nerve injury could be detected by observing the movement of the toes during the operation.Citation9,Citation11,Citation12 However, this method could not provide immediate feedback from the nerve root contact because the loss of motor function appeared only when the nerve root had been injured.
In our hospital, the surgeons raised the following question: Can pain be blocked while maintaining the “tactile sensation” so that patients can receive immediate feedback from the nerve root contact to avoid nerve injury? This idea of pain-tactile separation block is innovative and has not been mentioned in studies thus far. When the nerve root was contacted, the sensation was different from the tactile sensation generated by skin receptors. However, whether the sensation was slight pain or genuine tactile sensation, it could serve as feedback. Accordingly, unclear sensation from nerve root contact can be defined as sensation of the nerve root (SNR). Based on pharmacodynamics, we assumed that a lower concentration of local anaesthetics would maintain better SNR. The lower concentration limit, maximizing SNR while keeping pain to a tolerable level, was determined to be 0.294% (95% CI: 0.271%, 0.303%) in our previous study.Citation13 This result confirmed that epidural anaesthesia at an appropriate concentration could maintain SNR to provide feedback from nerve root contact.
Is the low concentration maximizing SNR the best option? The presence of mild intraoperative pain was still an unpleasant experience for patients. This prompted us to further explore how to enhance the analgesic efficacy. As the concentration increases, the analgesic efficacy becomes stronger, and the SNR becomes weaker. Therefore, an upper concentration limit allows epidural anaesthesia to both maximize analgesic efficacy and maintain SNR. In this study, the upper concentration limit was investigated using a biased coin design (BCD) and isotonic regression.Citation14
Methods
This study was part of the clinical trial that was conducted at Tongde Hospital of Zhejiang Province after Research Ethics Board approval (Zhe Tongde Expedited Review No. [2020]013) and is registered on the website of the Chinese Clinical Trial Registry (ChiCTR2000031593). The title of the clinical trial was
Effective concentration range of epidural anaesthesia for avoiding nerve injury during PELD: A prospective, double-blind, cohort study of the biased-coin design (BCD) and best drug dose.
The effective concentration range was the difference between the upper concentration limit and the lower concentration limit. The lower concentration limit, defined as the minimum effective concentration (MEC) at which the patients could tolerate their pain while providing feedback, has been published before.Citation13 The upper concentration limit was defined as the maximum tolerable concentration (MTC), ie, the highest concentration that could be administered before SNR was lost. As a rule, the 10% effective concentration (EC10), ie, the concentration that caused 10% of patients to lose SNR was used to estimate the MTC due to individual differences. This study was conducted in accordance with the Declaration of Helsinki. All participants were informed about the purpose of the trial.
Patients who received PELD from November 2020 to June 2022 were recruited after providing written informed consent. All of the participants were informed of the purpose of the trial. The inclusion criteria were as follows: ASA physical status I to III, age between 18 and 80 years, and elective PELD. The exclusion criteria were as follows: local anaesthetic allergy, contraindications to epidural anaesthesia, severe systemic disease, mental retardation or inability to communicate. The withdrawal criteria were as follows: a decision by the patient to withdraw from the study, unintentional nerve root contact during epidural puncture, occurrence of total spinal anaesthesia, erroneous insertion of the epidural catheter into the vessel or subarachnoid space, scope of anaesthesia not covering the surgical site, severe drug allergy, or manifestations of local anaesthetic toxicity.
The patients were routinely fasted before surgery and did not receive any preoperative medication. A puncture point was selected above the surgical site at a distance of four spinal segments, and the catheter was inserted into the epidural space 3 cm caudally. Then, a 12 mL solution of ropivacaine (Naropin®, AstraZeneca AB, Sodertalje, Sweden) was injected at a preset concentration, and the operation began after successful anaesthesia.
Once the nerve roots were visible, the surgeons deliberately poked the nerve roots with the probe to confirm whether they had SNR. When the nerve root was stimulated, the sudden sensation of innervation region was recognised as SNR, and these sensations ceased when the probe was withdrawn from the nerve root. If SNR was present, the occurrence of sensations in the innervation area upon contact with the nerve root, such as slight pain or numbness, was defined as a negative response. In contrast, a lack of sensory feedback upon contact with the nerve root was defined as a positive response.
If patients suffered from moderate or severe pain (visual analogue scale [VAS] > 3 points) at any time during the operation, they received 4 mg oxycodone for rescue analgesia followed by 2 mg oxycodone every 5 minutes until the pain level reached VAS <3 points, up to a maximum dose of 10 mg. Patients were instructed to take conscious deep breaths or received naloxone antagonism if respiratory amnesia occurred.
The primary outcome measure was the response from contact nerve root, including the respective numbers of patients with positive and negative responses at each concentration level when their nerve roots were contacted. The secondary outcome measures were the type and number of statements with SNR in patients with a negative response, each patient’s pain score during surgery, and the number of patients reporting VAS=0.
This was a double-blind study. The ropivacaine concentration for each patient was prepared by the investigator (Dr Jie Zhou) and concealed from all other participants, such as anaesthetists and patients. Each surgery was performed by three experienced anaesthetists (Dr Bingwei Hu, Dr Chen Zhou and Dr Hongwei Wang) and two surgeons (Dr Weixing Xu and Dr Weiguo Ding) from the same medical group with more than 15 years of clinical experience. At each surgery, the primary surgeon (Dr Weixing Xu) deliberately poked the nerve roots with the probe and inquired with the patient about any sudden sensations in the innervated area, such as numbness or pain, to confirm SNR. Anaesthetist assisted the surgeon in further to determine the characteristics of SNR and documented them on the case report form. All patients received post-operative follow-up care from a designated nurse (Miss Qing Jiang).
BCD was used in this study. The first patient was given 0.5% ropivacaine, which was speculated to be the probability of a 100% positive response to completely block all sensations. Then, the concentration of the next patient was decreased by 0.03% if the previous patient had a positive response, and the concentration was increased by 0.03% with a probability of 0.11 or remained unchanged with a probability of 0.89 if the previous patient had a negative response. In BCD, a sample size of 40 cases is usually considered sufficient to enable robust parameter estimation,Citation15 but some researchers hold that 45 positive responses must be observed before the sample size is sufficient.Citation16 The latter was adopted as a stopping rule. This study was stopped after the appearance of 45 negative responses because our design was symmetrical to the previous study. Beforehand, 44 envelopes were prepared with cards marked 1 or 0 (the random digits were generated by R); each time a patient had a negative response, one envelope was opened. The card marked 0 indicated the same concentration for the next patient, while the card marked 1 indicated a 0.03% increase in concentration.
Each patient was visited by the same nurse on the first postoperative day (24 hours after surgery), and complications such as pain, lower extremity muscle strength and paraesthesia were recorded. One week after surgery, each patient was interviewed by telephone by the same nurse.
Statistical analyses were performed using R (ver. 4.2.1) and SPSS (ver. 25). Graphs were generated using GraphPad Prism 8 (ver. 8.0.2). Some concentration levels were obtained in BCD. The maximum likelihood estimates (MLEs) of true positive rates at various concentration levels were used to fit the cumulative distribution function (CDF). Two models, isotonic regression and probit regression, were used to obtain point and interval estimations. Isotonic regression was performed using R with an R-script that was programmed by the investigator. A linear interpolation as a point estimation considered more accurate was obtained with the pooled adjacent violators algorithm (PAVA),Citation17 and the 95% CI was obtained using bootstrapping. Probit regression was performed using SPSS to obtain the estimators for comparison and verification.
Results
Fifty-nine patients were recruited, and three patients were excluded from the study. One of two patients withdrew from the study because the nerve root was unexpectedly contacted during the epidural puncture, and another patient received dexmedetomidine to reduce anxiety. Fifty-four patients were finally included in the study (). The details of their baseline characteristics are shown in .
Table 1 Baseline Characteristics of the 54 Patients Recruited. Values are the Mean (SD), Median (IQR [Range]) or Number (Proportion)
A concentration set composed of 54 objects was obtained for BCD (). By applying the PAVA of isotonic regression, adjusted positive rates were obtained from the MLEs of the true positive rates at five concentration levels (). The EC10, estimated by , was 0.434%, and the calculated 95% CI was 0.410–0.440% (). By probit regression, the MLEs of true positive rates were fitted CDF as follows:
(Φ was CDF of standard normal distribution, p was probability, and C was ropivacaine concentration). The calculated EC10 was 0.431% (95% CI: 0.399%, 0.444%) ().
Table 2 The Number of Positive Responses, MLEs of the True Positive Rates and Adjusted Positive Rates Calculated by the PAVA for Each Concentration Level of Ropivacaine
Figure 2 Responses of patients. The hollow circles indicate cases of negative response (possessing sensation of the nerve root), the solid circles indicate cases of positive response (losing sensation of the nerve root), and the diamonds indicate the cases dropped from the study. The black lines indicate the estimator of the 10% effective concentration (EC10) and the 95% CI. On the left side of the y-axis, the sample sizes are shown at different concentration levels, among which two levels (0.41% and 0.44%) account for a large majority of cases.
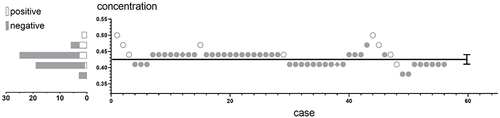
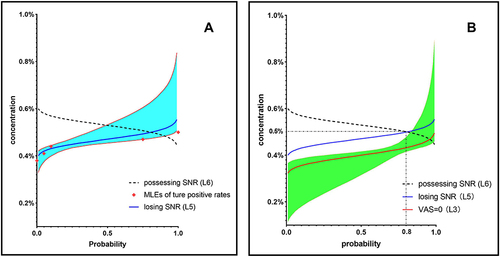
Figure 3 The curves of SNR loss and VAS=0. The possession of SNR (L6) as the target endpoint was replaced by the loss of SNR (L5), which is the real effect of local anaesthetics. (A) MLEs of true positive rates are plotted as red diamonds. The cumulative distribution function (CDF, blue line) and 95% CI (azure area) of SNR loss (L5) were obtained by probit regression. (B) The regression function (Orange line) and 95% CI (jade-green area) were obtained by probit regression to match the CDF of VAS=0 (L3).
Among the 45 patients with a negative response, the majority believed that multiple sensations from contacting nerve roots were indistinguishable and could not clearly describe the nature. Therefore, the tendency regarding the sensations mentioned in each statement was documented. Three types of sensation statements were reported when nerve roots were contacted: “tactile sensation”, radiculalgia, and numbness. All patients expressed a sense of mild acid-like burning. Twenty-six patients (57.8%) reported “tactile sensation” with the statement, “My legs are being touched”, when the nerve root was contacted (statement I). Six patients (13.3%) who considered no pain or difficulty distinguishing sensations reported “sudden numbness” in the innervation areas of the nerve roots (statement II). Thirteen patients (28.9%) reported radiculalgia, which was described as “sudden, electricity-like and bearable” (statement III) (). Among them, only two patients reported VAS > 3 points, and their statements were changed to statement I when the nerve roots were contacted again after 5 minutes of 4 mg of oxycodone. Nine patients did not possess SNR (positive response); that is, the patient reported no sensation as their feedback when the nerve roots were contacted. Two patients reported mild pain during skin incision or ligament separation. The patients who lost SNR (positive response) and reported “tactile sensation” or numbness ultimately rated their intraoperative pain as VAS=0, and their number for each concentration level is shown in .
Table 3 The Number of VAS=0 and Instances of Each Statement of SNR for Each Concentration Level of Ropivacaine
Nobody was unsatisfied with the anaesthesia. On the first postoperative day of follow-up, each patient was able to walk on their own. No abnormal muscle strength or significant paraesthesia was reported. At the telephone follow-up one week after surgery, each patient was satisfied with the anaesthesia without complications.
Discussion
Due to the limits of the operating space and field of view in percutaneous endoscopic transforaminal lumbar discectomy, it is difficult to completely avoid contacting or compressing nerve tissues. Therefore, surgeons hope that patients can provide timely feedback when their nerve roots are contacted to reduce the incidence and severity of nerve injury.
Through the accurate selection of local anaesthetic concentration, our study has convinced us that it is possible to achieve adequate analgesic efficacy during surgery while patients possess the SNR. The MTC for possessing the SNR was 0.434% (95% CI: 0.410%, 0.440%), as determined by isotonic regression. In our previous study, the MEC for tolerable pain was determined to be 0.294%, with a 95% CI of 0.271% - 0.303%. Therefore, to avoid nerve injury in PELD, the recommended effective concentration range of ropivacaine is 0.294%~0.434%. This method overcomes the drawback of lagging feedback, which arises when nerve injury is predicted by the movement of the patient’s toes. Patients who possess SNR can provide feedback to surgeons immediately when the nerve roots are contacted, and then the potentially harmful operations are terminated in a timely manner to avoid the occurrence of nerve injury.
In this study, two models were used to solve the point and interval estimations of MTC. The results of isotonic regression and probit regression were different, but the difference (0.434% vs 0.431%) was not clinically significant. Sequential design can reduce the necessary sample size and cost,Citation18 and BCD assembles dose levels near the target dose for any given quantile.Citation19 Therefore, subjects in our study were clustered in two concentration levels (0.41% and 0.44%) for probabilities that were 0.053 and 0.11 on either side of the target (Г= 0.1), and the sample sizes were 19 and 27, respectively (). The isotonic regression independently calculates the linear interpolation from two concentration levels with large sample sizes to provide credible results with narrower 95% CIs. In contrast, probit regression uses data from all concentration levels, such that the estimators are affected by each concentration level. In addition, probit regression theoretically requires the probability density function of the drug response to conform to a normal distribution, which is difficult to verify in a small-sample study such as a BCD study. Based on the above reasons, it is believed that both estimators of models predict MTC well, but the result of isotonic regression is recommended because it is theoretically more accurate than probit regression.
Can the pain-tactile separation block be confirmed, especially if the relevant studies are uninformative? We speculate that this phenomenon can be achieved by blocking peripheral nerves using a low concentration of ropivacaine. According to the anatomy of peripheral nerves, pain sensation is transmitted through Aδ fibers (1–4 μm) and C fibers (0.4–1.2 μm), while tactile sensation is transmitted by Aβ fibers (6–22 μm).Citation20 Since different nerve fibers have different degrees of susceptibility to local anaesthetics, pain is blocked at lower concentrations than tactile sensation. However, for epidural anaesthesia, blocking the spinal cord and nerve roots will be more complicated, and sensations from contacting nerve roots do not meet the definition of tactile sensation. Contacting the nerve roots will produce radiculalgia without the intervention of local anaesthetics, but the experience of radiculalgia from blocking nerves with a low concentration is unclear. Ideally, radiculalgia should be weakened to a sensation without pain, which is called “tactile” by surgeons. Our findings show that SNR was present in 45 patients, among whom 71.1% perceived sensation without pain in the innervation area as “tactile” sensation or numbness. The experience of radiculalgia at a low concentration was liable to be recognized as “tactile” in error because it was strange and vague to the patients who experienced an illusion of pain-tactile separation block. In essence, the weakened signal from radiculalgia is projected into the cerebral cortex, which generates the same experience as tactile sensation. Owing to the reasons above, the feedback from nerve root contact was finally explained as the sensation without pain instead of pain-tactile separation block. The pain-tactile separation block is the illusion of tactile sensation without pain.
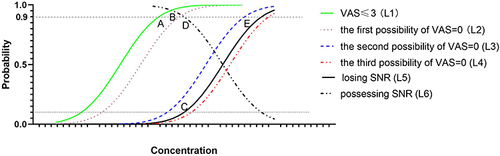
Theoretically, sensation without pain (VAS=0) as a feedback indicator is better than the effective concentration range, which includes MEC with VAS≤3 and MTC with SNR. Unfortunately, the actual experience from contacting nerve roots could not be predicted at the beginning of our study with the lack of evidence. This leads to difficulty in assigning the appropriate clinical endpoint for the outcome. Therefore, the possible endpoint was chosen to build a viable study design based on pharmacodynamics. Sensation without pain is best, but no studies provide grounds to infer whether other senses are present when VAS=0. One can be sure that a slight pain such as SNR can also provide feedback. Since the efficacy of SNR is inversely proportional to the local anaesthetic concentration, an MEC of maximum efficacy of SNR (curve L1) will be obtained when the pain is tolerable (VAS≤3). In contrast, it is more difficult to assign an endpoint for MTC that achieves both maximum analgesic efficacy and SNR. With an increase in concentration, the probability of possessing SNR will decrease (curve L6). In diametric opposition, the local anaesthetics block the nerve roots to lose SNR (curve L5) in reality. This is the reason why we use the EC10 for losing SNR instead of the EC90 for possessing SNR as the estimator of MTC. In theory, there are three possibilities for the CDF of VAS=0 (). The first one could be curve L2 to the right of L1 (VAS≤3), and VAS=0 is better than VAS≤3 as an endpoint for MEC under these circumstances. The second one is curve L3 on the left side of L5, in which case it cannot meet both the 90% of patients with VAS=0 and the 90% of patients with possessing SNR. The third is curve L4 to the right of L5, in which case the SNR is derived from slight pain and it is impossible to attain sensation without pain. Since the position of the CDF of VAS=0 could not be predicted prior to the study, we conservatively used VAS ≤3 as the endpoint of MEC and the loss of SNR as the endpoint of MTC to avoid inconclusive outcomes.
Figure 4 Theoretical cumulative distribution functions (CDFs) at different endpoints based on pharmacodynamics. L1 is the CDF of VAS≤3, and point B indicates 90% of patients with VAS≤3 as the estimator for MEC. The CDF of VAS=0 may be L2, L3 or L4. L5 is the CDF of those losing SNR, and L6 is the probability curve of those possessing SNR. There is a correlation wherein L5 is symmetric with L6 and L5 = 1–L6; thus, the concentration at which 10% of patients lose SNR (point C) is the same as the concentration at which 90% of patients possess SNR (point D) as an estimator for theMTC. a) If the CDF of VAS=0 is L2 close to L1, the concentrations for both VAS≤3 among 90% of patients (point A) and VAS=0 among 90% of patients (point B) are lower than the MTC (point C). The effective concentration range (=MTC-MEC) of BC is more accurate than that of AC, and VAS=0 is a better clinical endpoint for MEC than VAS≤3. b) If the CDF of VAS=0 is L3 near and to the left of L5, where the concentration in 90% of patients with VAS=0 (point E) would be larger than the MTC (point C), AC can be adopted as the effective concentration range only. c) If the CDF of VAS=0 is close to L4 and to the right of L5, only AC can be adopted as the effective concentration range. Furthermore, the SNR is lost when VAS=0, such that sensation without pain is not realized.
Can all patients possess the sensation without pain? Lack of evidence. In our study, SNR was present in 45 patients, 28.9% of whom still had mild pain, and it is unknown whether these patients could achieve sensation without pain at a lower concentration than MTC. It is unnecessary to explore the concentrations more precisely because mild and indistinguishable sensations are not sufficient to create unpleasant memories for patients. To verify the exact position of the CDF for VAS=0, the data from our study were secondary analyses. The statement of “tactile sensation” or “numbness” was categorized as VAS=0 (). Probit regression was used to obtain the function as , and then the probability of VAS=0 at 0.434% was predicted to be 80% (). This means that it is impossible to achieve 90% of patients possessing SNR if VAS=0 was used as the endpoint, which confirmed that the CDF of VAS=0 is curve L3. Therefore, using VAS≤3 as the endpoint of MEC was advisable in our previous study. We draw this conclusion cautiously because the SNR was described as unfamiliar and confusable to patients who reported “numbness”, which inevitably resulted in reporting bias.
In this study, oxycodone was used to relieve pain in patients with a VAS score > 3. Some evidences suggested that oxycodone is superior to other opioids for respiratory depression, which had not been seen with conventional doses of oxycodone in several studies,Citation21–24 and the current maximum 24-hour dose of oxycodone in a clinical study was 64 mg without respiratory depression.Citation25 Therefore, oxycodone was chosen as an intraoperative analgesic remedy in the prone position.
As an estimator of MTC, the EC10 still means that 10% of patients lose SNR. We would prefer to obtain a precise prediction model recommending an individual dose for each patient, which is unrealized and limited by current theory and knowledge. Although possessing SNR in the effective concentration range can alleviate concerns of nerve injury, confirming that epidural anaesthesia with low-concentration ropivacaine is superior to avoid nerve injury requires large-sample randomized controlled trials and long-term follow-up. Our study suggests that ropivacaine has an effective concentration range that maintains SNR, achieving the surgeon’s goal of immediate feedback from contact with the patient’s nerve roots. This approach is expected to help surgeons avoid causing nerve injury. Our study determined that the upper concentration limit was 0.434% (95% CI: 0.410%, 0.440%).
Conclusions
The concentration of ropivacaine used for epidural anaesthesia should not exceed 0.434% to avoid nerve injury in percutaneous endoscopic transforaminal lumbar discectomy.
Disclosure
The authors report no competing interests in this work.
Acknowledgments
Bingwei Hu and Xianhui Kang are co-first authors for this study. This research was approved by the Medical Ethics Committee of Tongde Hospital of Zhejiang Province (Zhe Tongde Expedited Review No. [2020]013) and is registered on the website of the Chinese Clinical Trial Registry (ChiCTR2000031593). We wish to thank the orthopaedist Leijun Yu for his first inspiring advice on the “tactile” feedback from nerve root contact. We would also like to express our gratitude to Nurse Qing Jiang for her excellent performance in the follow-up work.
References
- Zhou C, Zhang G, Panchal RR, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Physician. 2018;21(2):E105–E112.
- Zhu Y, Zhao Y, Fan G, et al. Comparison of the effects of local anesthesia and epidural anesthesia for percutaneous transforaminal endoscopic discectomy in elderly patients over 65 years old. Int J Surg. 2017;48:260–263. doi:10.1016/j.ijsu.2017.11.029
- Wu K, Zhao Y, Feng Z, Hu X, Chen Z, Wang Y. Stepwise local anesthesia for percutaneous endoscopic interlaminar discectomy: technique strategy and clinical outcomes. World Neurosurg. 2020;134:e346–e352. doi:10.1016/j.wneu.2019.10.061
- Ye XF, Wang S, Wu AM, et al. Comparison of the effects of general and local anesthesia in lumbar interlaminar endoscopic surgery. Ann Palliat Med. 2020;9(3):1103–1108. doi:10.21037/apm-20-623
- Mooney J, Laskay N, Erickson N, et al. General vs local anesthesia for Percutaneous Endoscopic Lumbar Discectomy (PELD): a systematic review and meta-analysis. Global Spine J. 2022;13(6):21925682221147868.
- Ren Z, He S, Li J, et al. Comparison of the safety and effectiveness of percutaneous endoscopic lumbar discectomy for treating lumbar disc herniation under epidural anesthesia and general anesthesia. Neurospine. 2020;17(1):254–259. doi:10.14245/ns.1938366.183
- Leppanen RE. Monitoring spinal nerve function with H-reflexes. J Clin Neurophysiol. 2012;29(2):126–139. doi:10.1097/WNP.0b013e31824ceec5
- Daniel JW, Botelho RV, Milano JB, et al. Intraoperative neurophysiological monitoring in spine surgery: a systematic review and meta-analysis. Spine. 2018;43(16):1154–1160. doi:10.1097/BRS.0000000000002575
- Zhu Y, Zhao Y, Fan G, et al. Comparison of 3 anesthetic methods for percutaneous transforaminal endoscopic discectomy: a prospective study. Pain Physician. 2018;21(4):E347–E353.
- Xu T, Tian R, Qiao P, Han Z, Shen Q, Jia Y. Application of continuous epidural anesthesia in transforaminal lumbar endoscopic surgery: a prospective randomized controlled trial. J Int Med Res. 2019;47(3):1146–1153. doi:10.1177/0300060518817218
- Fang G, Ding Z, Song Z. Comparison of the effects of epidural anesthesia and local anesthesia in lumbar transforaminal endoscopic surgery. Pain Physician. 2016;19(7):E1001–E1004.
- Kong M, Gao C, Cong W, Li G, Zhou C, Ma X. Percutaneous endoscopic interlaminar discectomy with modified sensation-motion separation anesthesia for beginning surgeons in the treatment of L5-S1 disc herniation. J Pain Res. 2021;14:2039–2048. doi:10.2147/JPR.S306319
- Hu B, Li L, Wang H, et al. Determining the minimum effective concentration of ropivacaine in epidural anesthesia for tolerable pain in transforaminal percutaneous endoscopic lumbar discectomy to avoid nerve injury: a double-blind study using a biased-coin design. Drug Des Devel Ther. 2022;16:315–323. doi:10.2147/DDDT.S334605
- Fang G, Wan L, Mei W, Yu HH, Luo AL. The minimum effective concentration (MEC 90) of ropivacaine for ultrasound-guided supraclavicular brachial plexus block. Anaesthesia. 2016;71(6):700–705. doi:10.1111/anae.13445
- Pace NL, Stylianou MP, Warltier DC. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
- Tran DQ, Dugani S, Dyachenko A, Correa JA, Finlayson RJ. Minimum effective volume of lidocaine for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. 2011;36(2):190–194. doi:10.1097/AAP.0b013e31820d4266
- Saranteas T, Finlayson RJ, Tran DQ. Dose-finding methodology for peripheral nerve blocks. Reg Anesth Pain Med. 2014;39(6):550–555. doi:10.1097/AAP.0000000000000157
- Iasonos A, Gönen M, Bosl GJ. Scientific review of Phase I protocols with novel dose-escalation designs: how much information is needed. J Clin Oncol. 2015;33(19):2221–2225. doi:10.1200/JCO.2014.59.8466
- Durham SD, Flournoy N, Rosenberger WF. A random walk rule for phase I clinical trials. Biometrics. 1997;53(2):745–760. doi:10.2307/2533975
- Lirk P, Berde CB. Local Anesthetics. In: Gropper MA, Miller RD, Cohen NH, et al., editors. Miller’s Anesthesia. 9th ed. Place: Elsevier; 2019:868–869.
- Kim NS, Kang KS, Yoo SH, et al. A comparison of oxycodone and fentanyl in intravenous patient-controlled analgesia after laparoscopic hysterectomy. Korean J Anesthesiol. 2015;68(3):261–266. doi:10.4097/kjae.2015.68.3.261
- Raff M, Belbachir A, El-Tallawy S, et al. Intravenous oxycodone versus other intravenous strong opioids for acute postoperative pain control: a systematic review of randomized controlled trials. Pain Ther. 2019;8(1):19–39. doi:10.1007/s40122-019-0122-4
- Dang SJ, Li RL, Wang J, et al. Oxycodone vs sufentanil in patient-controlled intravenous analgesia after gynecological tumor operation: a randomized double-blind clinical trial. J Pain Res. 2020;13:937–946. doi:10.2147/JPR.S236933
- Han L, Su Y, Xiong H, et al. Oxycodone versus sufentanil in adult patient-controlled intravenous analgesia after abdominal surgery: a prospective, randomized, double-blinded, multiple-center clinical trial. Medicine. 2018;97(31):e11552. doi:10.1097/MD.0000000000011552
- Silvasti M, Rosenberg P, Seppälä T, Svartling N, Pitkänen M. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta anaesthesiologica Scandinavica. 1998;42(5):576–580. doi:10.1111/j.1399-6576.1998.tb05169.x