Abstract
The potential anti-cancer effect of traditional Chinese medicine (TCM) monomers has been widely studied due to their advantages of well-defined structure, clear therapeutic effects, and easy quality control during the manufacturing process. However, clinical trial information on these monomers is scarce, resulting in a lack of knowledge regarding the research progress, efficacy, and adverse reactions at the clinical stage. Therefore, this study systematically reviewed the clinical trials on the anti-cancer effect of TCM monomers registered in the Clinicaltrials.gov website before 2023.4.30, paying special attention to the trials on tumors, aiming to explore the research results and development prospects in this field. A total of 1982 trials were started using 69 of the 131 TCM monomers. The number of clinical trials performed each year showed an overall upward trend. However, only 26 monomers entered into 519 interventional anti-tumor trials, with vinblastine (194, 37.38%) and camptothecin (146, 28.13%) being the most used. A total of 45 tumors were studied in these 519 trials, with lymphoma (112, 21.58%) being the most frequently studied. Clinical trials are also unevenly distributed across locations and sponsors/collaborators. The location and the sponsor/collaborator with the highest number of performed trials were the United States (651,32.85%) and NIH (77). Therefore, China and its institutions still have large room for progress in promoting TCM monomers in anti-tumor clinical trials. In the next step, priority should be given to the improvement of the research and development ability of domestic enterprises, universities and other institutions, using modern scientific and technological means to solve the problems of poor water solubility and strong toxic and side effects of monomers, so as to promote the clinical research of TCM monomers.
Introduction
To date, cancer remains a major public health challenge. At present, the incidence of six types of tumors, including adolescent colorectal cancer, continues to increase.Citation1,Citation2 Moreover, cancer is probably becoming the leading cause of premature death in most countries in the 21st century.Citation3 Common treatments include surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapy.Citation4,Citation5 However, these methods are more or less limited by the treatment conditions, the type/grade of tumor and the potential complications. In China, traditional Chinese medicine (TCM) has unique characteristics in treating cancer for thousands of years. Chinese medicinal monomers with well-defined structures have been isolated from Chinese herbs thanks to the help of modern scientific and technological means, showing superior anti-tumor effects.Citation6–12 Chinese medicinal monomers represent great research and development potential as new anti-tumor drugs due to the characteristics of clear structure, definite effects, and easy-to-control quality process.
Previous studies provided detailed analyses of the drug development profile in China,Citation13 the characteristics of clinical trials of new TCMs,Citation14 and the clinical trials on specific tumors.Citation15,Citation16 However, there are few reviews worldwide summarizing the characteristics of clinical trials that used anti-tumor herbal monomers. The clinicaltrials.gov website, which is one of the most influential platforms in the world, was released by the National Institutes of Health (NIH) in 2000 and includes more than 450,000 clinical studies from all 50 states and 221 countries. This clinical trial registration platform is one of the most influential platforms in the world, containing data of many anti-tumor clinical trials using active ingredients of TCMs worldwide, providing a support for their further analysis.
Therefore, this study systematically reviewed the clinical trials using anti-tumor TCM monomers registered in the Clinicaltrials.gov website before April 30, 2023. Moreover, this review focused on anti-tumor trials and explored the research results and development prospects in this field, thus providing a basis and decision-making references for pharmaceutical researchers, enterprises, institutions, government departments and other personnel.
Data Collection
Data Sources
All clinical trial data included in this study were obtained from the ClinicalTrials.gov database. Since this database was created and put into use, a number of initiatives have resulted in more complete clinical trial information being stored in it. Firstly, the International Committee of Medical Journal Editors (ICMJE) has required registered clinical trials as a prerequisite for submission to biomedical journals starting in 2005.Citation17 In addition, in 2007, Section 801 of the Food and Drug Administration (FDA) Amendments Act required ClinicalTrials.gov to include more types of clinical trials, additional trial registration information, and summary results including adverse events, providing penalties for violations.Citation18 Subsequently, ClinicalTrials.gov officially published the results database in 2008. A month later the World Medical Association Congress revised the Declaration of Helsinki, which supports prospective registration and public disclosure of research results. Therefore, the ClinicalTrials.gov website is the place to go to look for a more comprehensive collection of clinical trial data on TCM monomers worldwide.
Search Strategy and Inclusion Criteria
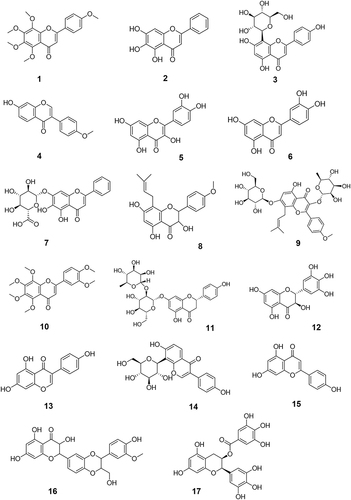
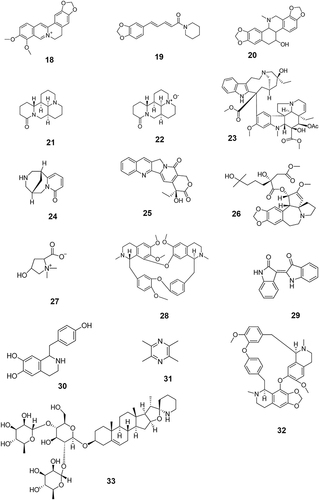
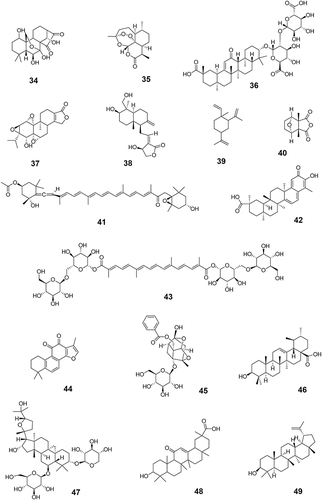
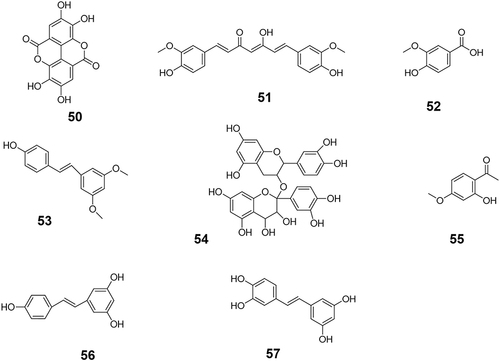
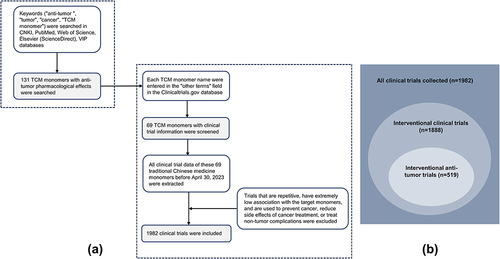
The first step was to investigate anti-tumor TCM monomers. The keywords “anti-tumor”, “tumor”, “cancer”, and “TCM monomers” were used to search the literature related to antitumor monomers in the databases of CNKI, PubMed, Web of Science, Elsevier (ScienceDirect) and VIP. A total of 131 monomers with proved anti-tumor effect by cell experiments, animal experiments or clinical trials were collected (Table S1). The monomers were classified into more than 10 types of compounds based on their structural characteristics including polysaccharides, quinones, phenylpropanoids, alkaloids, flavonoids, terpenoids, steroids, and phenols. Subsequently, the above monomers were searched in the Clinicaltrials.gov database, and data of 69 TCM monomers participating in clinical trials were retrieved (). The chemical structure of each of the 69 monomers is shown in . All clinical trial data of these 69 TCM monomers before April 30, 2023 were extracted, including NCT number, study type, phases, conditions, interventions, status, enrollment, start date, sponsor/collaborators and locations.
Table 1 Sixty-Nine Anti-Cancer TCM Monomers with Clinical Trials
Figure 5 The chemical structures of six other types of anti-tumor monomers with clinical trials data. The specific classification is as follows: 58–63 belong to phenylpropanoids; 64–65 belong to quinonoids; 66 belongs to polysaccharides; 67 belongs to steroid compounds; 68 belongs to macrocyclic ketone compounds and 69 belongs to inorganic salt compounds.
The following 3 aspects were considered before further analysis: 1) Duplicate clinical trials were removed. 2) Trials with very low correlation with the target monomers were excluded. For example, they mention only the names or abbreviations of the target monomers, rather than using them as research purposes. 3) Trials in which interventions were used to prevent cancer, mitigate side effects of cancer treatment, or treat non-oncologic complications were excluded. Quantity and percentage were used as categorical variables for various evaluation indicators, and the specific composition and time trend of each indicator were further analyzed. In addition, data such as type of intervention, sponsor/collaborators, and location often have multiple side-by-side entries. Therefore, only the elements ranked first are counted to facilitate the analysis. shows the search process of anti-tumor TCM monomers and the collection and exclusion process of anti-tumor clinical trials. In addition, the types of clinical trials that we focused on are shown in .
Results
Overall Results
Distribution of Types of Clinical Trials and Their Phases
A total of 1982 trials of three types were included, with the vast majority being interventional trials (1888, 95.26%), followed by observational trials (92, 4.64%) and expanded access trials (2, 0.10%). Among the observational studies, 55 were concluded, but only 1 multicenter retrospective study for classical Hodgkin’s lymphoma (cHL) produced results. In addition, 2 expanded access trials on green tea extract for small cell lung cancer and digital subtraction angiography in nanomedicine intervention for pancreatic cancer were inconclusive.
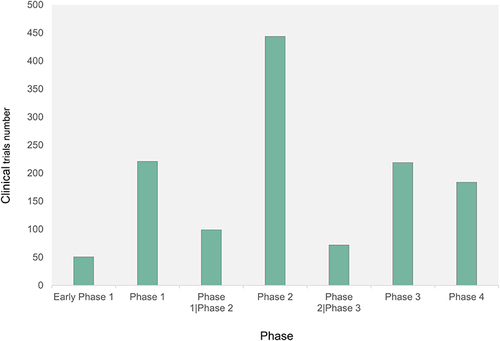
Excluding 598 (31.67%) clinical trials that did not meet clinical phasing criteria, the remaining 1290 interventional trials were in 7 phases (). Only 51 (2.70%) of the clinical trials were in early Phase 1, probably because the subjects generally do not obtain a therapeutic effect at this stage. Or it may be because of the limited toxicological studies of the drug at this time. Participants must assume a certain amount of safety risks, which leads to a limited number of participants to the trial, making difficult to carry out the study. In contrast, 444 (23.52%) clinical trials were in Phase 2.
Treatment Indications and Types of Interventions
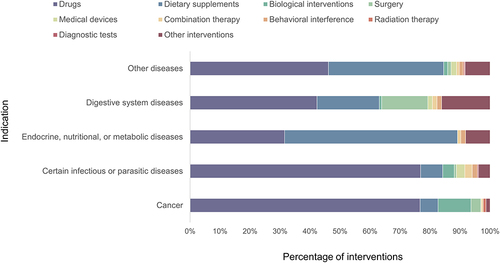
A total of 1945 clinical trials involved interventions. And a total of 1110 (56.00%) clinical trials involved interventions to test drugs. In addition, 491 (24.77%) clinical trials used dietary supplements. These are followed by lifestyle and dietary interventions (131, 6.61%), biological interventions (79, 3.99%), and surgery (53, 2.67%). However, trials using medical devices (27), combination therapy (21), behavioral interference (20), radiation therapy (9), and diagnostic tests (4) as intervention measures accounted for only 4.09% of the total.
The international Classification of Diseases 11th Revision (ICD-11) was used to categorize the indications involved in all interventional clinical trials due to the multi-targeting and other characteristics of TCM monomers. The indications of 157 trials were not found in ICD-11 for the time being. The remaining interventional clinical studies mainly focus on “cancers” (27.49%), “certain infectious or parasitic diseases” (15.10%), “endocrine, nutritional, or metabolic diseases” (9.75%) and “digestive system diseases” (6.89%). Further analysis was performed on the interventions for the main indications in interventional clinical trials (): 76.69% of clinical trials on “cancers”, 76.84% on “certain infectious or parasitic diseases”, and 42.31% on “digestive system diseases” were performed using drugs as intervention. However, interventions on “endocrine, nutritional, or metabolic diseases” were dominated by dietary supplements (57.61%), followed by drugs (31.52%). In addition, the details of other diseases are presented in Table S2.
Enrollment, Start Date, and Trial Status
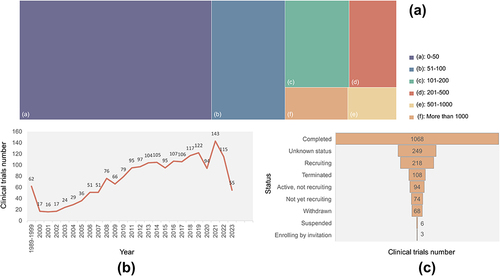
The enrollment in all trials was divided into 6 intervals. Excluding 30 trials that did not mention the “enrollment”, 49.09% (973) of the trials had participants ranging from 0 to 50. In contrast, only 8.78% (174) of the trials had more than 500 participants. Statistical analysis revealed that less than 100 clinical trials were annually performed from 1989 to 2011. However, the number of clinical trials since 2012 was more than 100, with a peak of 148 clinical studies performed in 2021. Although occasional fluctuations in the number of clinical trials were present each year from 1989 to 2022, the overall trend showed an increase. In addition, data from 2023 showed that 56 clinical trials have been performed or are expected to be performed this year. Furthermore, up to 56.66%(1123) of the trials were completed, 20.69% (410) of the trials may be performed in the future, with the status including “active, and not recruiting”, “enrolling by invitation”, “recruiting”, and “not yet recruiting”.
shows the number of enrollments (), the time of the start date (), and the status () of interventional clinical trials. In the scope of the intervention trials, the distribution trend of these three indicators is similar to the data of the total clinical trials. A range of 0–50 participants was present in 50.21% of the trials. The number of completed trials accounted for 56.57%. The number of clinical trials performed each year shows an overall upward trend. Moreover, both the number of total clinical trials and the number of interventional trials in 2020 decreased, probably related to the global pandemic of the new coronavirus infection.
Locations and Sponsors/Collaborators
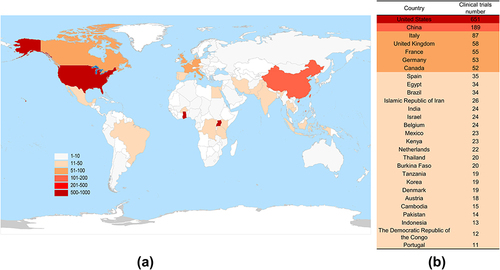
All clinical trials were performed in 87 countries, and shows geographical distribution the locations. shows the locations with over 10 clinical trials. The United States, China and Italy are the three countries with the largest number of registrations, with 651 (32.85%), 189 (9.54%) and 87 (4.39%), respectively. The regional distribution of interventional trials showed the same trend, with 636 (33.69%), 169 (8.95%) and 81 (4.29%) trials registered in the United States, China and Italy, respectively.
Figure 10 (a) Geographical distribution of clinical trials of TCM monomers registered by leading clinical trial units worldwide. (b) Locations with over 10 clinical trials.
The sponsors/collaborators of all clinical trials come from hundreds of organizations such as research institutes, hospitals, universities, and companies. Ten of these organizations have been involved in more than 10 clinical trials and have undertaken the largest number of interventional trials. The NIH was involved in the largest number of clinical trials, for a total of 77, of which 73 were interventional trials. Next, the University of Texas and the University of California were involved in 39 and 34 trials, respectively. All the 39 of the former and 32 of the latter were interventional trials. In addition, the company with the largest number of trials among the top 10 organizations was GlaxoSmithKline in the UK, which performed 18 interventional clinical trials.
Analysis of Cancer Treatment
Analysis of Interventional Clinical Trials
Monomers Involved and The Number of Their Respective Clinical Trials
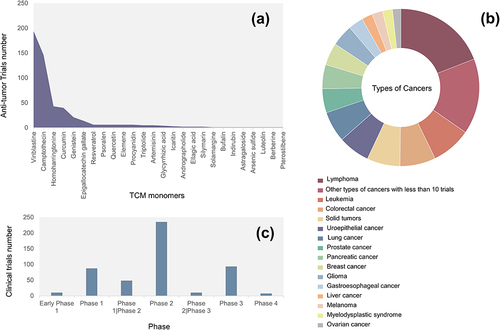
Interventional anti-tumor trials involved 519 trials of 26 TCM monomers (). Among them, vinblastine and camptothecin were the hotspots in the research on anti-tumor drugs, with 194 (37.38%) and 146 (28.13%) clinical trials related to them, respectively. They were followed by homoharringtonine (43, 8.29%), curcumin (40, 7.71%), genistein (21, 4.05%), and epigallocatechin gallate (14, 2.70%). None of the remaining 20 monomers was involved in more than 6 trials.
Types of Cancers
The many cancer subtypes involved in the trials were properly classified to facilitate the statistical analysis, hoping to clarify the research trend of each cancer. Except for four trials where tumor types were not identified, the remaining trials involved a total of 45 types of tumors. There are 15 types of tumors with 10 or more clinical trials (). Lymphoma was the most researched tumor, with 112 (21.58%) trials, of which 88 were on Hodgkin’s lymphoma. The next three types of tumors were involved in 40 or a bit more trials: leukemia (48, 9.25%), colorectal cancer (43, 8.29%), and unspecified type of solid tumors (40, 7.71%). In addition, the detail of other types of cancers with less than 10 trials are presented in Table S3.
Clinical Trial Phases and Start Date
shows the phases of the interventional anti-tumor trials. Most of the anti-tumor clinical trials were in phase 2 (235, 45.28%), and very few clinical trials entered Phase 4 (7, 1.35%). Moreover, only 58 clinical trials combined phase 1 and phase 2 or phase 2 and Phase 3. In addition, only 58 anti-tumor trials had been performed before 2000, but since 2000, the number of anti-tumor trials performed each year has increased, with 33 trials in 2017 and 2022, both peaking.
Distribution of Locations and Sponsors/Collaborators
A total of 486 clinical trials with location information were distributed in 30 countries. Among them, the United States (58.77%) and China (9.06%) performed the largest number of experiments. In addition, France (3.08%), Germany (2.70%), Italy (2.70%), Belgium (2.50%), the United Kingdom (2.50%), and Canada (2.12%) performed more than 10 trials. A total of 232 sponsors/collaborators were involved in these trials. The NIH performed the highest number of clinical trials, for a total of 51 (21.98%). A total of 98.28% sponsors/collaborators started 10 or less than 10 trials, with 147 sponsors/collaborators starting only 1 anti-tumor clinical trial.
Analysis of the Results of Clinical Trials with Outcomes
Analysis of the Results of Clinical Trials Related to Vinblastine
Ninety-eight anti-tumor clinical trials made their results available, and among them, 41 were related to vinblastine. Vinblastine is commonly used in the treatment of tumors such as Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, testicular cancer, breast cancer, Kaposi’s sarcoma, and renal cell carcinoma,Citation33 which is also demonstrated in the results of our statistical clinical trials. These 41 trials involved a total of eight types of tumors, with the highest number of trials being in lymphoma (23), with Hodgkin’s lymphoma (21) being the hotspot of research. This was followed by urothelial carcinoma (7), melanoma (6), leukemia (2), brain and central nervous system tumors (1), prostate cancer (1), renal carcinoma (1), and Ewing’s sarcoma (1).
Vinblastine is commonly used to treat cancer in combination with other drugs. Common chemotherapeutic regimens in clinical trials included ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), AVD (doxorubicin, vinblastine, and dacarbazine), Stanford V (doxorubicin, methylethylamine, vinblastine, vincristine, bleomycin, etoposide, and prednisone), and CVT (cisplatin, vinblastine, and temozolomide). Data from clinical trials that produced results showed that 13 trials investigating Hodgkin’s lymphoma used the ABVD regimen. For example, the trial NCT00003389 concluded that ABVD remains the standard approach in patients with advanced and locally extensive Hodgkin’s lymphoma,Citation34 and NCT00504504 concluded that “ABVD in combination with rituximab has good clinical activity in patients with advanced cHL”.Citation35 However, ABVD treatment also has some limitations, since some patients experienced complications and cancer recurrence.Citation36,Citation37 Thus, researchers start to combine ABVD with other drugs, including brentuximab vedotin + ABVD, rituximab + ABVD, or they modify ABVD to solve these problems, such as attempting the brentuximab vedotin + AVD scheme. The studies involved in the trial NCT01712490 found that patients receiving brentuximab vedotin +AVD treatment for Phase III or IV Hodgkin’s lymphoma had a better survival compared to patients receiving ABVD.Citation38 These changes resulted in satisfactory results.Citation35,Citation36,Citation39 MAVC, another chemotherapeutic regimen, is used in the treatment of uroepithelial cancer. An early study did not show that MVAC adjuvant chemotherapy did not have a statistically significant benefit in patients with lymphovascular invasion uroepithelial carcinoma.Citation40 However, a subsequent study found that dose-dense MVAC was well tolerated, showing some effectiveness in high-grade uroepithelial carcinoma and can be used in patients who are eligible for the treatment.Citation41
In clinical trials considering the progression-free survival rate as the first primary outcome to evaluate efficacy, 10 had results. Four clinical trials did not show accurate trial results for various reasons. The result of one of the remaining 6 items showed that patients with metastatic melanoma who received a low-dose chemotherapeutic regimen that included vinblastine had a median progression-free survival of 3 weeks. Another remaining one was a vinblastine-related treatment for high-risk patients with early childhood embryonal brain tumors, choroid plexus cancers, high-grade gliomas or ventricular meningiomas, with a median progression-free survival rate of 30.8%. In addition to the above two trials, the others among the 6 clinical trials showed that the median progression-free survival rate roughly ranged from 80% to 90% and were sometimes even higher when chemotherapy was combined with radiotherapy. The statistical analysis performed on 41 trials with high incidence of serious adverse reactions and other adverse reactions revealed that the main serious adverse reactions were granulocytopenia or neutropenia and fever (13), while the main other adverse reactions were fatigue (9), granulocytopenia or neutropenia and fever (12).
Analysis of the Results of Clinical Trials Related to Camptothecin
A total of 33 anti-tumor clinical trials involving camptothecin and showing results were performed, and involved 14 different types of cancers. In contrast to vinblastine, camptothecin is mostly used in the treatment of gastro-esophageal cancer (8), colorectal cancer (7), lung cancer (5) and gliomas (5). Camptothecin was also involved in clinical studies on the treatment of pancreatic cancer (4), rhabdomyosarcoma (3), solid tumors (2) and other tumors.
Since the anti-tumor mechanism of camptothecin was revealed in the 80s, the mechanism of action and conformational relationships of camptothecin and its derivatives have been studied more intensively, leading to a significant progress nowadays.Citation42 Irinotecan belongs to the water-soluble semi-synthetic derivatives of camptothecin, in the form of hydrochloride trihydrate, and is the first specific topoisomerase I inhibitor. Twenty-six trials involved irinotecan among the 33 trials with results. A phase 2 trial confirmed that the simultaneous inhibition of epidermal growth factor receptor (EGFR) and BRAF combined with irinotecan is effective in colorectal cancer with the brafv600e mutation.Citation43 In addition, clinical data suggested that irinotecan was used to treat pancreatic cancer, glioma, rhabdomyosarcoma, lung cancer, gastric cancer, esophageal cancer, biliary tract cancer, solid tumors, hepatoblastoma, neuroblastoma, pinealoblastoma, ganglion neuroblastoma, and tumors of the central nervous system. In addition, trials in this investigation involved CRLX101 (5), topotecan (2), DS-8201a (1) and NKTR-102 (1). CRLX101, also known as IT-101, is a camptothecin/polyethylene glycol-cyclodextrin nanomedicine currently in Phase II clinical studies. The results of the NCT03531827 trial suggested that a regimen of NLG207 (formerly CRLX101) co-administered with enzalutamide is effective for enzalutamide-resistant preclinical prostate cancer patients.Citation44 However, NLG207 12 mg/m2 in combination with enzalutamide was not well tolerated in patients with metastatic castration-resistant prostate cancer (mCRPC) following several lines of the standard of care therapy.Citation45 Topotecan was approved for marketing by the US FDA in 1996 and is used in the treatment of colon and ovarian cancer. The 2 studies (NCT00057837, NCT01803269) related to topotecan were both on small cell lung cancer, although none of these two trials published the results at this time. DS-8201a, also known as T-DXd or Enhertu, consists of three components: a humanized anti-HER2 antibody, an enzyme cleavage linker, and a comedogenic analogue topoisomerase I inhibitor (DXd); this is the first FDA-approved drug targeting HER2 mutations in non-small cell lung cancer. A Phase I, multicenter, non-randomized, open-label, multi-dose research trial showed promising results using T-DXd in solid tumors expressing or carrying a mutated HER2 (NCT02564900). Results from a phase 1 clinical trial using trastuzumab deruxtecan (DS-8201) showed a promising effect in solid tumors expressing HER2 or carrying the mutant form of it.Citation46 NKTR-102 is a polyethylene glycol conjugate of irinotecan, which is structurally modified by coupling a 4-arm polyethylene glycol to the hydroxyl group of irinotecan. A Phase II, single-arm, open-label study on NKTR-102 in bevacizumab-resistant high-grade gliomas (NCT01663012) was completed, but no results have been published.
The median progression-free survival in patients treated with camptothecin-related compounds was more than 2 months. The data on progression-free survival ranged from a low value of 0% (ie, all patients experienced disease progression) to 55% or more. The 33 clinical trials listed up to 25 serious adverse reactions, mainly diarrhea (3), neutropenia (2), thrombosis (2), seizures (2) and death (2). Other adverse reactions were predominantly anemia (12), neutropenia (7) and fatigue (4).
Analysis of the Results of Other Monomers-Related Clinical Trials
Homoharringtonine is one of the main active ingredients in plants of the family Cephalotaxus, such as Cephalotaxus sinensis, and it shows a significant anti-tumor activity.Citation47 It became the first new anti-leukemia drug recommended for clinical application in China in the 70s. Omacetaxine mepesuccinate, a semi-synthetic compound of homoharringtonine, has also been approved in the market by the FDA in 2012 for the treatment of patients with chronic myeloid leukemia resistant to tyrosine kinase inhibitors.Citation48,Citation49 Eight of the nine clinical trials involving homoharringtonine applied this drug in different types of leukemia, with two phase II trials (NCT00462943 and NCT00375219) demonstrating that ”subcutaneous administration of omacetaxine mepesuccinate may provide clinical benefit in patients with chronic-phase CML who are refractory or intolerant to treatment with multiple tyrosine kinase inhibitors”Citation50 and that “omacetaxine mepesuccinate may provide safe and effective treatment in patients with CML with T315I mutations”Citation51 respectively. The two clinical trials used the overall hematological response rate as the first primary outcome measure to study the efficacy of homoharringtonine in patients with advanced CML, and in patients with CML with the T315I mutation. Both results suggested that homoharringtonine is more effective in patients with CML in the chronic phase. The results of these two clinical trials also showed that the median overall hematological response rates for all participants were 41.0% and 59.2%. The dominant serious adverse reaction was reduction of granulocytes or neutrophils and fever caused by this reduction (5), in addition to pneumonia (1), sepsis (1), death (1), and atrial flutter (1). Other adverse reactions were thrombocytopenia (3), reduction of granulocytes or neutrophils and fever caused by this reduction (2), anemia (1), fatigue (1), vomiting (1) and respiratory failure (1).
Curcumin is a natural polyphenol monomer used in TCM, derived from the rhizome of the ginger family and araceae family. Previous studies found that curcumin possesses a variety of pharmacological properties such as anti-tumor, antibacterial, anti-inflammatory and antioxidant effects.Citation52,Citation53 Currently, two clinical trials show results. One of them is a phase II trial using curcumin in patients with advanced pancreatic cancer, which has been completed and the results are available (NCT00094445). The six-month survival rate of patients was 15.9% after 8 weeks of curcumin, and the main serious adverse effects and other adverse effects were anorexia and edema, respectively. The remaining one was a pilot study on curcumin with or without piperine in patients with multiple myeloma (NCT00113841). The combination of curcumin and piperine significantly reduced the percentage of NF-kB protein expression compared with the effect of using curcumin alone. No serious adverse reactions occurred to the patients in this trial, while other adverse reactions were mainly constipation.
Genistein is an isoflavone compound mainly found in leguminous plants such as soybeans, kudzu root, and yam root. A total of 7 genistein tests were included in the discussion scope of this article, with 6 being in phase 2 and 1 being in phase 1/2. Two trials published their results in journals: the first one was a phase II trial on isoflavone G-2535 as a cancer chemopreventive biomarker in preoperative patients with bladder cancer. It revealed that “genistein showed a potential bimodal effect in epidermal growth factor receptor (EGFR) phosphorylation of bladder cancer tissue (more effective at low doses), which should be further evaluated and may be used in combination with other drugs” (NCT00118040).Citation54 The other one was “genistein in combination with FOLFOX or FOLFOX-bevacizumab in metastatic colorectal cancer”. It concluded that “the addition of genistein to FOLFOX or FOLFOX-bevacizumab is safe, tolerated and has a significant therapeutic effect, which deserves validation in a larger clinical trial” (NCT01985763).Citation55 Since 2 of the 7 trials had 0 participants, the results were not available. The status of the other 5 studies was the following: 1) One used the number of adverse events and percentage change in tumor size as the main indicators of effectiveness, thus showing that patients who received genistein in combination with chemotherapy experienced varying degrees of adverse events, with the highest number of grade 1 adverse events and the lowest number of grade 3 adverse events, while the median change in tumor size was −43.0%. 2) Another trial used tissue content of calcitriol as the first primary outcome measure. The average levels of the treatment group and control group are 0.057ng/mL and 0.045ng/mL. 3) The third trial observed a 0.5% change in the percentage of Ki-67 expression in the tissues of all participants compared to the baseline after a pharmacological intervention, while the control group using placebo showed a change of 1.0%. In addition, four more “probably related toxic events” were observed in the treatment group than in the control group. 4) The fourth clinical study used the degree of phosphorylation of EGFR in tumors and benign tissues as the primary indicator of efficacy. The analysis of the combination of the two treatment groups in the trial showed that 67.57% of the tumors showed a strong phosphorylation of EGFR, while 18.92%, 13.51%, and 0.00% of the tumors showed a moderate, weak, and negative phosphorylation, respectively, whereas 93.33%, 0.00%, 6.67%, and 0.00% of the tumors of the control group showed a phosphorylation of EGFR, in a strong to weak degree in descending order. Similarly, the results showed that the phosphorylation of EGFR in the benign tissue in the treatment group was 4.55% strong, 27.27% moderate, 54.55% weak and 13.64% negative, while in the control group, the results were slightly different: 0.00% strong, 33.33% moderate, 44.44% weak and 22.22% negative. 5) The fifth clinical trial used the number of survived patients and the median overall survival as the primary outcome measures. The results showed that 10 patients survived and the median overall survival for the treatment group was 6.3 months. Participants in 4 of the 7 clinical trials experienced serious adverse reactions, particularly with neutrophils, supraventricular tachycardia, atrial fibrillation, and nausea. Other adverse effects were observed in all trials and were related to emergency right inguinal repair, rash, fatigue, elevated blood urea nitrogen (bun), hypertension, abdominal pain, and metabolic and nutritional disturbances.
Psoralen has photosensitizing activity and is usually used in conjunction with long-wave ultraviolet irradiation in the treatment of tumors, known as photochemotherapy (PUVA, Psoralen Ultra-Violet A). There is currently one preliminary study on pegylated interferon-α-2b in combination with PUVA in cutaneous T-cell lymphoma that was completed (NCT00724061), and its available results showed a dose-limiting toxicity grade of 0 for the dosing regimen. The data available for this trial showed that the main serious and other adverse reactions in participants were ventricular tachycardia, and fatigue. Extracorporeal photopheresis (ECP) therapy is an immunomodulatory therapy involving the separation of leukocytes from the patient’s peripheral blood, which were treated with PUVA in vitro, and then these processed leukocytes were injected back into the patient body to produce an immunosuppressive effect. Another completed phase 2 trial was on the effects of pentostatin administered prior to donor bone marrow transplantation in combination with ECP therapy and total body irradiation in the treatment of patients with myelodysplastic syndromes (NCT00045305). It used the median complete response rate as the primary indicator of efficacy and achieved a final result of 35.3%. The main serious adverse reactions and other adverse reactions observed in this clinical trial were leukopenia and anemia, respectively.
The primary outcome measures of the two trials on epigallocatechin gallate were “sustained reduction in m-protein of ≥ 25% from the baseline” and “epigallocatechin gallate levels in non-malignant bladder tissue”. In the first trial, no patients had sustained reductions of ≥ 25% in m-protein relative to baseline (NCT00942422). In the second study, the mean epigallocatechin gallate level in the non-malignant bladder tissue of patients who underwent polyphenol E+ transurethral resection or cystectomy was 1.72 ng/mL (NCT00666562). However, the mean epigallocatechin gallate level in the non-malignant bladder tissue of patients treated only with polyphenol E+ placebo was 0.50 ng/mL. In addition, the main serious adverse reaction and other adverse reactions in the former trial were hyperglycemia, and no serious adverse reactions were observed in the second trial, in which the other adverse reaction was headache.
The scientists investigating resveratrol used the number of subjects experiencing adverse events and serious adverse events as the first primary outcome measures. The results of only one trial showed that the probability of adverse events was 100% regardless of whether resveratrol (under the name of SRT501) was combined with another drug, whereas the probability of a serious adverse event decreased by almost 17% with SRT501 in combination with bortezomib compared to no combination. Its main serious adverse reactions and other adverse reactions were acute renal failure and nausea, respectively. In addition, a phase 2 study showed that the safety and efficacy of SRT501 for the treatment of patients with recurrent and/or refractory multiple myeloma are not optimistic.Citation56
The results of the studies on genistein, curcumin, psoralen, epigallocatechin gallate, and resveratrol are briefly statistically presented in .
Table 2 Partial Results of Anti-Cancer Clinical Trials of TCM Monomers (Genistein, Curcumin, Psoralen, Epigallocatechin Gallate, and Resveratrol)
Discussion
Unique TCM has played a non-negligible role in the treatment of many diseases in the last decade.Citation57–59 Monomers, which are the material basis of the medicinal effect of TCMs, have a clear chemical structure and numerous pharmacological activities.Citation60–63 In particular, their positive anti-tumor therapeutic effect has attracted the attention of researchers all over the world, and has gradually become the focus of new drug research and development, with an increasing number of clinical trials. By collating and analyzing all clinical trials recorded on Clinicaltrial.gov under specific search conditions, this work found that the number of interventional anti-tumor TCM monomeric clinical trials registered worldwide reached 1982 as of April 30, 2023, showing an increasing trend. However, the United States and its institution have undertaken the highest number of interventional anti-tumor trials both in terms of regional distribution and sponsors/collaborators. This means that China still has a lot of room for progress in promoting the registration of clinical trials on anti-tumor TCM monomers. It is worth noting that in recent years, the Chinese government has issued a series of measures to support the development of TCM, including the Law of the People’s Republic of China on TCM, Several Policies and Measures on Accelerating the Development of TCM, and the 14th Five-Year Plan for the Development of TCM. The Chinese research and development environment on TCM monomers will be greatly improved with the strong support of national policies, which is conducive to the research and development of new drugs based on TCM monomer.
The phases of the collected clinical trial showed that nearly half of the interventional anti-tumor clinical trials involve a phase 2 stage, but the number of phase 1 clinical trials is only a third of that of phase 2 trials, which may be due to the following reasons: 1) some phase 1 clinical trials were performed during a short period of time, which may last even a few months, resulting in the failure to register in a timely manner; 2) the results of some phase 1 clinical trials failed to meet investigators’ expectations, leading to the failure to register these trials; 3) a larger number of phase 1 clinical trials had good results and could be continued, leading to an increase in the number of phase 2 trials. On the other hand, the number of phase 3 trials is much lower than that of phase 2 trials, perhaps because phase 3 trials, as the final stage of the clinical development process, are not only the most time-consuming and difficult, but they also require a significant financial investment. Phase 4 clinical trials are post-market studies performed by the applicant to further monitor and evaluate the drug to ensure its long-term safety and efficacy. The smallest number of phase 4 clinical trial registrations may also be related to the willingness of applicants. In addition, the number of trials with clinical phases 1/2 and 2/3 were 9.25% and 1.93%, respectively. This suggests that even a small number of researchers chose a joint study approach in designing their trials, seamlessly linking two consecutive phases, during which adjustments are made to the trial program through dynamic analysis. All this is to achieve a reduction in the overall sample size, an effective reduction of the research cycle, and a savings of the associated resources and costs. Such a trial protocol with continuous phases can lead to faster and less expensive drug development and may represent another option for subsequent clinical trial researchers.
Although 131 anti-tumor TCM monomers were counted, only 52.67% of them entered the clinical trials, and less than 30 cases entered the interventional anti-tumor trials. This suggests that although many TCM monomers showed good anti-tumor activity in preclinical studies, they did not enter clinical trials for the time being due to a variety of reasons, such as low bioavailability,Citation64 certain toxic effects,Citation65 and anti-tumor mechanisms that are not yet fully defined. For example, the low bioavailability of baicalin limits its therapeutic effect;Citation66,Citation67 the water solubility, low bioavailability, low blood concentration, and oral absorption of ginsenosides limit their clinical application;Citation68,Citation69 and toosendanin causes liver cell death in rats and has a certain toxic effect.Citation70,Citation71 However, these problems can be solved by performing a structural modification of the monomers,Citation72,Citation73 changing the dosage form, and using them in combination with other drugs, so that more TCM monomers can enter the clinical trial stage.
Vinblastine is difficult to be directly used in clinical treatment due to myelosuppression, myalgia, mild to moderate nausea and vomiting, and large doses causing mucositis and painful glossitis. Therefore, it is often used in combination with other drugs in the treatment of clinical cancer. Camptothecin binds to the Top I gene to form a “drug-Topo I-DNA” ternary complex, leading to DNA single-strand breaks and preventing DNA replication, thus exerting anti-tumor effects.Citation74 However, the natural product camptothecin shows some problems such as poor water solubility and poor selectivity, leading to a random targeting.Citation74,Citation75 In recent years, researchers have sought to develop related derivatives through structural modification to overcome these problems, and have made some progress. Of the 5 camptothecin derivatives, both irinotecan and topotecan were approved by FDA. Moreover, DS-8201 was considered as a new star in the anti-cancer field with significant therapeutic effects on HER2-positive tumor patients. It was rapidly approved by the US FDA, as well as by the National Medical Products Administration of China (NMPA) for official listing in China on February 21, 2023. NKTR-102, as a polyethylene glycol conjugate of irinotecan, has better anti-tumor effects and higher safety, making it a promising drug. CRLX101 was identified in a clinical trial on the treatment of lung cancer as a viable combination drug strategy with extremely low toxicity and side effects.Citation76,Citation77 Thus, it is a promising camptothecin derivative. Structural modification, change of dosage form and combination of drugs to solve the drawbacks of the current anti-tumor monomer are conducive to speeding up the research and development of anti-tumor TCM monomer.
Although more efforts are needed to promote the introduction of TCM monomers into clinical trials, there are still some monomers in clinical research worth looking forward to. Minnelide is a water-soluble derivative of Triptolide A, which is a promising new anti-pancreatic cancer drug currently in phase II evaluation (NCT04896073); a concurrent phase 1 trial using Minnelide in combination with osimertinib is also ongoing in patients with EGFR-mutated NSCLC (non small cell lung carcinoma) (NCT05166616). In addition, a phase I/II investigational clinical trial on the tolerability, safety, and efficacy of curcumin liposomes in combination with radiotherapy and temozolomide is ongoing in the treatment of patients with newly diagnosed high-grade gliomas, being in the phase of recruiting (NCT05768919).
This study has some limitations. The first is the limitation in the information of the trials in the ClinicalTrials.gov database. On the one hand, some clinical studies may be registered on other platforms (including China Clinical Trial Registration Center, European Union Clinical Trials Register, and University Hospital Medical Information Network Center Clinical Trials Registry); thus, a small number of clinical trials are not registered and enrolled in this database and information is not available even though the ClinicalTrials.gov database includes most of the clinical trials from all over the world.Citation78 On the other hand, research information may be missing in some trials.Citation78 Second, the ClinicalTrials.gov database does not have an indication classification for drug item, and the use of ICD-11 to classify the indications for drugs in this study is somewhat subjective. Third, the manual search of all clinical trials on anti-tumor herbal monomers may be potentially subjected to selection bias. Fourth, some clinical trials involve multiple intervention types and may be performed in multiple locations, with multiple sponsor/collaborators. Therefore, data processing would become extremely complex if all data were included in the analysis. Considering this, this study chose to prioritize the first set of data for statistical analysis to obtain a general understanding of the trends, although this approach may result in discrepancies between the statistical data and actual outcomes.
Conclusion
As of 2023, the number of clinical trials on anti-tumor TCM monomers shows an increasing trend, and Lymphoma is a key type studied by anti-tumor trials of TCM monomers. Monomers such as vinblastine and camptothecin have been used in clinical practice for a long time. However, most of the anti-tumor monomers did not yet enter the clinical research, which is still a problem to be solved. In the future, researchers can consider improving this situation through structural modifications, combination therapy and other methods. At present, the clinical research on anti-tumor monomers in China is slightly backward. However, the strong support of national policies, the research and development level of Chinese enterprises, universities and other institutions will continue to improve, and the research and development on TCM monomers will achieve more results.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by Beijing Nova Program (20230484300) and the Fundamental Research Funds for the Central Public Welfare Research Institutes (CI2021A04309, ZZ13-YQ-059).
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA. 2024;74(1):12–49. doi:10.3322/caac.21820
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA. 2023;73(3):233–254. doi:10.3322/caac.21772
- Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi:10.1002/cncr.33587
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA. 2021;71(3):264–279. doi:10.3322/caac.21657
- Buyel JF. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol Adv. 2018;36(2):506–520. doi:10.1016/j.biotechadv.2018.02.002
- Naz I, Ramchandani S, Khan MR, Yang MH, Ahn KS. Anticancer Potential of Raddeanin A, a natural triterpenoid isolated from anemone raddeana regel. Molecules. 2020;25(5). doi:10.3390/molecules25051035
- Ren T, Bai XY, Yang MZ, et al. Gambogic acid suppresses nasopharyngeal carcinoma via rewiring molecular network of cancer malignancy and immunosurveillance. Biomed Pharmacother. 2022;150:113012. doi:10.1016/j.biopha.2022.113012
- Chen L, Wang J, Wu J, Zheng Q, Hu J. Indirubin suppresses ovarian cancer cell viabilities through the STAT3 signaling pathway. Drug Des Devel Ther. 2018;12:3335–3342. doi:10.2147/dddt.S174613
- Huang H, Chen AY, Rojanasakul Y, Ye X, Rankin GO, Chen YC. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. Journal of Functional Foods. 2015;15:464–475. doi:10.1016/j.jff.2015.03.051
- Chen J, Wen B, Wang Y, et al. Jervine exhibits anticancer effects on nasopharyngeal carcinoma through promoting autophagic apoptosis via the blockage of Hedgehog signaling. Biomed Pharmacother. 2020;132:110898. doi:10.1016/j.biopha.2020.110898
- Raza W, Luqman S, Meena A. Prospects of tangeretin as a modulator of cancer targets/pathways. Pharmacol Res. 2020;161:105202. doi:10.1016/j.phrs.2020.105202
- Guo C, He J, Song X, et al. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol Res. 2019;149:104463. doi:10.1016/j.phrs.2019.104463
- Lin L, Chen Y, Yan L, et al. Analysis of clinical trials of new drugs in China as of 2019. Drug Discov Today. 2020;25(12):2080–2088. doi:10.1016/j.drudis.2020.09.030
- Zhou Y, Yang J, He Y, et al. Characteristic analysis of clinical trials for new traditional Chinese medicines in mainland China from 2013 to 2021. Front Med. 2022;9:1008683. doi:10.3389/fmed.2022.1008683
- Li N, Huang HY, Wu DW, et al. Changes in clinical trials of cancer drugs in mainland China over the decade 2009–18: a systematic review. Lancet Oncol. 2019;20(11):e619–e626. doi:10.1016/s1470-2045(19)30491-7
- Chen H, Zhou Y, Han X, Shi Y. The changing landscape of anti-lymphoma drug clinical trials in mainland China in the past 15 years (2005–2020): a systematic review. Lancet Public Health. 2021;8:100097. doi:10.1016/j.lanwpc.2021.100097
- De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the international committee of medical journal editors. New Engl J Med. 2004;351(12):1250–1251. doi:10.1056/NEJMe048225
- Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database--update and key issues. New Engl J Med. 2011;364(9):852–860. doi:10.1056/NEJMsa1012065
- Wang ZL, Wang S, Kuang Y, Hu ZM, Qiao X, Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol. 2018;56(1):465–484. doi:10.1080/13880209.2018.1492620
- Shi Y, Shi X, Zhao M, Ma S, Zhang Y. Pharmacological potential of Astragali Radix for the treatment of kidney diseases. Phytomedicine. 2024;123:155196. doi:10.1016/j.phymed.2023.155196
- Ying J, Zhang M, Qiu X, Lu Y. The potential of herb medicines in the treatment of esophageal cancer. Biomed Pharmacother. 2018;103:381–390. doi:10.1016/j.biopha.2018.04.088
- Daneshvar S, Zamanian MY, Ivraghi MS, et al. A comprehensive view on the apigenin impact on colorectal cancer: focusing on cellular and molecular mechanisms. Food Sci Nutr. 2023;11(11):6789–6801. doi:10.1002/fsn3.3645
- Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majus L. (Papaveraceae). Pharmacol Res. 1996;33(2):127–134. doi:10.1006/phrs.1996.0019
- Ding Y, Chen S, Zhang F, et al. Chitinase is a potent insecticidal molecular target of camptothecin and its derivatives. J Agric Food Chem. 2023;71(4):1845–1851. doi:10.1021/acs.jafc.2c06607
- Li Y, Zeng ZW, Chen D, et al. Facilitated drug repurposing with artemisinin-derived PROTACs: unveiling PCLAF as a therapeutic target. J Med Chem. 2023;66(16):11335–11350. doi:10.1021/acs.jmedchem.3c00824
- Yang T, Yu R, Cheng C, et al. Cantharidin induces apoptosis of human triple negative breast cancer cells through mir-607-mediated downregulation of EGFR. J Transl Med. 2023;21(1):597. doi:10.1186/s12967-023-04483-y
- Wen F, Liu D, Wang M, et al. Celastrol induces premature ovarian insufficiency by inducing apoptosis in granulosa cells. Biomed Pharmacother. 2023;169:115815. doi:10.1016/j.biopha.2023.115815
- Liu H, Cheng H, Wang H, Wang Q, Yuan J. Crocin improves the renal autophagy in rat experimental membranous nephropathy via regulating the SIRT1/Nrf2/HO-1 signaling pathway. Renal Failure. 2023;45(2):2253924. doi:10.1080/0886022x.2023.2253924
- Xu Z, Zhou H, Zhang Y, et al. Recent pharmacological advances in the treatment of cardiovascular events with Astragaloside IV. Biomed Pharmacother. 2023;168:115752. doi:10.1016/j.biopha.2023.115752
- Yu KH, Kuo CY, Wu IT, et al. Novel (-)-arctigenin derivatives inhibit signal transducer and activator of transcription 3 phosphorylation and P-glycoprotein function resensitizing multidrug resistant cancer cells in vitro and in vivo. Eur J Pharmacol. 2023;960:176146. doi:10.1016/j.ejphar.2023.176146
- Watson M, Saitis T, Shareef R, Harb C, Lakhani M, Ahmad Z. Shikonin and Alkannin inhibit ATP synthase and impede the cell growth in Escherichia coli. Int J Biol Macromol. 2023;253(Pt 4):127049. doi:10.1016/j.ijbiomac.2023.127049
- Ye Q, Zhou X, Ren H, Han F, Lin R, Li J. An overview of the past decade of bufalin in the treatment of refractory and drug-resistant cancers: current status, challenges, and future perspectives. Front Pharmacol. 2023;14:1274336. doi:10.3389/fphar.2023.1274336
- Dhyani P, Quispe C, Sharma E, et al. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Can Cell Inter. 2022;22(1):206. doi:10.1186/s12935-022-02624-9
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J clin oncol. 2013;31(6):684–691. doi:10.1200/jco.2012.43.4803
- Younes A, Oki Y, McLaughlin P, et al. Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma. Blood. 2012;119(18):4123–4128. doi:10.1182/blood-2012-01-405456
- Park SI, Shea TC, Olajide O, et al. ABVD followed by BV consolidation in risk-stratified patients with limited-stage Hodgkin lymphoma. Blood Adv. 2020;4(11):2548–2555. doi:10.1182/bloodadvances.2020001871
- Stephens DM, Li H, Schöder H, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood. 2019;134(15):1238–1246. doi:10.1182/blood.2019000719
- Ansell SM, Radford J, Connors JM, et al. Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. New Engl J Med. 2022;387(4):310–320. doi:10.1056/NEJMoa2206125
- Kasamon YL, Jacene HA, Gocke CD, et al. Phase 2 study of rituximab-ABVD in classical Hodgkin lymphoma. Blood. 2012;119(18):4129–4132. doi:10.1182/blood-2012-01-402792
- von Rundstedt FC, Mata DA, Groshen S, et al. Significance of lymphovascular invasion in organ-confined, node-negative urothelial cancer of the bladder: data from the prospective p53-MVAC trial. BJU Int. 2015;116(1):44–49. doi:10.1111/bju.12997
- Margulis V, Puligandla M, Trabulsi EJ, et al. Phase II trial of neoadjuvant systemic chemotherapy followed by extirpative surgery in patients with high grade upper tract urothelial carcinoma. J Urol. 2020;203(4):690–698. doi:10.1097/ju.0000000000000644
- O’Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34(10):1500–1508. doi:10.1016/s0959-8049(98)00229-9
- Kopetz S, Guthrie KA, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J clin oncol. 2021;39(4):285–294. doi:10.1200/jco.20.01994
- Schmidt KT, Chau CH, Strope JD, et al. Antitumor activity of NLG207 (Formerly CRLX101) in combination with enzalutamide in preclinical prostate cancer models. Mol Cancer Ther. 2021;20(5):915–924. doi:10.1158/1535-7163.Mct-20-0228
- Schmidt KT, Karzai F, Bilusic M, et al. A single-arm phase II Study Combining NLG207, a nanoparticle camptothecin, with enzalutamide in advanced metastatic castration-resistant prostate cancer post-enzalutamide. Oncologist. 2022;27(9):718–e694. doi:10.1093/oncolo/oyac100
- Tsurutani J, Iwata H, Krop I, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase i study in multiple advanced solid tumors. Cancer Discovery. 2020;10(5):688–701. doi:10.1158/2159-8290.Cd-19-1014
- Wu Q, Chen P, Li J, Lin Z, Zhang Q, Kwok HF. Inhibition of bladder cancer growth with homoharringtonine by inactivating integrin α5/β1-FAK/Src axis: a novel strategy for drug application. Pharmacol Res. 2023;188:106654. doi:10.1016/j.phrs.2023.106654
- Lü S, Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J Hematol Oncol. 2014;7:2. doi:10.1186/1756-8722-7-2
- Chen Y, Li S. Omacetaxine mepesuccinate in the treatment of intractable chronic myeloid leukemia. Onco Targets Ther. 2014;7:177–186. doi:10.2147/ott.S41786
- Cortes J, Digumarti R, Parikh PM, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate for chronic-phase chronic myeloid leukemia patients resistant to or intolerant of tyrosine kinase inhibitors. Am J Hematol. 2013;88(5):350–354. doi:10.1002/ajh.23408
- Cortes J, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120(13):2573–2580. doi:10.1182/blood-2012-03-415307
- Firoz HM, Nanjundaiah S, Sadashiva CT, Neethumol B, Rashmi Y, Sreedrisya AK. Antiproliferative activity and apoptosis-inducing mechanism of Curcuma longa (Turmimax®) on HeLa cell lines. Braz J Biol. 2023;83:e275953. doi:10.1590/1519-6984.275953
- Jafari H, Namazi H. pH-sensitive biosystem based on laponite RD/chitosan/polyvinyl alcohol hydrogels for controlled delivery of curcumin to breast cancer cells. Colloids Surf B. 2023;231:113585. doi:10.1016/j.colsurfb.2023.113585
- Messing E, Gee JR, Saltzstein DR, et al. A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer Prev Res. 2012;5(4):621–630. doi:10.1158/1940-6207.Capr-11-0455
- Pintova S, Dharmupari S, Moshier E, Zubizarreta N, Ang C, Holcombe RF. Genistein combined with FOLFOX or FOLFOX-Bevacizumab for the treatment of metastatic colorectal cancer: phase I/II pilot study. Cancer Chemother Pharmacol. 2019;84(3):591–598. doi:10.1007/s00280-019-03886-3
- Popat R, Plesner T, Davies F, et al. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160(5):714–717. doi:10.1111/bjh.12154
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. doi:10.1182/blood-2007-07-102798
- Zhao J, Tostivint I, Xu L, et al. Efficacy of combined abelmoschus manihot and irbesartan for reduction of albuminuria in patients with type 2 diabetes and diabetic kidney disease: a multicenter randomized double-blind parallel controlled clinical trial. Diabetes Care. 2022;45(7):e113–e115. doi:10.2337/dc22-0607
- Zhou X, Zhao S, Liu T, et al. Schisandrol A protects AGEs-induced neuronal cells death by allosterically targeting ATP6V0d1 subunit of V-ATPase. Acta pharmaceutica Sinica B. 2022;12(10):3843–3860. doi:10.1016/j.apsb.2022.06.013
- Qian Y, Zheng Y, Jin J, et al. Immunoregulation in diabetic wound repair with a photoenhanced glycyrrhizic acid hydrogel scaffold. Adv Mater. 2022;34(29):e2200521. doi:10.1002/adma.202200521
- Wan S, Zhang J, Hou R, et al. A strategy for component-based Chinese medicines design approach of Polygonum orientale L. against hypoxia/reoxygenation based on uniform design-stepwise regression-simulated annealing. Biomed Pharmacother. 2021;135:111177. doi:10.1016/j.biopha.2020.111177
- Wang X, Yang C, Ru Y, et al. An optimal combination of five main monomer components in Wuzi Yanzong Pill that prevents neural tube defects and reduces apoptosis and oxidative stress. J Ethnopharmacol. 2023;313:116540. doi:10.1016/j.jep.2023.116540
- Zhang Z, Ji Y, Hu N, et al. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci. 2022;17(5):751–766. doi:10.1016/j.ajps.2022.07.006
- Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46(1):2–18. doi:10.4143/crt.2014.46.1.2
- Pan MS, Cao J, Fan YZ. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. ChinMed. 2020;15:55. doi:10.1186/s13020-020-00338-6
- Li N, Feng L, Tan Y, Xiang Y, Zhang R, Preparation YM. Characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia-reperfusion after i.v. administration in rats. Molecules. 2018;23(7). doi:10.3390/molecules23071747
- Zhao L, Wei Y, Huang Y, He B, Zhou Y, Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int j Nanomed. 2013;8:3769–3779. doi:10.2147/ijn.S51578
- Xie HT, Wang GJ, Lv H, et al. Development of a HPLC-MS assay for ginsenoside Rh2, a new anti-tumor substance from natural product and its pharacokinetic study in dogs. Eur J Drug Metab Pharmacokinet. 2005;30(1–2):63–67. doi:10.1007/bf03226409
- Yu H, Teng L, Meng Q, et al. Development of liposomal Ginsenoside Rg3: formulation optimization and evaluation of its anticancer effects. Int J Pharm. 2013;450(1–2):250–258. doi:10.1016/j.ijpharm.2013.04.065
- Zhang Y, Qi X, Gong L, et al. Roles of reactive oxygen species and MAP kinases in the primary rat hepatocytes death induced by toosendanin. Toxicology. 2008;249(1):62–68. doi:10.1016/j.tox.2008.04.005
- Zhuo Y, Zhang Y, Li M, et al. Hepatotoxic evaluation of toosendanin via biomarker quantification and pathway mapping of large-scale chemical proteomics. Food Chem Toxicol. 2021;153:112257. doi:10.1016/j.fct.2021.112257
- Li S, Wu X, Fan G, Du K, Deng L. Exploring cantharidin and its analogues as anticancer agents: a review. Curr Med Chem. 2023;30(18):2006–2019. doi:10.2174/0929867330666221103151537
- Zou M, Xu Y, Lin P, Zhou L, Xia X. Use of different ligand modification liposomes to evaluate the anti-liver tumor activity of cantharidin. J Liposome Res. 2023;33(3):283–299. doi:10.1080/08982104.2022.2163254
- Selas A, Martin-Encinas E, Fuertes M, et al. A patent review of topoisomerase I inhibitors (2016-present). Expert Opin Ther Patents. 2021;31(6):473–508. doi:10.1080/13543776.2021.1879051
- Lu J, Liu C, Wang P, et al. The self-assembling camptothecin-tocopherol prodrug: an effective approach for formulating camptothecin. Biomaterials. 2015;62:176–187. doi:10.1016/j.biomaterials.2015.05.046
- Sanoff HK, Moon DH, Moore DT, et al. Phase I/II trial of nano-camptothecin CRLX101 with capecitabine and radiotherapy as neoadjuvant treatment for locally advanced rectal cancer. Nanomedicine. 2019;18:189–195. doi:10.1016/j.nano.2019.02.021
- Tian X, Nguyen M, Foote HP, et al. CRLX101, a nanoparticle-drug conjugate containing camptothecin, improves rectal cancer chemoradiotherapy by inhibiting DNA repair and HIF1α. Cancer Res. 2017;77(1):112–122. doi:10.1158/0008-5472.Can-15-2951
- Tse T, Fain KM, Zarin DA. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ. 2018;361:k1452. doi:10.1136/bmj.k1452