Abstract
Purpose
Shivering occurs frequently after caesarean delivery. The present study aimed to investigate the ED50 and ED95 of an intravenous (i.v.) bolus of dexmedetomidine for treating severe shivering after caesarean delivery under combined spinal-epidural anaesthesia.
Patients and methods
Seventy-five parturients with severe shivering after caesarean delivery were randomized into one of the five groups to receive an i.v. bolus of 0.2 (Group D1), 0.25 (Group D2), 0.3 (Group D3), 0.35 (Group D4) or 0.4 (Group D5) μg/kg of dexmedetomidine. Effectiveness of shivering treatment was defined as a standardized shivering score decreasing to ≤1 within 10 min of dexmedetomidine injection. The ED50 and ED95 were determined by probit regression. Adverse effects were also compared among the groups.
Results
The ED50 and ED95 of i.v. dexmedetomidine to treat severe shivering were 0.23 (95% CI, 0.16–0.26) μg/kg and 0.39 (95% CI, 0.34–0.52) μg/kg, respectively. No difference in the incidence of adverse effects was found between groups.
Conclusion
An i.v. bolus of 0.39 μg/kg of dexmedetomidine will treat 95% of parturients experiencing severe shivering after caesarean delivery.
Introduction
Shivering, characterized by involuntary, repetitive activity of skeletal muscles, is one of the most common complications during and especially after caesarean delivery under neuraxial anaesthesia with an incidence of up to 50%–85%.Citation1–3 After caesarean delivery, shivering can cause maternal discomfort and anxiety, interfere with blood pressure and oxygen saturation monitoring as well as electrocardiograms.Citation4 Therefore, developing strategies for treating shivering may improve the care we provide for our parturients.
Several medication classes, including opioids, 5-hydroxytryptamine3 (5-HT3) receptor antagonists, α2-receptor agonists and N-methyl-D-aspartate (NMDA)-receptor antagonists, have been used to prevent or treat perioperative shivering based on the possible mechanisms by which the central thermoregulatory mechanisms can be modified.Citation5–8 However, many anti-shivering agents are restricted by their side effects. For example, meperidine and tramadol may induce nausea and vomiting, which is very distressing for the patient, and ketamine can lead to hypertension and tachycardia.Citation9–11 Dexmedetomidine, a highly specific and selective α2 adrenoreceptor agonist, is considered a valuable medication in the current obstetric anaesthesia practice with a favorable risk–benefit ratio.Citation12,Citation13 Previous studies have demonstrated that i.v. dexmedetomidine could effectively reduce the incidence and intensity of shivering after caesarean delivery under neuraxial anaesthesia.Citation14–16 However, the dose-response of i.v. dexmedetomidine for treating shivering in parturients during Caesarean delivery has not been studied.
We conducted a prospective, randomized, double-blinded clinical trial to determine the dose–response characteristics of an i.v. bolus of dexmedetomidine for treating severe shivering after Caesarean delivery in parturients undergoing combined spinal–epidural anaesthesia and to derive values for the bolus dose of dexmedetomidine that would be effective for treating severe shivering in 50% (ED50) and 95% (ED95) of parturients.
Methods
Study Design
The study protocol was approved by the Ethical Review Board of Women’s Hospital, School of Medicine, Zhejiang University (approval number IRB-20200254-R) and registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn) (registration no: ChiCTR2000039264, PI: Meijuan Yang, registered on 22nd October 2020). Written informed consent was obtained from all participants. This study adheres to the applicable CONSORT guidelines for randomized trials.
Parturients with a singleton pregnancy, American Society of Anaesthesiologists physical status (ASA II), ages between 20 and 45 years, and a gestational age ≥37 weeks scheduled for elective Caesarean delivery were enrolled in the study. Exclusion criteria were as follows: gestational hypertension, diabetes mellitus, obesity (body mass index >35 kg/m2), height <150 cm or >170 cm, an initial body temperature >38°C or <36°C, cardiopulmonary disease, fetal abnormality, contraindication to spinal anaesthesia, chronic users of adrenergic receptor blockers or calcium channel blockers, a known history of alcohol or substance abuse, and postpartum haemorrhage greater than 1000 mL.
Of the enrolled participants, the parturients who presented with severe shivering (shivering score >2, which was assessed using the method described by Crossley et al)Citation17 with a five-point rating scale: 0 = no shivering; 1 = no visible shivering but one or more of piloerection, peripheral vasoconstriction or peripheral cyanosis (other causes excluded); 2 = muscle activity in only one muscle group; 3 = moderate muscle activity in more than one muscle group, but not generalized shaking; 4 = violent muscle activity that involves the whole body) after delivery were randomized to one of the five groups in a 1:1:1:1:1 ratio and received i.v. dexmedetomidine 0.2 μg/kg (Group D1), 0.25 μg/kg (Group D2), 0.3 μg/kg (Group D3), 0.35 μg/kg (Group D4) or 0.4 μg/kg (Group D5) based on a computer-generated random number sheet. The patient’s group assignment code was placed in an opaque, sealed envelope, which was opened by an assistant who was not involved in the anaesthesia procedure or data collection. The assistant was also responsible for preparing the solution of dexmedetomidine. Parturients who did not have severe shivering were excluded. The randomization number for each excluded parturient was used for the next enrolled parturient.
Anaesthetic Procedure and Interventions
No anxiolytic was given to any parturient. The temperature and humidity of our operating room was maintained at 22–24°C and 60%, respectively. Upon arrival in the operating room, standard monitoring including non-invasive blood pressure (NIBP), pulse oximetry (SpO2), electrocardiogram (ECG) and tympanic membrane temperature was applied. The patient received supplemental oxygen at 5 L/min via a face mask and pre-load infusion of 10 mL/kg of unwarmed lactated Ringer’s solution and then at 10 mL/kg/h intraoperatively.
A combined spinal-epidural anaesthesia (CSEA) procedure was performed using a double-space technique with parturients in the left lateral decubitus position.Citation18 A 16-gauge Tuohy needle was used to puncture into the epidural space at the L1-2 interspace, and an 18-gauge nylon multiport epidural catheter was then inserted from 3 cm to 4 cm cephalad into the epidural space. The catheter was secured and flushed with 3mL saline (no local anaesthetic) if aspiration was negative for blood and cerebrospinal fluid (CSF). Thereafter, a 27-gauge pencil-tip needle was used to puncture into the subarachnoid space at the L3-4 interspace. With confirmation of CSF presence, 3 mL of 0.5% ropivacaine was injected into the subarachnoid space over 30 seconds. The parturient was then turned to the supine position with 15-degree of left uterine displacement. Additional epidural anesthetic was 5 mL of 2% lidocaine, repeated every 10 min if necessary. Surgery began when a sensory block of T6 was achieved. During the surgery, all parturients were covered with one standard layer of surgical drapes with no active warming.
After delivery, parturients who presented severe shivering (shivering score ≥3) received an i.v. bolus of dexmedetomidine, which was diluted with normal saline to 5 mL, immediately after shivering. The dose of dexmedetomidine given to the parturient was determined according to the group assignment. The duration of injection lasted at least 2 minutes. Effectiveness of shivering treatment was defined when the shivering score decreased to ≤1 within 10 min, ineffective treatment was defined when the shivering score did not decrease to ≤1 within 10 min. Parturients with ineffective shivering treatment were given an i.v. bolus of 0.5 mg/kg tramadol.
After neonatal delivery and umbilical cord clamping, 10IU oxytocin were injected into the uterine wall, followed by an infusion of 10IU/h oxytocin to prevent uterine atony. If the uterine tone was found to be inadequate, secondline uterotonics (0.1mg of intravenous carbetocin, which was based on obstetrician’s request) were administered as needed. Postoperative pain was managed by the administration of 2 mg morphine in a volume of 3 mL through the epidural catheter at the end of the surgery, and followed by parturient controlled epidural analgesia with 0.1% ropivacaine mixed with 0.4μg/mL sufentanil using a PCA pump (Jiangsu Apon Medical Technology Co., LTD).
Measurements
NIBP was measured at 5-min intervals (and at 2-min intervals during the first 10 mins after dexmedetomidine injection) and heart rate (HR) and SpO2 were continuously monitored during the intraoperative period. Tympanic membrane temperature was measured and recorded at 4 time-points [T0: parturient’s arrival in operating room; T1: the time severe shivering occurred; T2: 10 mins after dexmedetomidine injection; T3: 20 mins after dexmedetomidine injection]. The baseline systolic blood pressure (SBP) and HR were determined by calculating the mean of 3 consecutive measurements at 3-min intervals. Hypotension was defined as a decrease in SBP ≥ 20% from the baseline or to ≤90 mmHg, which was treated with rapid i.v. infusion of lactated Ringer’s solution and/or a bolus of 4 μg i.v. norepinephrine. Reactive hypertension, defined as an increase in SBP ≥ 20% from the baseline, was treated with a bolus of nitroglycerine 50 μg if the hypertension lasted over 5 mins. Bradycardia, defined as a HR of < 50 bpm, was treated with 0.5 mg atropine intravenously.
The shivering score was assessed at 2-min intervals during the procedure. The sedation score was graded as: 1 = awake, oriented, cooperative; 2 = drowsy; 3 = eyes closed but arousable to command or physical stimuli; 4 = eyes closed and unarousable to physical stimuli. The sedation score was assessed at 5-min of intervals at 5 minutes after dexmedetomidine injection, for a total of 30 minutes. Sedation and shivering score are recorded by anesthesiologist and assistant who did not know about the group allocation.
Side effects related to anaesthesia or dexmedetomidine injection such as respiratory depression (defined as SpO2 <90% or respiratory rate <10 bpm), reactive hypertension, hypotension, bradycardia, estimated blood loss, nausea and vomiting were treated and recorded.
Statistical Analysis
Sample size was calculated with the Cochran–Armitage Test using PASS® software (Version 11.0.7, NCSS, LLC, Kaysville, UT).Citation19 Based on the results of our preliminary study, which showed that the percentage of success in treatment of shivering was 40%, 50%, 80%, 90% and 90%, respectively, for 5 groups with a bolus of 0.2, 0.25, 0.3, 0.35 or 0.4 μg/kg i.v. dexmedetomidine, a sample size of 10 parturients per group (50 parturients in total) would have 90% power to detect a linear trend among groups using a z-test with continuity correction and a significance level of 0.05. Considering the possible dropouts, we increased the sample size to 15 parturients per group (75 parturients in total).
Analysis was performed using IBM SPSS statistics for Windows version 22.0 (IBM Corp, Armonk NY) and GraphPad Prism version 8.4.0 (GraphPad Software Inc., San Diego, CA). For continuous variables, Kolmogorov–Smirnov test was used for assessment of normality. The normally distributed variables were presented as mean ± standard deviation (SD) and analysed by one-way analysis of variance (ANOVA) with Bonferroni post hoc tests for pairwise comparisons, and the non-normally distributed variables were presented as median and interquartile range (IQR) and were analyzed using the Mann–Whitney U-test. Categorical data were analysed using Chi-square test. The ED50 and ED95 of i.v. dexmedetomidine for treating shivering were calculated using probit regression. A P value of < 0.05 (two-sided) was considered statistically significant.
Results
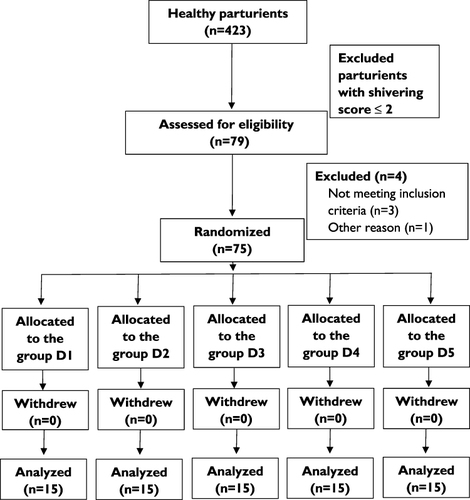
The recruitment scheme is shown in . A total of 423 parturients undergoing elective caesarean delivery were assessed for eligibility, of which 318 presented with shivering (shivering score ≥1) and 79 parturients with shivering score ≥3. The incidence of shivering and severe shivering after caesarean delivery were 75.2% and 18.7%, respectively. Of the parturients with severe shivering, 4 patients did not meet inclusion criteria. Finally, a total of 75 subjects (15 subjects in each group) were included in the study randomization. There were no significant differences in age, height, weight, gestational age and surgical duration among the groups ().
Table 1 Demographic Characteristics and Operative Data
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) Diagram showing parturient recruitment and flow.
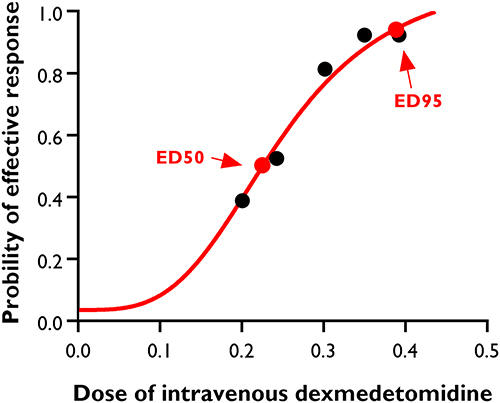
The number of parturients with effective control of severe shivering after caesarean delivery was 6 (40%), 9 (60%), 12 (80%), 14 (93.3%) and 14 (93.3%) in Group D1, Group D2, Group D3, Group D4 and Group D5, respectively (P < 0.001). The dose–response curve of i.v. dexmedetomidine for treating severe shivering is shown in . The values for ED50 and ED95 were 0.23 μg/kg (95% CI, 0.16–0.26 μg/kg) and 0.39 μg/kg (95% CI, 0.34–0.52 μg/kg), respectively.
Figure 2 Dose–response curve of intravenous dexmedetomidine for treating severe shivering after caesarean delivery. The values for ED50 and ED95 calculated by probit regression model were 0.23 μg/kg (95% CI, 0.16–0.26 μg/kg), and 0.39 μg/kg (95% CI, 0.34–0.52 μg/kg) respectively.
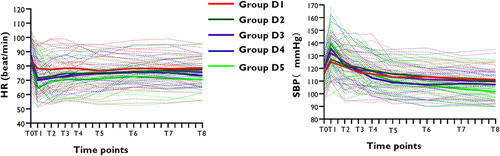
There were no differences among groups in HR and SBP at the observed time points (, P > 0.05). The trends of mean SBP mostly increased at 1min, then decreased at 3, 5min, and finally stabilized at a certain level in each group. Conversely, the trends of mean HR decreased at 1min, then increased at 3, 5min, and finally stabilized at a certain level in each group.
Figure 3 Changes in the heart rate and systolic blood pressure with time. Systolic blood pressure and heart rate were monitored at the following time points: before dexmedetomidine injection, 1min, 3min, 5min, 7min, 10min, 15min, 20min after dexmedetomidine injection, and at the end of surgery (T0, T1, T2, T3, T4, T5, T6, T7 and T8). The solid line showed the mean heart rate and systolic blood pressure in each group at different time points. The dotted line showed the heart rate and systolic blood pressure of each patient in each group at different time points.
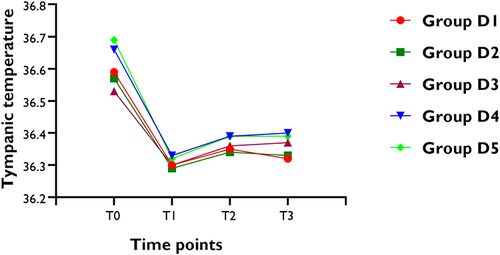
shows changes in tympanic membrane temperature in five groups. There were no clinically meaningful or statistically significant differences among groups in the mean values of tympanic membrane temperature at the four observed time points. However, in each group, the tympanic membrane temperature was lower at the time of severe shivering happened (before dexmedetomidine injection) compared to parturient arrival in the operating room (all P < 0.05).
Figure 4 Changes of tympanic membrane temperature (°C). T0 = Time of arrival in operating room; T1 = the time severe shivering occurred; T2 =10 mins after dexmedetomidine injection; T3 = 20 mins after dexmedetomidine injection.
There were no significant differences in the incidence of hypotension, reactive hypertension, bradycardia, SpO2 < 90%, sedation score at 3 or 4, nausea and vomiting, and atropine use among groups (). Only 1 parturient in group D4 and 2 parturients in group D5 experienced reactive hypertension. One parturient in group D5 had bradycardia requiring atropine. No parturient had a sedation score of 4.
Table 2 Intra-Operative Adverse Events
Discussion
In the present prospective, randomized, double-blinded dose–response study, we found that the ED50 and ED95 values of an i.v. bolus of dexmedetomidine for treating severe shivering were 0.23 μg/kg (95% CI, 0.16–0.26 μg/kg) and 0.39 μg/kg (95% CI, 0.34–0.52 μg/kg) after caesarean delivery under combined spinal-epidural anaesthesia. What is novel about our findings is that we demonstrate the dose response of dexmedetomidine for treating shivering and provide a reference of dexmedetomidine dosage for controlling shivering after caesarean delivery in clinical practice.
Studies have shown that i.v dexmedetomidine could prevent or treat shivering safely after caesarean delivery.Citation15,Citation16,Citation20,Citation21 Lamontagne et al suggested that a single intravenous bolus of 30 μg dexmedetomidine could significantly decrease shivering in 90% of patients within 10 min, as compared with 23% in the control group during caesarean delivery associated with neuraxial anaesthesia.Citation15 Recently, Sween et al found that prophylactic administration of a single bolus of i.v. dexmedetomidine 10 μg could reduce the incidence of shivering without notable side effects.Citation20 But i.v. dexmedetomidine 10 μg reduced the incidence of shivering by only about 50%, the effective rate was low. Abdel-Ghaffar al. reported that compared to dexmedetomidine 0.2 µg/kg or 0.5 µg/kg group, dexmedetomidine 0.3 µg/kg effectively treated shivering under spinal anesthesia with modest hemodynamic and sedation effects.Citation16 In the present study, the ED95 of dexmedetomidine for treating severe shivering was 0.39 μg/kg, which is close to the dosage of dexmedetomidine used in previous studies.
The mechanism of the anti-shivering effect of dexmedetomidine is not well explored. It was proposed that dexmedetomidine, through its action on the α2-adrenoceptor in the hypothalamus, could inhibit the spontaneous firing rate of neurons, suppress sympathetic tone with attenuation of the neuro-endocrine, decrease central thermo-sensitivity, and consequently reduce the vasoconstriction and shivering thresholds.Citation22,Citation23 In addition, dexmedetomidine probably modulates the function of the thermoregulatory centre via binding α2-receptors, and then exhibits an anti-shivering effect.Citation24,Citation25 While the proposed mechanism could not be verified with the results of the present study, which showed the mean body temperature was comparable among the groups, future studies should explore this mechanism.
A slow bolus administration of dexmedetomidine over 10 mins has been well described,Citation26,Citation27 but this method goes with some obvious disadvantages, such as inconvenient preparation and time-consuming. A rapid bolus injection, if proven to be hemodynamically appropriate, would allow a more timely and optimum administration of the drug to avert and treat shivering. Bloor et al showed that when 0.5 μg/kg dexmedetomidine was administered intravenously over the course of 2 minutes, arterial blood pressure stabilized in healthy volunteers.Citation28 Jooste et al showed that after administration of i.v. dexmedetomidine bolus of either 0.25μg/kg or 0.5μg/kg over 5 seconds, systolic blood pressure, diastolic blood pressure, and systemic vascular resistance all increased at 1 minute, and decreased significantly to near baseline for both doses by 5 minutes. Cardiac output, central venous pressure, and pulmonary vascular resistance did not change significantly. The HR decreased after dexmedetomidine injection and remained below the baseline measurement after 5 minutes.Citation29 John et al also found that after a rapid bolus of 0.5μg/kg dexmedetomidine, a statistically significant change in hemodynamics was observed, but no patients required any intervention for hemodynamic changes.Citation30 Previous research showed that dexmedetomidine administered to healthy volunteers develops nonlinear clearance, which may result in higher than expected plasma concentrations and side effects.Citation31,Citation32 Considering the above factors, we injected the dexmedetomidine with a bolus dose less than 0.5 μg/kg in 2–3 min for the present study.
Similar to previous study, a biphasic dose–response relation between dexmedetomidine and the hemodynamic effects was observed in our study.Citation26,Citation33 The reason for this observation is that with i.v. administration of dexmedetomidine, peripheral α2B receptor of the postsynaptic membrane of the vascular smooth muscle are activated to cause vasoconstriction. Afterwards, the central sympathomimetic actions of the α2 receptor are achieved, and there is a moderate decrease in blood pressure and heart rate. Therefore, dexmedetomidine exerts a predictable effect on haemodynamics.Citation28,Citation34 Dexmedetomidine caused peripheral vasoconstriction and bradycardia when the plasma concentration exceeded 1000 pg/mL.Citation35 The hemodynamic effects of dexmedetomidine are related to the rate of infusion and dose. In this study, the hemodynamic instability was transient, and we found that the changes in blood pressure and HR were reversible over time. Only one patient in group D4 and two patients in group D5 developed reactive hypertension, and no one needed nitroglycerine. One patient in group D5 had bradycardia requiring atropine injection. While considering that hypertension and bradycardia only occurred in high-dose group (0.35μg/kg or 0.4μg/kg) and nonlinear clearance of dexmedetomidine, we recommended to start using dexmedetomidine at 0.3μg/kg and giving slowly to (> 2min) avoid hemodynamic instability.
With regard to side effects, we found that no parturients developed grade 4 sedation after dexmedetomidine administration. Sedation with dexmedetomidine is characterized as short term and ease of arousability to verbal stimulus.Citation36 It has even been demonstrated that with high doses of α2-adrenergic agonist infusion, respiratory variables are minimally changed.Citation37 Similarly, there were no parturients who had respiratory depression in the present study. There was no statistical significance in the incidence of nausea and vomiting between the five groups. The exact aetiology of perioperative nausea and vomiting remains unknown. Nausea and vomiting can be aggravated by a variety of factors including uterine manipulation, cerebral ischemia, blocking of sympathetic nerves, hypotension and peritoneal closure in parturients undergoing caesarean delivery.Citation38,Citation39 Intravenously injected dexmedetomidine leads to enhanced stability of the sympathetic adrenergic system, and then results in steady haemodynamics. The stable hemodynamic effects may reduce the incidence of nausea and vomiting. In this study, the incidence of nausea and vomiting was low.
There are limitations in our study that should be considered. First, we did not measure body temperature postoperatively. As a result, we were unable to detect the effect of dexmedetomidine on body temperature through possible modulation of the thermoregulatory centre. Second, because measuring shivering on the shivering scale is subjective and subject to inter-observer variability, it is possible that the results could be different should the study be repeated with different observers. Although the majority of the data were measured by two researchers, a reliable and objective way for detecting shivering is still needed. Third, the sample population consisted of healthy parturients, so it is unknown if the data are generalizable to other operations or parturients with cardiovascular disease. Forth, we did not record the duration of dexmedetomidine and shivering score postoperatively. More future work needs to be performed.
Conclusions
The ED50 and ED95 of i.v. dexmedetomidine for treating severe shivering after caesarean delivery were 0.23 μg/kg and 0.39 μg/kg, respectively. Considering that no obvious adverse effects of dexmedetomidine were found in the parturients of Group D5 (0.4 μg/kg of dexmedetomidine) and the incidence of the adverse effects was comparable among groups, we recommended a bolus of the ED95 (0.39 μg/kg) of dexmedetomidine for treating severe shivering after caesarean delivery.
Ethics and Consent Statements
Our research was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants who joined the study, and approval for the study protocol was granted by the relevant institutional review board and ethics committee. We ensured confidentiality and privacy of participant data throughout the study while adhering to all applicable data protection regulations.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
Mei-Juan Yang and Shu-Xi Li contributed equally to this work. The authors thank all the staff of the Department of Anesthesia and Operation Theatre, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, China, for their help with this study. This study was supported by grants from the National Natural Science Foundation of China (81471126, 81870868).
Data Sharing Statement
We are committed to sharing the raw data collected during the clinical trial, including demographic information, clinical measurements, and treatment outcomes. The data can be obtained from the corresponding author ([email protected]) on reasonable request. Access to the data will be granted for research purposes only and is subject to compliance with applicable ethical guidelines and data protection regulations. Prior permission is required for any commercial use or sharing of the data. The data will be released upon publication of the manuscript and will remain accessible for a period of 2 years after publication.
References
- Carpenter L, Baysinger CL. Maintaining perioperative normothermia in the patient undergoing cesarean delivery. Obstet Gynecol Surv. 2012;67(7):436–446. doi:10.1097/OGX.0b013e3182605ccd
- Roy JD, Girard M, Drolet P. Intrathecal meperidine decreases shivering during cesarean delivery under spinal anesthesia. Anesth Analg. 2004;98(1):230–234. doi:10.1213/01.ANE.0000093251.42341.74
- Jun JH, Chung MH, Jun IJ, et al. Efficacy of forced-air warming and warmed intravenous fluid for prevention of hypothermia and shivering during caesarean delivery under spinal anaesthesia: a randomised controlled trial. Eur J Anaesth. 2019;36(6):442–448. doi:10.1097/EJA.0000000000000990
- De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96(2):467–484. doi:10.1097/00000542-200202000-00036
- Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387(10038):2655–2664. doi:10.1016/S0140-6736(15)00981-2
- Crowley LJ, Buggy DJ. Shivering and neuraxial anesthesia. Reg Anesth Pain Med. 2008;33(3):241–252. doi:10.1016/j.rapm.2007.11.006
- Mahmood MA, Zweifler RM. Progress in shivering control. J Neurol Sci. 2007;261(1–2):47–54. doi:10.1016/j.jns.2007.04.038
- Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93(5):1288–1292. doi:10.1097/00000539-200111000-00052
- Ameta N, Jacob M, Hasnain S, Ramesh G. Comparison of prophylactic use of ketamine, tramadol, and dexmedetomidine for prevention of shivering after spinal anesthesia. J Anaesth Clin Pharm. 2018;34(3):352–356. doi:10.4103/joacp.JOACP_211_16
- Kundra TS, Kuthiala G, Shrivastava A, Kaur P. A comparative study on the efficacy of dexmedetomidine and tramadol on post-spinal anesthesia shivering. Saudi J Anaesth. 2017;11(1):2–8. doi:10.4103/1658-354X.197344
- Liu ZX, Xu FY, Liang X, et al. Efficacy of dexmedetomidine on postoperative shivering: a meta-analysis of clinical trials. Can J Anaesth. 2015;62(7):816–829. doi:10.1007/s12630-015-0368-1
- Bautista L, George RB. Dexmedetomidine for every Cesarean delivery...maybe not? Can J Anaesth. 2019;66(7):751–754. doi:10.1007/s12630-019-01355-2
- Sng BL, Dabas R, Sia AT. Intravenous dexmedetomidine use in obstetric anaesthesia: a weapon in our armoury? Int J Obstet Anesth. 2018;36:1–2. doi:10.1016/j.ijoa.2018.09.003
- Yu G, Jin S, Chen J, Yao W, Song X. The effects of novel α2-adrenoreceptor agonist dexmedetomidine on shivering in patients underwent caesarean section. Biosci Rep. 2019;39(2). doi:10.1042/BSR20181847
- Lamontagne C, Lesage S, Villeneuve E, Lidzborski E, Derstenfeld A, Crochetiere C. Intravenous dexmedetomidine for the treatment of shivering during Cesarean delivery under neuraxial anesthesia: a randomized-controlled trial. Can J Anaesth. 2019;66(7):762–771. doi:10.1007/s12630-019-01354-3
- Abdel-Ghaffar HS, Mohamed SA, Fares KM, Osman MA. Safety and efficacy of dexmedetomidine in treating post spinal anesthesia shivering: a randomized clinically controlled dose-finding trial. Pain Physician. 2016;19(4):243–253. doi:10.36076/ppj/2019.19.243
- Crossley AW, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia. 1994;49(3):205–207. doi:10.1111/j.1365-2044.1994.tb03422.x
- Tang Y, Yang M, Fu F, Huang X, Feng Y, Chen X. Comparison of the ED50 of intrathecal hyperbaric ropivacaine co-administered with or without intrathecal dexmedetomidine for cesarean section: a prospective, double-blinded, randomized dose-response trial using up-down sequential allocation method. J Clin Anesth. 2020;62:109725. doi:10.1016/j.jclinane.2020.109725
- Nam JM. A simple approximation for calculating sample sizes for detecting linear trend in proportions. Biometrics. 1987;43(3):701–705. doi:10.2307/2532006
- Sween LK, Xu S, Li C, et al. Low-dose intravenous dexmedetomidine reduces shivering following cesarean delivery: a randomized controlled trial. Int J Obstet Anesth. 2021;45:49–55. doi:10.1016/j.ijoa.2020.11.004
- Liu J, Wang Y, Ma W. Shivering prevention and treatment during cesarean delivery under neuraxial anesthesia: a systematic review. Minerva Anestes. 2018;84(12):1393–1405. doi:10.23736/S0375-9393.18.12478-3
- Kamibayashi T, Maze M, Weiskopf R, Weiskopf R, Todd M. Clinical uses of α2-adrenergic agonists. Anesthesiology. 2000;93(5):1345–1349. doi:10.1097/00000542-200011000-00030
- Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87(4):835–841. doi:10.1097/00000542-199710000-00017
- Paris A, Ohlendorf C, Marquardt M, et al. The effect of meperidine on thermoregulation in mice: involvement of alpha2-adrenoceptors. Anesth Analg. 2005;100(1):102–106. doi:10.1213/01.ANE.0000139355.86522.D1
- Hunter JC, Fontana DJ, Hedley LR, et al. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122(7):1339–1344. doi:10.1038/sj.bjp.0701520
- Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382–394. doi:10.1097/00000542-200008000-00016
- Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78(5):813–820. doi:10.1097/00000542-199305000-00002
- Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77(6):1134–1142. doi:10.1097/00000542-199212000-00014
- Jooste EH, Muhly WT, Ibinson JW, et al. Acute hemodynamic changes after rapid intravenous bolus dosing of dexmedetomidine in pediatric heart transplant patients undergoing routine cardiac catheterization. Anesth Analg. 2010;111(6):1490–1496. doi:10.1213/ANE.0b013e3181f7e2ab
- Hauber JA, Davis PJ, Bendel LP, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg. 2015;121(5):1308–1315. doi:10.1213/ANE.0000000000000931
- Alvarez-Jimenez R, Weerink MAS, Hannivoort LN, et al. dexmedetomidine clearance decreases with increasing drug exposure: implications for current dosing regimens and target-controlled infusion models assuming linear pharmacokinetics. Anesthesiology. 2022;136(2):279–292. doi:10.1097/ALN.0000000000004049
- Colin PJ, Hannivoort LN, Eleveld DJ, et al. Dexmedetomidine pharmacodynamics in healthy volunteers: 2. Haemodynamic profile. Br J Anaesth. 2017;119(2):211–220. doi:10.1093/bja/aex086
- Takata K, Adachi YU, Suzuki K, Obata Y, Sato S, Nishiwaki K. Dexmedetomidine-induced atrioventricular block followed by cardiac arrest during atrial pacing: a case report and review of the literature. J Anesth. 2014;28(1):116–120. doi:10.1007/s00540-013-1676-7
- Wang CY, Chen F, Wu J, et al. The association of the optimal bolus of dexmedetomidine with its favourable haemodynamic outcomes in adult surgical patients under general anaesthesia. Br J Clin Pharmacol. 2020;86(1):85–92. doi:10.1111/bcp.14137
- Potts AL, Anderson BJ, Holford NH, Vu TC, Warman GR. Dexmedetomidine hemodynamics in children after cardiac surgery. Paediatr Anaesth. 2010;20(5):425–433. doi:10.1111/j.1460-9592.2010.03285.x
- Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesth. 2008;21(4):457–461. doi:10.1097/ACO.0b013e328305e3ef
- Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136–1142. doi:10.1046/j.1365-2044.1999.01114.x
- Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–448. doi:10.1213/ANE.0000000000004833
- Jelting Y, Klein C, Harlander T, Eberhart L, Roewer N, Kranke P. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: challenges and solutions. Local Reg Anesth. 2017;10:83–90. doi:10.2147/LRA.S111459
