Abstract
Background
Anemia in patients with cancer who are undergoing active therapy is commonly encountered and may worsen quality of life in these patients. The effect of blood transfusion is often temporary and may be associated with serious adverse events. Erythropoiesis-stimulating agents are not effective in 30%–50% of patients and may have a negative effect on overall survival.
Aims
To assess the efficacy and feasibility of intravenous iron therapy in patients with cancer who have non-iron-deficiency anemia and who are undergoing treatment with chemotherapy without the use of erythropoiesis-stimulating agents.
Methods
Adult patients with solid cancers and non-iron-deficiency anemia were included. Ferric sucrose at a dose of 200 mg was given in short intravenous infusions weekly for a total of 12 weeks. Hemoglobin level was measured at baseline, every 3 weeks, and 2 weeks after the last iron infusion (week 14). Adverse events related to intravenous iron were prospectively reported.
Results
Of 25 patients included, 19 (76.0%) completed at least three iron infusions and 14 (56.0%) finished the planned 12 weeks of therapy. The mean hemoglobin level of the 25 patients at baseline was 9.6 g/dL (median, 9.9 g/dL; range, 6.9 g/dL 10.9 g/dL). The mean change in hemoglobin level for the 15 patients who completed at least 9 treatments was 1.7 g/dL (median, 1.1 g/dL; range, −1.9 g/dL to 3.2 g/dL); it reached 2.1 g/dL (median, 1.3 g/dL; range, −0.2 g/dL to 4.6 g/dL; P = 0.0007) for the 14 patients who completed all 12 weekly treatments. Five (20.0%) patients were transfused and considered as treatment failures. No treatment-related adverse events were reported.
Conclusion
Intravenous iron treatment alone is safe and may reduce blood transfusion requirements and improve hemoglobin level in patients with cancer who are undergoing anticancer therapy. Further randomized studies are needed to confirm these findings.
Introduction
Anemia is a common complication of cancer and its treatment. It occurs in more than 50% of patients and may reach 90% in certain types of cancers, especially in patients undergoing chemotherapy and/or radiation therapy.Citation1
Anemia is defined as an inadequate circulating level of hemoglobin (Hb) (Hb <12 g/dL) and may arise as a result of the underlying disease, bleeding, poor nutrition, chemotherapy, or radiation therapy. Preliminary studies suggest that survival and loco-regional control after radiation therapy, especially in head and neck cancers, may be compromised by anemia.Citation2–Citation4
Anemia often worsens symptoms such as fatigue, weakness, and dyspnea, and thus may have a negative effect on quality of life (QOL) and performance status in patients with cancer. Thus, to improve physical functioning, QOL, and prognosis in patients with cancer, it would be reasonable to take a proactive approach in identifying populations who need treatment for cancer-associated anemia (CAA) and provide timely management.
Blood transfusion is an effective way to replace depleted Hb within a short period, but the effect is, unfortunately, temporary and can cause serious adverse risks and increased mortality. In randomized clinical trials in patients with CAA, erythropoiesis-stimulating agents (ESAs) produced significant increases in Hb level, decreased transfusion requirements, and improved QOL.Citation5–Citation7 However, 30%–50% of patients do not respond to such agents. In addition, the use of ESAs often causes concern about severe adverse reactions.Citation6,Citation8 In several studies, ESAs were found to shorten overall survival time, or time to tumor progression in patients whose Hb level reached more than 12 g/dL. These studies included patients with different primary cancers, such as breast, lung, head and neck, cervix, and lymphomas.Citation9–Citation11 The lack of response to erythropoietin stimulation in patients with cancer is partly attributed to the functional iron deficiency state, in which the high rate of erythropoiesis exceeds the delivery of usable iron, despite adequate iron stores.Citation12 Absolute iron deficiency, in contrast, occurs when iron delivery is impaired because iron stores are depleted (serum ferritin, <100 ng/mL; transferring saturation, <20%).Citation13 Hepcidin, a peptide hormone produced by the liver, is up-regulated in chronic inflammatory states including cancer. Hepcidin inhibits iron transport across cell membranes, thus decreasing the accessibility of stored iron and gastrointestinal absorption of dietary iron, leading to an increased frequency of iron-restricted erythropoiesis.Citation14–Citation16
Many randomized trials examined the role of intravenous (IV) iron in addition to ESAs in the treatment of anemia in patients with cancer. Many of these studies showed improvement in ESA response, time to maximal response, reduction in ESA dose, and improvement in QOL parameters (when measured) in favor of the combination over ESAs alone. The observed benefit was independent of baseline iron parameters.Citation17–Citation21 One study found a 36% reduction in the number of patients transfused.Citation21
This pilot study assessed the efficacy and feasibility of IV iron monotherapy in patients with cancer who have anemia and who are undergoing treatment with chemotherapy and/or radiation therapy without the use of ESAs.
Patients and methods
Study design
Patients received the study treatment for 12 weeks followed by a 4-week follow-up period. Eligible patients were at least 18 years old, about to start a cycle of chemotherapy and/or radiation therapy within 1 week of inclusion, and had a nonmyeloid malignancy, Hb levels of 11.0 g/dL or less, a life expectancy of more than 24 weeks, and an Eastern Cooperative Oncology Group performance status of 0–2. Patients were also required to have a serum ferritin level of 100 ng/mL or higher or transferrin saturation (TSAT) levels of 15% or higher and to have received no ESAs or IV iron therapy within 30 days and no oral iron therapy (27 mg/day or more) within 7 days before enrollment. Patients were excluded for leukoerythroblastic features on blood film, hemolysis, gastrointestinal bleeding, folate or vitamin B12 deficiency, elevated serum ferritin (≥900 ng/mL) or transferrin saturation (TSAT) (≥35%) levels, pregnancy or lactation, liver dysfunction (grade 2 or higher based on National Cancer Institute Common Toxicity Criteria), renal dysfunction (serum creatinine levels ≥2.0 mg/dL), active infection requiring systemic antibiotics, personal or family history of hemochromatosis, comorbidities precluding study participation, hypersensitivity to IV iron, red blood cell transfusion within the last 2 weeks, or any investigational agent within 30 days before enrollment. Patients were not allowed to take any vitamin, mineral, or herbal supplements containing 27 mg or more of iron per day or 100 mg vitamin C per day during the study or follow-up period. Blood transfusions were permitted at the primary physician’s discretion if Hb levels decreased to 8 g/dL or less, and such patients were considered treatment failures. Changes to the chemotherapy plan were permitted. Written informed consent was provided by all patients before study participation, and the protocol and supporting documents were approved by the institutional review board of King Hussein Cancer Center. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as contained in the US Code of Federal Regulations that governs the protection of human subjects and the obligations of clinical investigators.
Treatment
Patients received 200 mg ferric hydroxide sucrose diluted in 100 mL normal saline and infused over the course of 1 hour weekly for a total of 12 weeks. The first dose was given during the first clinic visit (±4 days from the initiation of chemotherapy or radiation therapy). TSAT was monitored, as protocol mandated withholding iron therapy when TSAT levels were higher than 50%.
Assessment
At the first clinic visit (week 1; baseline), a blood sample was obtained for laboratory assessments before the study treatment was started. Patients attended weekly clinic visits for treatment and assessment; and returned for follow-up visits at week 14 which included a complete physical examination. Complete blood count and TSAT were done every 3 weeks, and again 2 weeks after last treatment (week 14). Complete laboratory assessment (Hb, serum ferritin, reticulocyte count, transferrin, TSAT, serum iron, total iron binding capacity, red cell indices, white blood cell count with differential, platelet count, and serum chemistries) were done at week 1 and at week 14 (end of study).
Adverse events were assessed at each clinic visit until study completion or withdrawal, and during the 30 days after the last study treatment.
Statistical analysis
Hb test results were presented as mean, median, and range through all 12 weeks. The same was done to show the changes of Hb level every 3 weeks. Comparison between means of Hb level were made between the baseline Hb and Hb levels in the following weeks, using t-test. A significance criterion of P < 0.05 was used in the analysis. All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC, USA).
Results
Twenty-five patients (17 women and 8 men) were eligible, consented, and included in the study; their mean age (standard deviation, [SD]) was 56 years (13.0 years). All patients were receiving active chemotherapy, and two were also receiving active radiotherapy. Chemotherapy varied according to the primary cancer and included anthracycline, platinum, taxanes, cyclophosphamide, high-dose ifosfamide, vincristine, vinblastine, bleomycin, and others. Many of the included patients had their chemotherapy treatment as second- or third-line therapy. Patients’ characteristics, including age, primary tumor, and active anticancer treatment are summarized in .
Table 1 Characteristics of the 25 patients with cancer who received intravenous iron therapy for non-iron-deficiency anemia
One patient died during the study from his tumor (after week 2), and five patients withdrew from the study because of inconvenience (three after week 3, and two after week 4). Nineteen (76.0%) patients completed a minimum of three treatments, 15 (60.0%) completed nine treatments, and 14 (56.0%) completed all twelve planned weekly treatments.
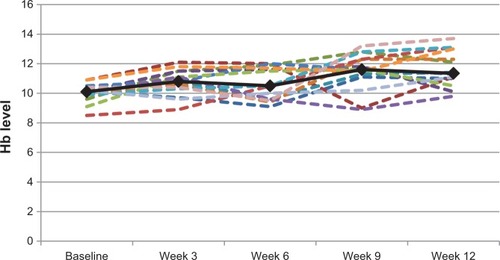
As seen in , the mean Hb level of the 25 patients at baseline was 9.6 g/dL (median, 9.9 g/dL; range, 6.9 g/dL–10.9 g/dL). For the 15 patients who completed at least nine treatments, the mean change in their Hb level was 1.7 g/dL (median, 1.1 g/dL; range, −1.9 g/dL to 3.2 g/dL). For the 14 patients who completed the whole treatment period (12 weeks), the mean Hb level change was 2.1 g/dL (median, 1.3 g/dL; range, −0.2 g/dL to 4.6 g/dL; P = 0.0007). Eight (42.1%) of the 19 patients who completed at least three iron infusions had a more than 1 g/dL increase in their Hb level. Hemoglobin level changes for the 14 patients who completed twelve iron infusions are shown in .
Table 2 Means and medians of hemoglobin levels (g/dL) of the 25 patients with cancer
Figure 1 Hemoglobin (Hb) level changes for the 14 patients who completed 12 iron infusions (dotted lines) and their mean hemoglobin level change (solid line).
No IV iron-related adverse events were reported among patients during the study or the follow-up period.
TSAT was monitored during the study period, and no patients had TSAT levels increase to more than 50%. The highest ferritin level among patients who completed at least nine IV iron treatments was 1,170 ng/mL; the mean level at the end of study period for the whole group was 379 ng/mL.
Five (20.0%) patients received blood transfusions and were considered treatment failures (three after week 3, transfused at Hb levels of 6.9 g/dL, 7.8 g/dL, and 5.4 g/dL; one after week 4, transfused at an Hb level of 8.2 g/dL; and one after week 9, transfused at an Hb level of 7.2 g/dL). The decision for transfusion was made by the primary treating physician.
Discussion
Anemia is extremely common in patients with cancer. Low Hb levels are associated with diminished QOL and possibly decreased overall survival.Citation2 Successful treatment of anemia has undeniable benefits for patients, often yielding dramatic symptomatic improvement. Although the role of ESAs is well-established in treating CAA, big concerns were recently raised about the negative effect of ESAs on survival in some patients with cancer.Citation9–Citation11 Concerns about the risk for thromboembolism in patients with cancer with higher Hb levels who are receiving ESA were also addressed in many trials.Citation22,Citation23 In addition, the possible immunosuppressive effects of blood product transfusions that may have relevance to neoplasia progression were addressed before.Citation24, Citation25
In our pilot study, we tested the feasibility of using iron supplementation alone to treat anemia in patients with cancer who are undergoing chemotherapy without the use of ESAs or blood transfusion, which could be a valid alternative, especially for patients with curable cancers.
Oral iron is easier to administer and relatively inexpensive, but low patient adherence, poor enteral absorption, and poor tolerance because of a wide range of troublesome gastrointestinal adverse effects limit its overall effectiveness.Citation26 Anemia of chronic disease may occur in patients with cancer and is associated with an increase in hepcidin levels, which decreases oral iron absorption and bone marrow iron use, negating any possible effect of regular doses of oral iron.Citation15
IV iron therapy significantly improves response to epoetin alfa when compared with oral iron or no iron in anemic patients with cancer who are receiving chemotherapy.Citation17–Citation21 Oral iron supplements with ESAs showed no significant benefit over ESAs alone in treating CAA.Citation21
Sodium ferric gluconate and iron sucrose appear to have more favorable safety profiles over iron dextran. A large prospective safety comparison trial failed to show serious anaphylactoid reactions,Citation27 which is confirmed in our study, in which no patients developed reactions and no patients withdrew from the study because of adverse effects.
Given that the mean Hb increase using ESAs with IV iron in one large controlled trial was 2.4 g/dL,Citation21 the results obtained in our study are clinically significant. These findings should be further confirmed and better assessed in larger studies, in which questions such as the optimal timing of IV iron therapy with respect to chemotherapy and the optimal total dose of IV iron should be determined.
The use of IV iron monotherapy was recently reviewed by a group in Germany that studied the use of ferric carboxymaltose to replace ESA and blood transfusions as a treatment for CAA. Iron-deficient patients treated with ferric carboxymaltose alone (n = 233) had a median of 1.4 g/dL increase in hemoglobin levels compared with those receiving additional treatment with ESAs (n = 46; median, 1.6 g/dL). Our study, however, is peculiar in using iron therapy in a non-iron-deficiency state.Citation28
Iron overload after IV iron therapy, with potential concerns about the risk of developing secondary cancers and infection, might be raised. The highest serum ferritin level in the present study in patients who completed at least 9 weeks of IV iron therapy was 1,170 ng/mL. Most of the literature addressing cancer and infections in iron-overloaded patients comes from patients with hemochromatosis or patients who are undergoing hemodialysis. Published reviews report an increase in hepatocellular carcinoma only in patients with hemochromatosis after they develop cirrhosis.Citation29 Similarly data supporting the association between IV iron therapy and higher infection rate are weak and not well-supported.Citation30 In fact, anemia itself is a risk factor for infections in patients receiving hemodialysis.Citation31 A multivariate analysis of associations between iron and mortality in more than 58,000 patients receiving hemodialysis reported no increased death rate from serum ferritin levels as high as 1,200 ng/mL.Citation30
The increasing cost of therapy in patients with cancer is of grave concern, which could be an additional benefit of IV iron over the use of ESAs in such patients.
To further address many of the questions raised, our team is planning a bigger trial for IV iron in patients with cancer who have anemia to confirm the results discussed in this pilot trial. In addition, we will be looking into predictors of response to IV iron, such as serum hepcidin level.
Conclusion
IV iron therapy alone is safe and may be effective in improving Hb levels in patients with cancer who are undergoing active anticancer therapy. Further randomized trials are needed to address many of the questions raised in our pilot study.
Disclosure
The authors report no conflicts of interest in this work.
References
- Henry DH Supplemental iron: a key to optimizing the response of cancer-related anemia to rHuEPO? Oncologist 1993 4 275 278
- Knight K Wade S Balducci L Prevalence and outcomes of anemia in cancer: a systematic review of the literature Am J Med 2004 116 Suppl 7A 11S 26S 15050883
- Cella D Kallich J McDermott A Xu X The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials Ann Oncol 2004 15 6 979 986 15151958
- Harrison LB Chadha M Hill RJ Hu K Shasha D Impact of tumor hypoxia and anemia on radiation therapy outcomes Oncologist 2007 6 492 508
- Demetri GD Kris M Wade J Degos L Cella D Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group J Clin Oncol 1998 16 10 3412 3425 9779721
- Gabrilove JL Cleeland CS Livingston RB Sarokhan B Winer E Einhorn LH Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing J Clin Oncol 2001 19 11 2875 2882 11387360
- Littlewood TJ Bajetta E Nortier JW Vercammen E Rapoport B Epoetin Alfa Study Group Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial J Clin Oncol 2001 19 11 2865 2874 11387359
- Glaspy J Bukowski R Steinberg D Taylor C Tchekmedyian S Vadhan-Raj S Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. Procrit Study Group J Clin Oncol 1997 15 3 1218 1234 9060566
- Henke M Laszig R Rübe C Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial Lancet 2003 362 9392 1255 1260 14575968
- Leyland-Jones B BEST Investigators and Study Group Breast cancer trial with erythropoietin terminated unexpectedly Lancet Oncol 2003 4 8 459 460 12901958
- Bohlius J Langensiepen S Schwarzer G Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis J Natl Cancer Inst 2005 97 7 489 498 15812074
- Macdougall IC Strategies for iron supplementation: oral versus intravenous Kidney Int Suppl 1999 69 S61 S66 10084288
- KDOQI KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target Am J Kidney Dis 2007 50 3 471 530 17720528
- Cazzola M Mercuriali F Brugnara C Use of recombinant human erythropoietin outside the setting of uremia Blood 1997 89 12 4248 4267 9192747
- Weiss G Goodnough LT Anemia of chronic disease N Engl J Med 2005 352 10 1011 1023 15758012
- Andrews NC Anemia of inflammation: the cytokine-hepcidin link J Clin Invest 2004 113 9 1251 1253 15124013
- Hedenus M Birgegård G Näsman P Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study Leukemia 2007 21 4 627 632 17252006
- Pedrazzoli P Farris A Del Prete S Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha J Clin Oncol 2008 26 10 1619 1625 18375891
- Bastit L Vandebroek A Altintas S Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia J Clin Oncol 2008 26 10 1611 1618 18375890
- Auerbach M Silberstein PT Webb RT Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia Am J Hematol 2010 85 9 655 663 20661916
- Henry DH Dahl NV Auerbach M Tchekmedyian S Laufman LR Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy Oncologist 2007 12 2 231 242 17296819
- Steensma DP Management of anemia in patients with cancer Curr Oncol Rep 2004 6 4 297 304 15161584
- Wun T Law L Harvey D Sieracki B Scudder SA Ryu JK Increased incidence of symptomatic venous thrombosis in patients with cervical carcinoma treated with concurrent chemotherapy, radiation, and erythropoietin Cancer 2003 98 7 1514 1520 14508840
- Shao W Edelman LS Sullivan DJ Nelson EW Shelby J Long-term cytokine alterations following allogeneic blood transfusion J Investig Med 1998 46 4 161 167
- Santin AD Bellone S Parrish RS Influence of allogeneic blood transfusion on clinical outcome during radiotherapy for cancer of the uterine cervix Gynecol Obstet Invest 2003 56 1 28 34 12867765
- Fishbane S Frei GL Maesaka J Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation Am J Kidney Dis 1995 26 1 41 46 7611266
- Fishbane S Safety in iron management Am J Kidney Dis 2003 41 Suppl 5 18 26 12776310
- Steinmetz T Tschechne B Harlin O Clinical experience with ferric carboxymaltose in the treatment of cancer- and chemotherapy-associated anaemia Ann Oncol 2013 24 2 475 482 23071262
- Kowdley KV Iron, hemochromatosis, and hepatocellular carcinoma Gastroenterology 2004 127 5 Suppl 1 S79 S86 15508107
- Besarab A Frinak S Yee J An indistinct balance: the safety and efficacy of parenteral iron therapy J Am Soc Nephrol 1999 10 9 2029 2043 10477157
- Kalantar-Zadeh K Regidor DL McAllister CJ Michael B Warnock DG Time-dependent associations between iron and mortality in hemodialysis patients J Am Soc Nephrol 2005 16 10 3070 3080 16033854