Abstract
Purpose
Dexmedetomidine (Dex) is a potent and highly selective α2-adrenergic receptor agonist. Within an appropriate dose range, Dex can effectively attenuate the surgical stress response, provide intraoperative hemodynamic stability, and improve the patient recovery quality. High-dose Dex can delay patient awakening from anesthesia and increase the incidence of bradycardia. This randomized controlled trial aimed to investigate the effects of low-dose intravenous Dex premedication in patients undergoing laparoscopic cholecystectomy (LC).
Material and Methods
In total, 100 patients undergoing LC were equally randomized into Group C (premedication with saline) and Group D (premedication with 0.5 µg/kg Dex). The patients were premedicated with saline or Dex, depending on the group, before anesthesia induction. Following this, anesthesia induction and endotracheal intubation was performed, and anesthesia was maintained during surgery. Following the completion of the surgery, the patients were transferred the post-anesthesia care unit (PACU) and stayed there until they met the PACU discharge criteria. The hemodynamic parameters, consumption of anesthetics, surgical duration, postoperative awakening time, extubation time, postoperative pain, and complications were recorded.
Results
No significant differences were observed in the heart rate (HR) and mean arterial pressure (MAP) between the two groups before premedication (P>0.05). The MAP and HR immediately after endotracheal intubation and immediately after extubation were significantly lower in Group D than in Group C (P<0.05 for both). The incidence of bradycardia was significantly higher in Group D than in Group C (P<0.05), while atropine was used in neither group. Propofol and remifentanil consumption was significantly lower in Group D than in Group C (P<0.05). The postoperative awakening and extubation times were significantly shorter in Group D than in Group C (P<0.05). The postoperative visual analog scale scores for pain and incidence of nausea, vomiting, and cough were significantly lower in Group D than in Group C (P<0.05 for all).
Conclusion
Our data suggest that premedication with dexmedetomidine (0.5 µg/kg) before general anesthesia induction can effectively attenuate intraoperative stress response and postoperative pain, maintain perioperative hemodynamic stability, and decrease the incidence of adverse events, which might be an effective and safe anesthetic protocol during LC worthy of further clinical application.
Introduction
Laparoscopic cholecystectomy (LC) is considered the standard treatment for gallbladder disease.Citation1 Compared to open cholecystectomy, LC is the preferred treatment approach because it is associated with less surgical trauma, shorter hospital stays, and faster postoperative recovery.Citation2 However, the elevated intra-abdominal pressure due to pneumoperitoneum can cause various stress responses that affect patient prognosis and present a severe challenge in anesthetic management.Citation3 Therefore, the search for an optimal anesthetic protocol to minimize adverse reactions during LC remains critical.
Dexmedetomidine (Dex), a potent and highly selective α2-adrenergic receptor agonist, presents sedative, analgesic, anesthetic, and sympatholytic properties, without causing respiratory depression, when used in an appropriate dose range.Citation4 Numerous studies have suggested that Dex can effectively attenuate the surgical stress response and provide intraoperative hemodynamic stability.Citation5 Furthermore, it has been shown to reduce anesthetic requirements and improve the quality of patient recovery.Citation6,Citation7 Hence, it has been widely used as an adjuvant during general anesthesia.
Nevertheless, the clinical effects of Dex remain controversial. Some studies have shown that the anesthesia recovery time is prolonged and the incidence of bradycardia increases significantly after intravenous Dex infusion.Citation8–10 This is mainly attributed to the different doses and methods of Dex administration. Although the complications are always transient and reversible, timely attention is required to avoid serious adverse consequences. To enhance the value of Dex for clinical application and improve the quality of general anesthesia, the more appropriate protocol of Dex administration need to be explored. The elimination half-life of Dex is approximately 2 h, with a rapid distribution half-life of approximately 6 min.Citation11 Most LC procedures are completed within 1 h; therefore, to reduce postoperative complications and shorten postoperative recovery time, we prefer preoperative Dex loading to intraoperative continuous Dex infusion.
According to our previous data, the anesthesia awakening time was prolonged and the incidence of bradycardia increased significantly when 1.0 µg/kg of Dex was administered before general anesthesia induction. This prospective, double-blind, randomized controlled trial aimed to determine the efficacy of low-dose (0.5 µg/kg) intravenous Dex premedication on hemodynamics and adverse events during general anesthesia. We aimed to confirm the efficacy of low-dose intravenous Dex premedication in patients under general anesthesia during LC and to provide a data reference for the clinical application of Dex in further research.
Materials and Methods
Ethics and Trial Registration
The study protocol was approved by the Medical Ethics Committee of the Sir Run Run Hospital, Nanjing Medical University (Ethics Number: 2019-SR-023), and the study was registered in the Chinese Clinical Trial Registry (ChiCTR2100054687). All participants signed an informed consent form. This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. Written informed consent was obtained from all participants.
Inclusion and Exclusion Criteria
This prospective, double-blind, randomized controlled trial was performed at Sir Run Run Hospital between January 1, 2022, and December 31, 2022. The inclusion criteria were as follows: age 18–64 years with planned LC; American Society of Anesthesiologists (ASA) physical status of Grade I or II, and body mass index (BMI) of 20–30 kg/m2.
The exclusion criteria were as follows: severe cardiovascular or cerebrovascular disease; severe endocrine, liver, kidney, neurological, or blood system disease; preoperative respiratory infection or asthma; history of opioid addiction; allergy to the drugs used in this study; and psychosocial disease or cognitive dysfunction.
Additionally, patients who were enrolled in this study but fulfilled one of the following criteria were excluded: incomplete case report form or lost to follow-up and failed to undergo an effectiveness and safety assessment; LC converted to open surgery; severe hemodynamic instability during surgery; and surgical duration >2 h.
Randomization and Blinding
This was a double-blinded study. All patients provided written informed consent, and they were divided into two groups using a computer-generated random number table via restricted randomization. This list was kept in a sealed envelope, and only the nursing staff without any relation to the research could access it. Based on the randomization results, the patients were assigned to different groups. In Group C, patients were premedicated before anesthesia induction by administering normal saline placebo in 10 min. In Group D, patients were premedicated before anesthesia induction by administering Dex (0.5 µg/kg) diluted in normal saline in 10 min. To maintain blinding, an independent research nurse prepared and distributed the medications in identical syringes labeled only with the study numbers.
Anesthetic Procedures
All patients fasted for at least 8 h before the operation, and no preoperative medication was administered. Peripheral venous access was established immediately after entering the operation room. Electrocardiography findings, noninvasive blood pressure, heart rate (HR), pulse oxygen saturation (SpO2), and bispectral index (BIS) were routinely monitored. For invasive monitoring of the arterial blood pressure, the radial artery was punctured. Depending on the allotted group, premedication with normal saline or Dex was administered before anesthesia induction in 10 min, and oxygen was administered via a mask at 2–3 L/min. Following this, anesthesia induction was achieved through intravenous administration of midazolam (0.03 mg/kg), propofol (2 mg/kg), sufentanil (0.4 µg/kg), and rocuronium (0.6 mg/kg). Endotracheal intubation was performed 3 min after anesthesia induction, and mechanical ventilation (oxygen flow: 2 L/min, fraction of inspired oxygen: 60%) was performed with a tidal volume of 8 mL/kg. The respiratory rate was adjusted to 10–15 breaths/min to maintain an end-tidal carbon dioxide of 35–40 mmHg and SpO2 of 97–100%.
Anesthesia was maintained with a continuous infusion of propofol (4–12 mg/kg/h) and remifentanil (0.1–0.5 µg/kg/min) to ensure a BIS score within 40–60 and mean arterial pressure (MAP) within 20% of the baseline value. Additional cisatracurium doses were administered during surgery as needed. Ephedrine (6 mg) was injected when the MAP was <65 mmHg or 20% of the baseline level, and atropine (0.5 mg) was injected when the HR was <45 beats/min. Other perioperative adverse events were recorded and managed in accordance with the clinical operation standards. The anesthesia depth and vital signs remained stable throughout the surgery. Both propofol and remifentanil infusions were discontinued at the end of the surgery, and oxycodone 5 mg and ondansetron 8 mg were administered. Following the completion of the surgery, the patients were transferred to the post-anesthesia care unit (PACU), and no antagonists were administered to antagonize the residual muscle relaxants. After resumption of spontaneous respiration, tracheal extubation was performed when patients fully regained consciousness and could lift their head. Then they were transferred to the ward when they met the PACU discharge criteria after observation.
Outcomes
The MAP and HR were recorded immediately before premedication of normal saline or Dex (T1), immediately before anesthesia induction (T2), immediately after endotracheal intubation (T3), at the beginning of surgery (T4), at the beginning of pneumoperitoneum (T5), 15 min after pneumoperitoneum (T6), immediately at the end of surgery (T7), immediately after extubation (T8). Perioperative characteristics, such as the amount of infused fluid, estimated blood loss, consumption of propofol and remifentanil, surgical duration (from the start of skin incision to the end of the operation), awakening time (from anesthetic discontinuation to recovery of consciousness), and tracheal extubation time (from anesthetic discontinuation to tracheal extubation) were recorded. The incidence of perioperative complications, such as nausea, vomiting, agitation, hypotension (systolic blood pressure <90 mmHg or diastolic blood pressure <60 mmHg), and bradycardia (HR <60 beats/min), was recorded. The occurrence and severity of cough during tracheal extubation were recorded (grade 0: no cough; grade 1: mild, single cough; grade 2: moderate [frequent cough lasting <5 s, with no effect on extubation]; grade 3: severe [continuous cough lasting ≥5 s, affecting extubation]). The resting visual analog scale (VAS) scores were recorded at 1, 2, 6, 12, and 24 h postoperatively.
Statistical Analysis
SPSS 26.0 (SPSS, Inc., IL, USA) was used for all statistical analyses. The normality of the data distribution was examined by the Shapiro–Wilk test. Normally distributed data are expressed as mean ± standard deviation (x±s), non-normally distributed data are expressed as median and interquartile range, categorical variables are presented as n (%). Data were compared between groups using unpaired t-tests, chi-squared tests, Fisher’s exact test, or Mann–Whitney U-test, as appropriate. P values<0.05 were considered statistically significant.
Results
Patient Inclusion and Characteristics
shows the flow diagram for this study. In total, 100 patients fulfilled the inclusion criteria, and they were randomized equally into group C (n=50) and group D (n=50). Their demographic data and clinical characteristics are shown in . There was no significant difference in sex, age, BMI, ASA grade, surgical duration, infusion volume, and blood loss between the two groups (P>0.05).
Table 1 The Demographic Characteristic of Patients
Hemodynamic results
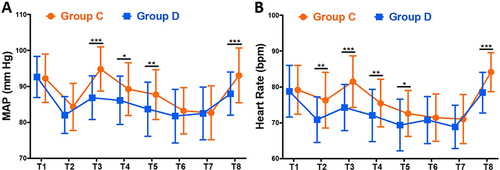
shows the hemodynamic results. No significant differences were observed in the MAP and HR between the two groups at T1 (P>0.05). The MAP at T3-T5 was significantly lower in Group D than in Group C (P<0.05). The HR at T2-T5 was significantly lower in Group D than in Group C (P<0.05). The MAP and HR at T8 were significantly lower in Group D than in Group C (P<0.001).
Figure 2 The results of repeated measurements of hemodynamic parameters. Mean arterial pressure (A) and heart rate (B) of patient in the perioperative period. *P<0.05, **P<0.01, ***P<0.001.
Consumption of Propofol and Remifentanil
shows the consumption of propofol and remifentanil. Propofol and remifentanil consumptions were significantly lower in Group D than in Group C (P<0.05).
Table 2 Consumptions of Propofol and Remifentanil
Postoperative Awakening Time and Extubation Time
shows the postoperative awakening and extubation time. The postoperative awakening time and extubation time were significantly shorter in Group D than in Group C (P<0.05).
Table 3 Postoperative Awakening Time and Extubation Time
Incidence of Perioperative Adverse Events
shows the incidence of perioperative adverse events. No significant difference was observed in the incidence of hypotension between the two groups (P>0.05). The incidence of bradycardia was significantly higher in Group D than in Group C (P<0.01), while atropine was used in neither group. The incidence of nausea and vomiting were significantly lower in Group D than in Group C (P<0.05). No significant difference was observed in the incidence of agitation between the two groups (P>0.05).
Table 4 Incidence of Adverse Events
Incidence of Cough During tracheal extubation
shows the incidence of cough during tracheal extubation. The total incidence of cough during tracheal extubation was significantly lower in Group D than in Group C (P<0.0001). The incidence of both mild and moderate cough was significantly lower in Group D than in Group C (P<0.01). No patient in either group experienced severe cough.
Table 5 Incidence of Cough During tracheal extubation
Comparison of VAS Scores at Different Time Points
shows the postoperative VAS scores. At 1, 2, and 6 h postoperatively, the VAS scores were significantly lower in Group D than in Group C (P<0.001). However, no significant difference was observed in the VAS scores between the groups at 12 and 24 h postoperatively (P>0.05).
Table 6 Comparison of Postoperative VAS Scores
Discussion
Our findings in this study demonstrate that premedication with intravenous Dex 0.5 µg/kg before anesthesia induction is effective in attenuating intraoperative stress response and maintaining stable hemodynamics, thereby reducing the incidence of perioperative complications in patients undergoing LC under general anesthesia. Moreover, premedication with Dex can effectively decrease the requirement for propofol and remifentanil, shorten the anesthesia awakening and extubation time, and relieve postoperative pain. Although the incidence of perioperative bradycardia increased with Dex premedication, it was mild and no active intervention was required. Thus, our data suggest that premedication with Dex 0.5 µg/kg before general anesthesia induction might be an effective and safe anesthetic protocol during LC, with great clinical significance and worthy of further clinical application.
Although LC is relatively less traumatic than open cholecystectomy, intubation, pneumoperitoneum, and extubation during general anesthesia are noxious stimuli that cause a significant stress response.Citation3 This leads to elevated concentrations of catecholamines, such as norepinephrine and epinephrine in the blood, inducing hemodynamic fluctuations that eventually result in serious complications.Citation12,Citation13 Given the elimination half-life of Dex is approximately 2 h, with a rapid distribution half-life of approximately 6 min.Citation11 Moreover, LC is a short procedure, the continuous intraoperative infusion of Dex may prolong the anesthesia recovery time; therefore, we prefer premedication with Dex before anesthesia induction.
It has been suggested that Dex decreases the propofol and opioid requirements during general anesthesia.Citation14–16 In this study, the consumption of propofol and remifentanil in Group D decreased by 14% and 8.8%, respectively, when compared with Group C, which was similar to that reported in previous studies. This implies that premedication with Dex can significantly reduce the requirement for anesthetics. Furthermore, our data suggest that premedication with Dex can effectively attenuate the perioperative stress response, maintain hemodynamic stability, and relieve postoperative pain. This is mainly attributed to the sedative and analgesic properties of Dex. Propofol induces sedation by potentiating the activity of γ-aminobutyric acid (GABA) receptors.Citation17,Citation18 The catecholamine release in the preoptic area of the hypothalamus is decreased by Dex, disinhibiting the GABAergic inhibitory projections to the major arousal nuclei in the midbrain and pons and reducing noradrenergic signaling in the hypothalamus and cortex, thereby inhibiting sympathetic nerve activity and achieving a sedative effect.Citation19 Furthermore, Dex induces analgesia by blocking the transmission of pain signals in the descending medullary-spinal noradrenergic pathway and prevents relaying of the peripheral stimuli.Citation20,Citation21 These findings demonstrate the synergistic effect of Dex with propofol and opioids and the attenuation of the stress response. Therefore, Dex is widely considered an excellent adjunct to general anesthesia.
Coughing caused by extubation during emergence is a common complication following general anesthesia, which could lead to hemodynamic disorders, laryngospasm, wound disruption, and bleeding.Citation22 Dex has been frequently used to reduce the incidence of cough during emergence from general anesthesia owing to its unique sedative, analgesic, and anti-sympathetic effects.Citation23 Our data demonstrated that premedication with Dex 0.5 µg/kg significantly reduced the incidence and severity of cough. Previous studies have revealed that the incidence of cough during emergence from general anesthesia is closely relate to Dex dose.Citation24 Although a larger dose of Dex could be more effective for sedation, it delays the postoperative recovery time is frequently reported.Citation25 However, other studies have shown that Dex has no significant effect on anesthesia recovery time.Citation26 Therefore, the effects of Dex on the awakening and tracheal extubation times remain controversial. Our previous findings displayed that premedication with Dex 1.0 µg/kg before anesthesia induction prolonged postoperative recovery time and extubation time significantly, which corroborates the findings of other studies. The proposed mechanism is that Dex quantitatively binds to α2-adrenergic receptors in the central and peripheral nervous systems, resulting in sedation and non-rapid eye movement sleep.Citation27 Once the Dex dose exceeds a certain threshold, excessive sedation occurs, leading to delayed recovery from general anesthesia.Citation27 Premedication with Dex 0.5 µg/kg was selected in this study based on the previous data and references, and the results demonstrate that the postoperative recovery time and extubation time were significantly shortened with Dex premedication. Besides 0.5 µg/kg of Dex being an appropriate dose that does not cause excessive sedation, the reduced consumption of propofol and remifentanil associated with its use is another advantage.
Dex has been widely used over the past few decades, and bradycardia is one of the most frequently reported adverse events associated with it, which should be valued.Citation28 The mechanism involved may be related to the high dose of Dex reducing norepinephrine release, inhibiting atrioventricular node and sinoatrial node functions, and exciting the vagus nerve.Citation29,Citation30 Our results revealed that the incidence of bradycardia was significantly higher in Group D than in Group C, whereas use of atropine was not required, implying that no intervention was needed in most cases of bradycardia induced by low-dose Dex. It has been reported that hypotension is another common adverse effect of Dex.Citation31,Citation32 Our study found no significant difference in the incidence of hypotension between the two groups. Moreover, it has been demonstrated that a rapid infusion of Dex with a large dose may activate peripheral α2- and α1-adrenergic receptors, causing vasoconstriction and a sudden increase in blood pressure.Citation33 However, no such phenomenon was observed in our study. This can be attributed to the appropriate dose of Dex used, and the patients included in this study were ASA grade I or II with excellent cardiovascular reserve. Administration of Dex could reduce the incidence of postoperative nausea and vomiting after general anesthesia.Citation34 Our data showed that the incidence of nausea and vomiting within 24 h postoperatively was 36% and 26% in Group C and 14% and 8% in Group D, respectively. However, the specific mechanism of Dex reduces incidence of nausea and vomiting remained elusive. Possible mechanism may be related to the inhibitory effect of Dex on sympathetic nerve activity, reduction in plasma catecholamine concentrations.Citation35 Additionally, as an adjuvant for general anesthesia, Dex can significantly reduce anesthetic consumption, which is another important factor in reducing the incidence of nausea and vomiting. Agitation during postoperative recovery is a common complication of general anesthesia. Previous studies have suggested that Dex could reduce the incidence of agitation during the recovery period.Citation36,Citation37 This study showed that two patients (4%) experienced postoperative agitation in Group C, while none of the Group D patients experienced postoperative agitation, which corroborates the findings of previous studies. Postoperative pain is a major trigger for agitation, and our findings demonstrated that premedication with Dex can effectively reduce the initial 6-h postoperative VAS scores, thereby alleviating the postoperative discomfort of patients. Although the detailed mechanism of agitation alleviation by Dex has not been elucidated, it may be closely related to its sedative, analgesic, and anti-anxiety effects.
This study has some limitations. First, the sample size was relatively small, with certain limitations and statistical deviations. Second, we did not evaluate the effects of different doses of Dex premedication. According to our previous data, the anesthetic effect of Dex with different doses was identified, and premedication with Dex 0.5 µg/kg was considered the optimal dose for subsequent research. Finally, the patients included in this study were relatively young and healthy, and the effects of Dex premedication may differ in older patients and those with coexisting diseases. Further studies with larger sample sizes are warranted to verify our results.
Conclusion
Premedication with dexmedetomidine 0.5 µg/kg before anesthesia induction can effectively attenuate intraoperative stress response and postoperative pain, maintain perioperative hemodynamic stability, reduce the anesthesia recovery time, reduce anesthetic requirements, and decrease the incidence of adverse events. Therefore, we confirmed that low-dose intravenous Dex premedication can provide satisfactory anesthetic effects and be beneficial to the postoperative outcomes of patients undergoing LC under general anesthesia, which is worthy of further clinical application.
Abbreviations
Dex, dexmedetomidine; LC, laparoscopic cholecystectomy; HR, heart rate; MAP, mean arterial pressure; VAS, visual analog score; BMI, body mass index; SpO2, pulse oxygen saturation; BIS, bispectral index; PACU, post-anesthesia care unit; ASA, American Society of Anesthesiologists; GABA, γ-aminobutyric acid.
Data Sharing Statement
All data generated during the current study has been deidentified. Further inquiries about the datasets can be directed to the corresponding author: Professor Ning Yin on reasonable request.
Ethics
Clinical Trials Registration: ChiCTR2100054687.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Appreciate for the support from the department of general surgery of the Sir Run Run Hospital, Nanjing Medical University.
Additional information
Funding
References
- Sun F, Zheng S, Wu J. Quality of information in gallstone disease videos on tiktok: cross-sectional study. J Med Internet Res. 2023;25:e39162. doi:10.2196/39162
- Kim HY, Choi JB, Min SK, Chang MY, Lim GM, Kim JE. A randomized clinical trial on the effect of a lidocaine patch on shoulder pain relief in laparoscopic cholecystectomy. Sci Rep. 2021;11(1):1052. doi:10.1038/s41598-020-80289-y
- Zhang B, Xu Z, Gu W, et al. Postoperative complications and short-term prognosis of laparoscopic pancreaticoduodenectomy vs. open pancreaticoduodenectomy for treating pancreatic ductal adenocarcinoma: a retrospective cohort study. World J Surg Oncol. 2023;21(1):26. doi:10.1186/s12957-023-02909-x
- Kong H, Li M, Deng CM, Wu YJ, He ST, Mu DL. A comprehensive overview of clinical research on dexmedetomidine in the past 2 decades: a bibliometric analysis. Front Pharmacol. 2023;14:1043956. doi:10.3389/fphar.2023.1043956
- Hu J, Lv B, West R, et al. Comparison between dexmedetomidine and propofol on outcomes after coronary artery bypass graft surgery: a retrospective study. BMC Anesthes. 2022;22(1):51. doi:10.1186/s12871-022-01589-6
- Prathapadas U, Hrishi AP, Appavoo A, Vimala S, Sethuraman M. Effect of low-dose dexmedetomidine on the anesthetic and recovery profile of sevoflurane-based anesthesia in patients presenting for supratentorial neurosurgeries: a randomized double-blind placebo-controlled trial. J Neurosci Rural Pract. 2020;11(2):267–273. doi:10.1055/s-0040-1703968
- Jiang X, Tang X, Liu S, Liu L. Effects of dexmedetomidine on evoked potentials in spinal surgery under combined intravenous inhalation anesthesia: a randomized controlled trial. BMC Anesthes. 2023;23(1):36. doi:10.3892/ijmm.2020.4584
- Choi EK, Seo Y, Lim DG, Park S. Postoperative nausea and vomiting after thyroidectomy: a comparison between dexmedetomidine and remifentanil as part of balanced anesthesia. Korean J Anesthes. 2017;70(3):299–304. doi:10.4097/kjae.2017.70.3.299
- Bedirli N, Akçabay M, Emik U. Tramadol vs dexmedetomidine for emergence agitation control in pediatric patients undergoing adenotonsillectomy with sevoflurane anesthesia: prospective randomized controlled clinical study. BMC Anesthes. 2017;17(1):41. doi:10.1186/s12871-017-0332-4
- Gao PF, Li SY, Li Y, Zhao L, Luo Q, Ji Y. The comparison of ketamine-dexmedetomidine (ketadex) and ketamine-propofol (ketofol) for procedural sedation in pediatric patients: a meta-analysis of randomized controlled trials. Heliyon. 2022;8(10):e11166. doi:10.1016/j.heliyon.2022.e11166
- Chen Y, Bian W, Xu B. Pretreatment with dexmedetomidine alleviates lung injury in a rat model of intestinal ischemia reperfusion. Mol Med Rep. 2020;21(3):1233–1241. doi:10.3892/mmr.2020.10942
- Silpa AR, Koshy KA, Subramanian A, Pradeep KK. Comparison of the efficacy of two doses of dexmedetomidine in attenuating the hemodynamic response to intubation in patients undergoing elective cardiac surgery: a randomized double-blinded study. J Anaes Clin Pharmacol. 2020;36(1):83–87. doi:10.4103/joacp.JOACP_235_18
- Miyazaki H, Miura D, Koguchi Y, Takamatsu C, Sakaguchi Y. Intraoperative serum catecholamine levels in a pregnant woman with pheochromocytoma undergoing cesarean delivery with combined spinal-epidural anesthesia: a case report. Cureus. 2022;14(5):e24727. doi:10.7759/cureus.24727
- Yu P, Zhang J, Zou Y, Wang J. Effect of preventive analgesia with nalbuphine and dexmedetomidine in endoscopic sinus surgery. Pain Res Manag. 2022;2022:2344733. doi:10.1155/2022/2344733
- Gu Y, Yang F, Zhang Y, et al. The effects of different doses of dexmedetomidine on the requirements for propofol for loss of consciousness in patients monitored via the bispectral index: a double-blind, placebo-controlled trial. BMC Anesthes. 2020;20(1):96. doi:10.1186/s12871-020-01013-x
- Dutta A, Sethi N, Sood J, et al. The effect of dexmedetomidine on propofol requirements during anesthesia administered by bispectral index-guided closed-loop anesthesia delivery system: a randomized controlled study. Anesth Analg. 2019;129(1):84–91. doi:10.1213/ANE.0000000000003470
- Kim SH, Kim N, Kim EH, Suh S, Choi SH. Propofol requirement in patients with growth hormone-secreting pituitary tumors undergoing transsphenoidal surgery. J Clin Med. 2019;8(5):571. doi:10.3390/jcm8050571
- Kang FC, Chen YC, Wang SC, So EC, Huang BM. Propofol induces apoptosis by activating caspases and the MAPK pathways, and inhibiting the akt pathway in tm3 mouse leydig stem/progenitor cells. Int J Mol Med. 2020;46(1):439–448. doi:10.3892/ijmm.2020.4584
- Yoo H, Iirola T, Vilo S, et al. Mechanism-based population pharmacokinetic and pharmacodynamic modeling of intravenous and intranasal dexmedetomidine in healthy subjects. Eur J Clin Pharmacol. 2015;71(10):1197–1207. doi:10.1007/s00228-015-1913-0
- Yeo J, Park S. Effect of dexmedetomidine on the development of mechanical allodynia and central sensitization in chronic post-ischemia pain rats. J Pain Res. 2018;11:3025–3030. doi:10.2147/JPR.S184621
- Kong D, Bai J, Ma S, Li C, Yang L, Kong X. Effects of dexmedetomidine hydrochloride on hemodynamics, postoperative analgesia and cognition in cesarean section. Exp Ther Med. 2018;16(3):1778–1783. doi:10.3892/etm.2018.6363
- Ye Q, Wang F, Xu H, Wu L, Gao X. Effects of dexmedetomidine on intraoperative hemodynamics, recovery profile and postoperative pain in patients undergoing laparoscopic cholecystectomy: a randomized controlled trial. BMC Anesthes. 2021;21(1):63. doi:10.1186/s12871-021-01283-z
- Hu S, Li Y, Wang S, Xu S, Ju X, Ma L. Effects of intravenous infusion of lidocaine and dexmedetomidine on inhibiting cough during the tracheal extubation period after thyroid surgery. BMC Anesthes. 2019;19(1):66. doi:10.1186/s12871-019-0739-1
- Aouad MT, Zeeni C, Al Nawwar R, et al. Dexmedetomidine for improved quality of emergence from general anesthesia: a dose-finding study. Anesth Analg. 2019;129(6):1504–1511. doi:10.1213/ANE.0000000000002763
- Kim JH, Ham SY, Kim DH, Chang CH, Lee JS. Efficacy of single-dose dexmedetomidine combined with low-dose remifentanil infusion for cough suppression compared to high-dose remifentanil infusion: a randomized, controlled, non-inferiority trial. Int J Med Sci. 2019;16(3):376–383. doi:10.7150/ijms.30227
- Di M, Han Y, Yang Z, et al. Tracheal extubation in deeply anesthetized pediatric patients after tonsillectomy: a comparison of high-concentration sevoflurane alone and low-concentration sevoflurane in combination with dexmedetomidine pre-medication. BMC Anesthes. 2017;17(1):28. doi:10.1186/s12871-017-0317-3
- Qiu G, Wu Y, Yang Z, et al. Dexmedetomidine activation of dopamine neurons in the ventral tegmental area attenuates the depth of sedation in mice. Anesthesiology. 2020;133(2):377–392. doi:10.1097/ALN.0000000000003347
- Zhu SJ, Wang KR, Zhang XX, Zhu SM. Relationship between genetic variation in the α2A-adrenergic receptor and the cardiovascular effects of dexmedetomidine in the Chinese Han population. J Zhejiang Univ Sci B. 2019;20(7):598–604. doi:10.1631/jzus.B1800647
- Sairaku A, Nakano Y, Suenari K, et al. Dexmedetomidine depresses sinoatrial and atrioventricular nodal function without any change in atrial fibrillation inducibility. J Cardiovasc Pharmacol. 2016;68(6):473–478. doi:10.1097/FJC.0000000000000434
- Tan C, Yan S, Shen J, et al. Effects of dexmedetomidine on cardiac electrophysiology in patients undergoing general anesthesia during perioperative period: a randomized controlled trial. BMC Anesthes. 2022;22(1):271. doi:10.1186/s12871-022-01811-5
- Chen Z, Shao DH, Ma XD, Mao ZM. Dexmedetomidine aggravates hypotension following mesenteric traction during total gastrectomy: a randomized controlled trial. Ann Saudi Med. 2020;40(3):183–190. doi:10.5144/0256-4947.2020.183
- Ruder TL, Donahue KR, Colavecchia AC, Putney D, Al-Saadi M. Hemodynamic effects of dexmedetomidine in adults with reduced ejection fraction heart failure. J Intensive Care Med. 2021;36(8):893–899. doi:10.1177/0885066620934416
- Li J, Tang H, Tu W. Mechanism of dexmedetomidine preconditioning on spinal cord analgesia in rats with functional chronic visceral pain. Acta Cir Bras. 2022;37(2):e370203. doi:10.1590/acb370203
- Koo JM, Chung YJ, Lee M, Moon YE. Efficacy of dexmedetomidine vs. remifentanil for postoperative analgesia and opioid-related side effects after gynecological laparoscopy: a prospective randomized controlled trial. J Clin Med. 2023;12(1):350. doi:10.3390/jcm12010350
- Xie C, Zhang C, Sun H, Lu Y. Effects of dexmedetomidine on postoperative nausea and vomiting in adult patients undergoing ambulatory thyroidectomy: a randomized clinical trial. Front Med Lausanne. 2021;8:781689. doi:10.3389/fmed.2021.781689
- Song J, Liu S, Fan B, Li G, Sun Q. Perioperative dexmedetomidine reduces emergence agitation without increasing the oculocardiac reflex in children: a systematic review and meta-analysis. Medicine. 2021;100(18):e25717. doi:10.1097/MD.0000000000025717
- Tang Y, Song Y, Tian W, Chen G, Gu Y. A systematic review and meta-analysis on the efficacy and safety of dexmedetomidine combined with sevoflurane anesthesia on emergence agitation in children. Transl Pediatr. 2022;11(7):1156–1170. doi:10.21037/tp-22-172