Abstract
Background
Thoracic paravertebral block (TPVB) analgesia can be prolonged by local anesthetic adjuvants such as dexmedetomidine. This study aimed to evaluate the two administration routes of dexmedetomidine on acute pain and chronic neuropathic pain (NeuP) prevention compared with no dexmedetomidine.
Methods
A total of 216 patients were randomized to receive TPVB using 0.4% ropivacaine alone (R Group), with perineural dexmedetomidine 0.5 μg·kg−1 (RD0.5 Group) or 1.0 μg·kg−1 (RD1.0 Group), or intravenous (IV) dexmedetomidine 0.5 μg·kg−1·h−1 (RDiv Group). The primary outcome was the incidence of chronic NeuP, defined as a Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain score > 12 points at 3-month after surgery.
Results
(1) For the primary outcome, RD0.5 Group and RD1.0 Group demonstrated a decreased incidence of chronic NeuP at 3-month after surgery; (2) Compared with R Group, RDiv Group, RD0.5 Group, and RD1.0 Group can reduce VAS scores at rest and movement and Prince-Henry Pain scores at 12 and 24-h after surgery, the consumption of oral morphine equivalent (OME) and improve QOD-15 at POD1; (3) Compared with RDiv Group, RD0.5 Group and RD1.0 Group can reduce VAS scores at rest and movement and Prince-Henry Pain scores at 12 and 24-h after surgery, the consumption of postoperative OME and improve QOD-15 at POD1; (4) Compared with RD0.5 Group, RD1.0 Group effectively reduced VAS scores at rest at 12 and 24-h after surgery, VAS scores in movement and Prince-Henry Pain scores at 12-h after surgery. However, RD1.0 Group showed an increased incidence of drowsiness.
Conclusion
Perineural or IV dexmedetomidine are similarly effective in reducing acute pain, but only perineural dexmedetomidine reduced chronic NeuP. Moreover, considering postoperative complications such as drowsiness, perineural dexmedetomidine (0.5 μg·kg−1) may be a more appropriate choice.
Clinical Trial Registration
Chinese Clinical Trial Registry (ChiCTR2200058982).
Introduction
Chronic postsurgical pain (CPSP) can sometimes last for a long period after surgery and has been identified as a disabling consequence that seriously impacts patient health and quality of life.Citation1 Its prevalence has been reported to range between 3% and 85%, depending on the type of operation.Citation2 CPSP is one of the more common complications following thoracic surgery, and a meta-analysis reported that chronic pain at 3 and 6-month after thoracic surgery was 57% and 47%, respectively.Citation2–4
CPSP is composed of both nociceptive and neuropathic (also called “neurogenic”) components.Citation5 A significant proportion of CPSP is neuropathic pain (NeuP). NeuP is results from a specific injury or illness that affects the somatosensory system.Citation6,Citation7 Compared with non-neuropathic chronic pain, NeuP is generally more intense.Citation6,Citation8 According to the updated classification of chronic pain in the International Statistical Classification of Diseases and Related Health Problems (ICD)-11, NeuP is first categorized into peripheral and central NeuP.Citation7 The proposal suggests that chronic NeuP can occur after peripheral nerve injury, with exacerbation observed post-surgery.Citation9–11 Previous research has demonstrated that intraoperative nerve impairment during thoracotomy can lead to the development of chronic NeuP.Citation11 Video-assisted thoracoscopic surgery (VATS) minimizes damage to the chest wall during lung resection, thus decreasing the pain associated with conventional thoracotomy. However, patients undergoing VATS may still endure considerable pain in the absence of adequate analgesia, which can be severe and long-lasting.Citation12
CPSP typically starts with acute postoperative pain and quickly transforms into long-term chronic postoperative pain when it is poorly controlled, which may be related to the inflammatory response and peripheral or central sensitization caused by secondary inflammation or injury of nerves.Citation13,Citation14 Preoperative, intraoperative, and postoperative factors can all lead to trigger central sensitization (CS), which can result in hyperalgesia and chronic pain.Citation15 In order to reduce chronic pain, a concept called pre-emptive analgesia has been proposed as perhaps the best timing for regional anesthesia.Citation16 The use of thoracic paravertebral block (TPVB) for thoracic procedures is well accepted, providing effective analgesia and alleviating the inflammatory response as well as the incidence of chronic pain after surgery.Citation17,Citation18
Various analgesic adjuvants have been utilized in combination with local anesthetics to extend the duration of their effects, with varying degrees of success. Dexmedetomidine is a highly selective drug α2-adrenergic receptor agonist that can prolong analgesia in a variety of routes, including neuraxial, perineural, and intravenous (IV) administration.Citation19 Among these routes, perineural dexmedetomidine was found to significantly prolong the duration of analgesia after single-injection peripheral nerve blocks (PNBs) by inhibiting the activation of Ih current (hyperpolarizing cation current).Citation20 Compared with perineural dexmedetomidine, IV dexmedetomidine can also prolong analgesia; however, to date, the results are inconsistent.Citation20,Citation21
We hypothesized that perineural dexmedetomidine in TPVB and IV dexmedetomidine would be helpful to decrease both acute pain and chronic NeuP at 3-month after surgery, compared to TPVB without dexmedetomidine.
Methods
Trial Design
This single-center, randomized controlled study was prospectively registered with the Chinese Clinical Trial Registry (https://www.chictr.org.cn, ChiCTR2200058982; principal investigator: Yanwu Jin; date of registration: April 21, 2022). This study was approved by the institutional ethics committee of the Second Hospital of Shandong University (No: KYLL-2022P254) and written informed consent was obtained from all patients before enrollment. The guidelines outlined in the Declaration of Helsinki were followed and this manuscript adhered to the applicable CONSORT guidelines.
Patient Recruitment
Between May 6, 2022, and February 4, 2023, a total of 224 adult patients who were undergoing thoracoscopic resection of lung lesions under general anesthesia (GA) were recruited at the second hospital of Shandong University, a tertiary care academic health sciences center fully affiliated with Shandong University.
Inclusion criteria were: (1) American Society of Anesthesiologists (ASA) physical status I–III; (2) Age 18–65 years, no limitation to gender; (3) Patients scheduled to undergo thoracoscopic unilateral pulmonary lesion resection (cuneiform/segmental/lobectomy); (4) Consenting to study participation;
Exclusion criteria were as follows: (1) Patient refusal; (2) Existing contraindications to TPVB such as coagulopathy and local and systemic infection. Coagulopathy was defined as a prothrombin time or activated partial prothrombin time that exceeded standard values or an international normalized ratio (INR) ≥ 1.4, or a platelet count < 80×109 L−1; (3) BMI > 28 kg/m2 or < 18 kg/m2; (4) Allergic to experimental drugs or general anesthetics; (5) Liver and kidney insufficiency; (6) History of chronic pain or opioid use; (7) History of surgery within the last 6 months; (8) Mental or nervous system diseases and language problems; (9) Pregnant or lactating patients; (10) Sick sinus syndrome (SSS), severe bradycardia (HR < 50/min) or atrioventricular block (AVB) II or above.
Randomization and Blinding
Patients were randomly assigned to one of the four groups (1:1:1:1) using a computer-generated randomization code and the sealed opaque envelope method. The assignment was carried out by a clinician who was not involved in the study. On the day of the surgery, the anesthesiologist (CLG) opened the opaque envelopes and each patient received the corresponding protocol. Prior to the surgery, TPVB was performed by the anesthesiologist (HZ) in the pre-anesthesia room, and GA was administered by another anesthesiologist (LZ) in the operating room. Postoperative follow-up was conducted by two certified nurses and an anesthesiologist (YCZ) who were also blinded to the patient group assignments. The TPVB injection mixture was identical in appearance and was prepared using an aseptic technique by one investigator (SSZ) who was informed of the group assignments but had no further involvement in patient care or data collection. Meanwhile, saline or dexmedetomidine infusion prepared by the investigator (SSZ) were all labeled “Intravenous Medication”, to which the anesthesiologist (LZ) was blinded. In this way, the anesthesiologist and the surgical team were all blinded to group allocation.
Assigned Interventions
Patients providing informed consent who met all eligibility criteria were randomized (1:1:1:1) into the following four groups: R Group, RDiv Group, RD0.5 Group, and RD1.0 Group.
R Group: TPVB injection mixture [ropivacaine 1% + 0.9% saline]; IV infusion 0.9% saline;
RDiv Group: TPVB injection mixture [ropivacaine 1% + 0.9% saline]; IV infusion 0.5 μg·kg−1·h−1 dexmedetomidine;
RD0.5 Group: TPVB injection mixture [ropivacaine 1% + dexmedetomidine 0.5 μg·kg−1 + 0.9% saline]; IV infusion 0.9% saline;
RD1.0 Group: TPVB injection mixture [ropivacaine 1% + dexmedetomidine 1.0 μg·kg−1 + 0.9% saline]; IV infusion 0.9% saline;
The dosing regimen was individualized based on the patient’s weight. The final ropivacaine concentration was 0.4%, and the total volume of TPVB injection in each group was calculated according to the weight of the patient (0.4% ropivacaine 0.3 mL·kg−1). To facilitate the comparison of the IV infusion of each group, dexmedetomidine IV infusion was administered at a concentration of 4 μg·mL−1 and a rate of 0.125 mL·kg−1·h−1 (0.5 μg·kg−1·h−1) in RDiv Group, and the remaining three groups were given 0.9% saline at a rate of 0.125 mL·kg−1·h−1 for 1 hour. Furthermore, to account for dead space in the block needle tubing in each group, 2 mL of 0.9% saline was used before TPVB medications were administered at the paravertebral spaces in each group.
Presurgical and Surgical Procedures
At 1-h before surgery, subjects were admitted to the pre-anesthesia room. After establishing IV access and successful local anesthesia, radial artery puncture and catheterization were performed. Standard monitors (electrocardiogram, invasive arterial pressure, and continuous oxygen saturation) were applied and supplemental oxygen was delivered via nasal cannula. The patients were placed in a standard lateral position for TPVB before induction of anesthesia. Before performing the nerve block, all patients received IV midazolam 0.02 mg·kg−1 or sufentanil 0.08 μg·kg−1 for anxiolysis and analgesia, as needed. Under complete aseptic measures, a 5–2 MHz low-frequency probe (C5-1B convex transducer, Wisonic Navi, Shenzhen, China) was placed in the oblique parasagittal plane to distinguish the intended transverse process.
The highlighted pleura and superior costotransverse ligament were identified, and a 21-G×100 mm short bevel non-echogenic nerve block needle (PAJUNK Gmbh Medizintechnologie, Geisingen, Germany) was carefully advanced using an in-plane technique, moving from a lateral to medial direction. The needle was slowly inserted until its tip punctured the superior costotransverse ligament and entered the paravertebral space. The position of the needle tip was confirmed by hydrodissection under direct ultrasound visualization via inject a small volume of saline (1mL). Following the verification of the anatomical location and absence of blood aspiration, medications for TPVB were administered at the paravertebral spaces between the fifth and sixth thoracic vertebrae. Ultrasound subsequently confirmed the presence of a faint echogenic shadow outside the pleura, accompanied by downward displacement of the pleura resulting from the administered mixtures. TPVB injection mixtures were identical in appearance and prepared by an investigator (SSZ) using an aseptic technique. In order to allow enough time for the TPVB to take effect, the sensory block level was tested 30 min before anesthesia induction, and patients with primary block failure were excluded (block level < T3~T8). The performance time of TPVB was defined as the sum of imaging and needling times.Citation22 US-guided TPVB was performed by the same experienced anesthesiologist (HZ) who was blinded to group allocation.
Intraoperative Management
Once in the operating room, all patients were continuously monitored for oxygen saturation, electrocardiogram, heart rate, non-invasive blood pressure, invasive blood pressure, temperature, and entropy using multichannel monitors (Datex-Ohmeda S/5 Avance). GA was induced with IV midazolam (0.02 mg·kg−1) for anxiolysis, IV sufentanil (0.4 μg·kg−1), IV propofol (1.5–2.0 mg·kg−1) and the neuromuscular block was achieved with cisatracurium (0.2 mg·kg−1). After induction, the trachea was intubated and mechanical ventilation was initiated. Intraoperatively, anesthesia was maintained by 1%~3% sevoflurane, propofol (3–10 mg·kg−1·h−1), and remifentanil (0.1–0.3 μg·kg−1·h−1) to maintain a suitable depth of anesthesia (Entropy index, EI 40–60). IV Sufentanil boluses (5 µg) were administered every 30 min as needed to maintain invasive blood pressure and heart rate within 10% of the patient’s baseline upon arrival at the hospital. Bradycardia (HR < 40 beats·min−1) was treated with atropine 0.6 mg IV. Muscle relaxation was reversed using glycopyrrolate (0.3 mg·kg−1) and neostigmine (0.04 mg·kg−1). About 30 min before emergence from anesthesia, all patients were administered ketorolac 30 mg IV to prevent acute pain and standard anti-emetic prophylaxis with the serotonin antagonist ondansetron 8 mg IV.
Surgery
The same surgical team performed unilateral thoracoscopic pulmonary lesion resection (cuneiform/segmental/lobectomy) on all subjects, who were placed in the lateral recumbent position. One or two ports were set up at different intercostal space levels according to the location of the tumor and lung nodule. All procedures were performed under minimally invasive thoracoscopic techniques, and none used rib expansion or any of the mechanical retractors used in conventional thoracotomy. At the end of surgery, a chest tube drain was placed at the surgical incision site. No postoperative administration of local anesthetic infiltration was performed on the wound.
Postoperative Management
All patients were admitted to the Thoracic Surgery Postoperative Observational Unit, which consists of a post-anesthesia care unit (PACU) and a level two surgical step-down unit. They were observed for the initial 48 hours following surgery before being transferred to a regular hospital floor.
The postoperative analgesic regimen consisted of routine intravenous administration of 50 mg of flurbiprofen every 12 h and patient-controlled intravenous analgesia (PCIA). The PCIA pump was set up with sufentanil 2 μg·kg−1 + ondansetron 8 mg + 0.9% saline = 100 mL; 2 mL·h−1 as basal infusion, control single dose 0.5 mL, lock-on time 15 min. The PCIA pump was started immediately after the operation upon arrival in the Thoracic Surgery Postoperative Observational Unit.
Postoperative pain in the PACU was defined as a visual analog scale (VAS; 10 cm-scale where 0 = no pain and 10 = worst pain) pain severity score > 4 after two boluses of PCIA and was treated with rescue analgesia as needed [oral combination acetaminophen (325 mg) oxycodone (5 mg) tablet or intramuscular pethidine 50 mg]. The rescue antiemetic ondansetron 0.1 mg·kg−1 was given intravenously if patients showed nausea or vomiting.
Patients were discharged at the discretion of the surgical team and in accordance with standard discharge guidelines. After patient recruitment, the nursing staff trained the patients and their families on how to conduct LANSS assessments. The LANSS pain scores were collected through a combination of telephone interviews and outpatient follow-ups at two time points, specifically 3-month and 6-month after surgery. The patients were followed up until 6-month after surgery.
Outcomes
The primary outcome was the incidence of chronic NeuP defined as a LANSS score > 12 points at 3-month after surgery.Citation23,Citation24
Secondary outcomes included: (1) LANSS score at 6-month after surgery; (2) Prince-Henry Pain Score at 1, 12, 24, 48-h after surgery; (3) VAS pain scores at rest and during movement at 1, 3, 6, 12, 24 and 48-h after surgery; (4) Quality of Recovery-15 (QoR-15) with a maximum score of 150 points at preoperative,Citation25 postoperative day 1 (POD1) and day 2 (POD2); (5) Intraoperative and postoperative oral morphine equivalent (OME);Citation26,Citation27 (6) Number of rescue analgesia and PCIA attempts; (7) Incidence of adverse effects at 24-h after surgery.
The additional indicators were recorded (eg concentrations of sevoflurane, number and location of thoracoscopic incisions, surgical, anesthesia, hospital, and postoperative chest tube drainage time). Surgical incisions, either single or double port, were performed at distinct intercostal space levels and denoted by “T”. Furthermore, the pre-operative Hospital Anxiety and Depression Scale score was recorded. Sleep quality in the last month was assessed by the Pittsburgh Sleep Quality Index before surgery and the adverse effects were monitored 24 hours following surgery (eg, nausea; vomiting; and drowsiness).Citation28,Citation29
Sample Size Calculation
The Power Exploration and Sample Size (PASS) 15.0 program (NCSS, LLC., Kaysville, UT, United States) was employed to calculate the sample size of this research. A LANSS score > 12 points was considered neuropathic, causing the patient’s pain.Citation23 According to our pilot study of 45 patients, the incidence of LANSS score exceeding 12 points at 3-month after surgery in R Group, RDiv Group, RD0.5 Group, and RD1.0 Group were 46.7%, 26.7%, 20%, and 13.3%, respectively. Assuming a two-tailed α error = 0.05 and β error = 0.1 with a power of 0.90, the sample size required per group was at least 45 participants. Accounting for a dropout rate of 20% to accommodate for incomplete data or loss to follow-up and increasing the sample scale, the research finally included 56 patients in every group.
Statistical Analysis
The SPSS for Windows statistical package (version 19.0; SPSS Inc., IBM, Chicago, IL, USA) was used in our calculations. The normality of continuous variables, expressed as mean (standard deviation, SD) or median (interquartile range, IQR), was assessed using the Kolmogorov–Smirnov test. A one-way ANOVA was used for data conforming to a normal distribution. For cardinal variables with non-normal distribution, differences were assessed using the Kruskal–Wallis H-test. Categorical variables were presented as numbers (n/%) and assessed using χ2 or Fisher’s exact test where appropriate. The threshold of statistical significance of the two-tailed P value for the one-way ANOVA and χ2 test comparison among groups was set at 0.0083 according to the Bonferroni correction. A repeated-measures analysis of variance was used to test the difference in continuous variables over time (eg, VAS score, Prince-Henry Pain, and QoR-15). For repeated outcome measurements, the P values were corrected using the Bonferroni–Holm step-down adjustment.
Results
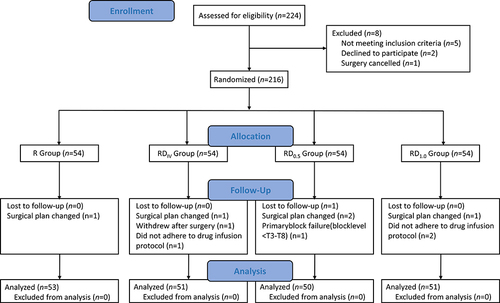
The study recruited 224 patients at this tertiary hospital who were scheduled for thoracoscopic unilateral pulmonary lesion resection under general anesthesia between May 2022 and February 2023. Eight patients were excluded, including 5 who did not meet inclusion criteria, 2 who declined, and 1 who had their surgery canceled. A total of 216 patients were included in the study and then randomized into the 4 groups; among them, 205 patients completed the study, as shown in the Consolidated Standards of Reporting Trials (CONSORT) flowchart (). No significant differences in demographic and operative data were observed among the four groups ().
Table 1 Patient Demographic and Operative Characteristics
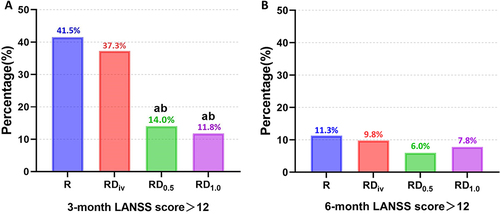
The follow-up of the chronic NeuP incidence is shown in . At 3-month after surgery, RD0.5 Group and RD1.0 Group showed a significantly lower incidence of LANSS pain score >12 in comparison to R Group and RDiV Group (). However, no significant difference was found between groups at 6-month after surgery ().
Figure 2 Incidence of LANSS pain score >12. (A) 3-month after surgery; (B) 6-month after surgery.
From 12 to 24h postoperatively, the VAS scores at rest and movement and Prince-Henry Pain scores in RDiV Group, RD0.5 Group, and RD1.0 Group were significantly lower compared with those in R Group (). Compared with RDiv Group, RD0.5 Group and RD1.0 Group can reduce VAS scores at rest and movement and Prince-Henry Pain scores at 12 and 24-h after surgery (). Compared with RD0.5 Group, RD1.0 Group effectively reduced VAS scores at rest at 12 and 24-h after surgery, VAS scores in movement and Prince-Henry Pain scores at 12-h after surgery. No differences were detected between groups at the other time points.
Table 2 Outcomes Between Groups
At POD1, the QoR-15 score in RDiV Group, RD0.5 Group, and RD1.0 Group were significantly higher compared with those in R Group. Compared with RDiV Group, the QoR-15 in RD0.5 Group and RD1.0 Group both demonstrated higher scores. However, no significant difference in QoR-15 was observed between groups preoperatively and at POD2 (). The intraoperative OME and postoperative OME within the 0–24 hour period, as well as the overall postoperative OME, exhibited significantly lower levels in RDiV Group, RD0.5 Group, and RD1.0 Group compared to R Group (). Furthermore, the postoperative OME in RD0.5 Group and RD1.0 Group were lower compared with RDiV Group from 0 to 24-h and totally postoperatively (). The number of PCIA attempts, rescue analgesia received, and anti-emetics required were similar among the four groups (). In addition, the overall cumulative incidence of drowsiness was highest in RD1.0 Group, while no significant difference was observed in other postoperative adverse reactions at 24-h after surgery ().
Table 3 The Incidence of Adverse Effects at 24 h After Surgery
Discussion
This randomized study was designed to evaluate the two administration routes of dexmedetomidine on acute pain and chronic NeuP prevention compared with no dexmedetomidine. This trial is the first to compare the incidence of chronic NeuP between the two routes of dexmedetomidine administration in TPVB in patients undergoing thoracic surgery. In addition, two concentrations of dexmedetomidine (0.5 ug·kg−1 and 1.0 ug·kg−1) were compared for the perineural administration route. Both perineural and IV dexmedetomidine groups effectively reduced acute pain, resulting in lower perioperative analgesic drug consumption and prolonged duration of analgesia compared with patients receiving ropivacaine alone. Nonetheless, only perineural dexmedetomidine reduced the incidence of chronic NeuP at 3-month after surgery.
According to the recently updated criteria of CPSP, it is defined as either a continuation of acute postoperative pain or pain that occurs after an asymptomatic period, lasting for at least 3 to 6 months duration and significantly affecting the health-related quality of life.Citation30 A large proportion of CPSPs can be attributed to NeuP, which can be evaluate by LANSS score, as intraoperative intercostal nerve neuropraxia is the most common cause for CPSP following such procedures.Citation31,Citation32 In the newly released ICD-11 classification, NeuP was defined as “pain initiated or caused by a primary lesion, dysfunction, or transitory perturbation of the peripheral or central nervous system”.Citation7 An important classification of NeuP is NeuP after peripheral nerve injury.Citation7 Surgery, especially thoracic surgery, increases the risk of NeuP, which may involve different degrees of nerve injury, such as transection, stretching, and compression.Citation11,Citation33–36 However, the precise mechanism underlying its occurrence remains elusive and likely involves a complex interplay between peripheral and central nervous system alterations.Citation37,Citation38
Our results suggested that chronic NeuP at 3 and 6-month after thoracic surgery were 41.5% and 11.3% with those receiving ropivacaine alone, respectively, which is compatible with that of previous studies.Citation24 At 3-month after surgery, perineural dexmedetomidine showed a significantly lower incidence of LANSS pain score > 12 in comparison to IV dexmedetomidine. This finding indicates that perineural administration of dexmedetomidine exhibits a certain degree of central and peripheral neuroprotective effects and contributes to the reduction in the incidence of chronic NeuP compared to IV administration.
Prolonged sensory block may provide longer analgesia and avoidance of premature return to moderate to severe pain. A recent meta-analysis revealed that perineural dexmedetomidine improves the postoperative pain severity of patients and prolongs the duration of analgesia in TPVB.Citation39 IV dexmedetomidine can also have the same effect as perineural dexmedetomidine in some studies.Citation19,Citation21 This study revealed that compared with ropivacaine alone, perineural or IV dexmedetomidine can reduce VAS scores at rest and movement and Prince-Henry Pain scores at 12 and 24-h after surgery, and reduce the consumption of intraoperative and postoperative OME, which was consistent with previous studies.Citation40 QoR-15 is a validated quality assessment tool, and the results demonstrated that perineural and IV dexmedetomidine can improve the QOD-15 score at POD1 compared with ropivacaine alone, which has achieved minimum clinically important difference (MCID) (6.0).Citation41
Another important result of our study was the effect of perineural dexmedetomidine compared to IV dexmedetomidine. Most importantly, perineural dexmedetomidine was found to reduce chronic NeuP at 3-month after surgery. In addition, compared with IV dexmedetomidine, perineural dexmedetomidine can reduce VAS scores at rest and movement and Prince-Henry Pain scores at 12 and 24-h after surgery, the consumption of postoperative OME and improve QOD-15 at POD1, which is in accordance with previous studies.Citation42 These findings suggest that compared with IV dexmedetomidine, perineural dexmedetomidine has analgesic advantages and can effectively reduce the incidence of acute pain and chronic NeuP.
The mechanism by which dexmedetomidine, as an adjuvant to local anesthetics, enhances the effect of analgesic is considered multifactorial and remains controversial. It was previously summarized in our review.Citation43 (1) Peripheral level: perineural dexmedetomidine can maintain the hyperpolarized state of cells by inhibiting the activation of Ih current (a non-inactivating, inwardly rectifying current carried by both Na+and K+ ions, which is also called the pacemaker current because it is believed to play a significant role in cell excitability) and activates α2-adrenoceptor in peripheral blood vessels, constricting blood vessels around the injection site;Citation20,Citation44 (2) Spinal cord level: dexmedetomidine inhibits the release of excitatory neurotransmitters such as glutamate and substance P in the spinal;Citation45 (3) Supraspinal level: dexmedetomidine may have a certain amount of systemic absorption and inhibit the descending noradrenergic pathway in the medulla or reduce sympathetic nerve signals, thereby partly achieving its analgesic effect from the central level.Citation46 Due to this systemic absorption, perineural dexmedetomidine may have similar effects as IV administration.
It is well-known that the contents of the thoracic paravertebral space include the intercostal neurovascular bundle.Citation47 TPVB is a technique used to block the conduction of somatosensory and motor nerves by injecting local anesthetics into the spinal nerve in the paravertebral space. The analgesic effects of perineural dexmedetomidine may be attributed to a combination of perineural effects as well as varying degrees of systemic absorption.Citation21
Another important finding of our study was the effect of perineural dexmedetomidine 0.5 μg·kg−1 compared to 1.0 μg·kg−1. Our study revealed that compared with 0.5 μg·kg−1, 1.0 μg·kg−1 perineural dexmedetomidine could reduce VAS scores at rest at 12 and 24-h, VAS score in movement, and Prince-Henry Pain score at 12-h after surgery. Additionally, it is noteworthy that despite the presence of some intergroup disparities in postoperative acute pain, the Bonferroni correction P-values indicate that these variances are not clinically relevant. Consequently, increasing the dose of perineural dexmedetomidine to 1.0 μg·kg−1 is not necessary. Furthermore, 1.0 μg·kg−1 perineural dexmedetomidine could increase the incidence of drowsiness compared to 0.5 μg·kg−1. Two previous meta-analyses found that perineural dexmedetomidine was associated with a significant increase in postoperative sedation within 24 hours, which may contribute to an increase in drowsiness, which was consistent with our study.Citation48,Citation49 1.0 μg·kg−1 perineural dexmedetomidine can be rapidly absorbed into the blood, which may be related to the rapid absorption of the drug due to the abundant venous plexus in paravertebral space, thereby leading to drowsiness or excessive sedation. Many studies have shown that the maximum safe dose of dexmedetomidine is 2 μg·kg−1, but we should be aware of the risk of intraoperative transient bradycardia and hypotension.Citation50 According to our result, a dose of dexmedetomidine of 0.5 μg·kg−1 appears to provide an optimal balance between adequate postoperative analgesia and the adverse effects of TPVB in thoracic surgery.
Limitations
Nevertheless, the limitations of the present study should be acknowledged. Although this is a prospective randomized controlled study, it is a single-center trial with a relatively small sample size, which inevitably lacks external validity. In addition, several outcomes were subjective, such as the LANSS score and VAS score, which may have introduced variability and bias. In order to minimize these effects, the investigators provided the patients with a thorough preoperative explanation of the evaluation protocol after recruitment. Thirdly, the incidence of CPSP was not assessed in the long-term follow-up of patients undergoing thoracic surgery. While NeuP is a frequent component of CPSP after VATS and a marker of severity, some patients with CPSP after VATS do not have NeuP. Therefore, although we found that perineural dexmedetomidine decreased the incidence of NeuP at 3-month after surgery, its impact on the overall incidence of CPSP is unknown. Furthermore, despite setting an IV dexmedetomidine group and two groups of perineural dexmedetomidine, the optimal dose of dexmedetomidine for IV and perineural administration requires further study.
Conclusion
In conclusion, our results suggest that perineural and IV dexmedetomidine are both effective in reducing acute pain and the consumption of analgesics. However, only perineural dexmedetomidine can reduce chronic NeuP in the context of TPVB in patients undergoing thoracoscopic resection of lung lesions under GA. Moreover, considering postoperative complications such as drowsiness, 0.5 μg·kg−1 perineural dexmedetomidine may be a more appropriate choice.
Ethics Approval and Consent to Participate
The study was conducted with Institutional Review Board approval from the Second Hospital of Shandong University in China (No: KYLL-2022P254), and the guidelines outlined in the Declaration of Helsinki were followed. Written informed consent was obtained from all study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors thank all the research assistants and patients for their time and efforts in this prospective study. The authors also thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77–86. doi:10.1093/bja/aen099
- Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi:10.1016/s0140-6736(19)30352-6
- Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014;15(9):887–897. doi:10.1016/j.jpain.2014.06.005
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/s0140-6736(06)68700-x
- Harden RN. Chronic Neuropathic Pain: mechanisms. Diagnosis Treatment. 2005;11(2):111–122. doi:10.1097/01.nrl.0000155180.60057.8e
- Bouhassira D. Neuropathic pain: definition, assessment and epidemiology. Revue neurologique. 2019;175(1–2):16–25. doi:10.1016/j.neurol.2018.09.016
- Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59. doi:10.1097/j.pain.0000000000001365
- Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
- Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(1):95–102. doi:10.1016/j.pain.2012.09.010
- Høimyr H, Rokkones KA, von Sperling ML, Finnerup K, Jensen TS, Finnerup NB. Persistent pain after lymph node excision in patients with malignant melanoma is neuropathic. Pain. 2011;152(12):2721–2728. doi:10.1016/j.pain.2011.07.009
- Benedetti F, Vighetti S, Ricco C, et al. Neurophysiologic assessment of nerve impairment in posterolateral and muscle-sparing thoracotomy. J Thoracic Cardiovasc Surg. 1998;115(4):841–847. doi:10.1016/s0022-5223(98)70365-4
- Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Werner MU, Kehlet H. Quantitative sensory testing of persistent pain after video-assisted thoracic surgery lobectomy. Br J Anaesth. 2012;108(1):126–133. doi:10.1093/bja/aer325
- Clarke H, Poon M, Weinrib A, Katznelson R, Wentlandt K, Katz J. Preventive analgesia and novel strategies for the prevention of chronic post-surgical pain. Drugs. 2015;75(4):339–351. doi:10.1007/s40265-015-0365-2
- Droog W, Walbeehm ET, Konijn JB, et al. A Systematic Review on Long-Term Postsurgical Pain Outcomes; What Is the Effect of Upper Extremity Regional Anesthesia? Anesthesia Analg. 2023. doi:10.1213/ane.0000000000006529
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi:10.1126/science.288.5472.1765
- Katz J, Clarke H, Seltzer Z. Review article: preventive analgesia: quo vadimus? Anesthesia Analg. 2011;113(5):1242–1253. doi:10.1213/ANE.0b013e31822c9a59
- Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013;111(5):711–720. doi:10.1093/bja/aet213
- Lönnqvist PA. Pre-emptive analgesia with thoracic paravertebral blockade? Br J Anaesth. 2005;95(6):727–728. doi:10.1093/bja/aei268
- Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesthesia Analg. 2013;117(1):271–278. doi:10.1213/ANE.0b013e318290c566
- Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115(4):836–843. doi:10.1097/ALN.0b013e318221fcc9
- Abdallah FW, Dwyer T, Chan VW, et al. IV and Perineural Dexmedetomidine Similarly Prolong the Duration of Analgesia after Interscalene Brachial Plexus Block: a Randomized, Three-arm, Triple-masked, Placebo-controlled Trial. Anesthesiology. 2016;124(3):683–695. doi:10.1097/aln.0000000000000983
- Bravo D, Aliste J, Layera S, et al. A multicenter, randomized comparison between 2, 5, and 8 mg of perineural dexamethasone for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. 2019;44(1):46–51. doi:10.1136/rapm-2018-000032
- Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92(1–2):147–157. doi:10.1016/s0304-3959(00)00482-6
- Abd-Elshafy SK, Abdallal F, Kamel EZ, et al. Paravertebral Dexmedetomidine in Video-Assisted Thoracic Surgeries for Acute and Chronic Pain Prevention. Pain Physician. 2019;22(3):271–280.
- Zubrzycki M, Liebold A, Skrabal C, et al. Assessment and pathophysiology of pain in cardiac surgery. J Pain Res. 2018;11:1599–1611. doi:10.2147/JPR.S162067
- Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118(6):1332–1340. doi:10.1097/ALN.0b013e318289b84b
- Li YW, Li HJ, Li HJ, et al. Delirium in Older Patients after Combined Epidural-General Anesthesia or General Anesthesia for Major Surgery: a Randomized Trial. Anesthesiology. 2021;135(2):218–232. doi:10.1097/aln.0000000000003834
- Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733–737. doi:10.1002/pds.3945
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
- Wu Y, Levis B, Sun Y, et al. Accuracy of the Hospital Anxiety and Depression Scale Depression subscale (Hads-D) to screen for major depression: systematic review and individual participant data meta-analysis. BMJ. 2021;373:n972. doi:10.1136/bmj.n972
- Werner MU, Kongsgaard UE. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113(1):1–4. doi:10.1093/bja/aeu012
- Richardson J, Sabanathan S. Pain management in video assisted thoracic surgery: evaluation of localised partial rib resection. A new technique. J Cardiovascular Surgery. 1995;36(5):505–509.
- Sharma S, Higgins C, Cameron P, et al. Validation of the Nepali Version of the Self-reported Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) in Adults With Chronic Pain and Predominantly Low-literacy Levels. J Pain. 2022;23(3):424–433. doi:10.1016/j.jpain.2021.09.004
- Andersen KG, Aasvang EK, Kroman N, Kehlet H. Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta Anaesthesiologica Scandinavica. 2014;58(10):1240–1248. doi:10.1111/aas.12393
- Petersen PL, Bredahl P, Perch M, Møller CH, Finnerup NB, Nikolajsen L. Chronic pain after bilateral thoracotomy in lung transplant patients. Scandinavian j Pain. 2019;19(2):271–277. doi:10.1515/sjpain-2018-0126
- Buchheit T, Pyati S. Prevention of chronic pain after surgical nerve injury: amputation and thoracotomy. Surgical Clinics North Am. 2012;92(2):393–407, x. doi:10.1016/j.suc.2012.01.005
- Reddi D. Preventing chronic postoperative pain. Anaesthesia. 2016;71 Suppl 1:64–71. doi:10.1111/anae.13306
- Themistocleous AC, Crombez G, Baskozos G, Bennett DL. Using stratified medicine to understand, diagnose, and treat neuropathic pain. Pain. 2018;159 Suppl 1(Suppl 1):S31–s42. doi:10.1097/j.pain.0000000000001301
- Goswami R, Anastakis DJ, Katz J, Davis KD. A longitudinal study of pain, personality, and brain plasticity following peripheral nerve injury. Pain. 2016;157(3):729–739. doi:10.1097/j.pain.0000000000000430
- Tang R, Liu YQ, Zhong HL, et al. Evidence basis for using dexmedetomidine to enhance the quality of paravertebral block: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13:952441. doi:10.3389/fphar.2022.952441
- Ding W, Chen Y, Li D, et al. Investigation of single-dose thoracic paravertebral analgesia for postoperative pain control after thoracoscopic lobectomy - A randomized controlled trial. Int j Surgery. 2018;57:8–14. doi:10.1016/j.ijsu.2018.07.006
- Myles PS, Myles DB. An Updated Minimal Clinically Important Difference for the QoR-15 Scale. Anesthesiology. 2021;135(5):934–935. doi:10.1097/aln.0000000000003977
- Abo-Zeid MA, Bakrey SA, Elbadrawy RE. Intravenous versus perineural dexmedetomidine as adjuvant in adductor canal block for total knee arthroplasty. Korean j Anesthesiol. 2023. doi:10.4097/kja.22579
- Chen Z, Liu Z, Feng C, Jin Y, Zhao X. Dexmedetomidine as an Adjuvant in Peripheral Nerve Block. Drug Des Devel Ther. 2023;17:1463–1484. doi:10.2147/dddt.S405294
- Shirasaka T, Kannan H, Takasaki M. Activation of a G protein-coupled inwardly rectifying K+ current and suppression of Ih contribute to dexmedetomidine-induced inhibition of rat hypothalamic paraventricular nucleus neurons. Anesthesiology. 2007;107(4):605–615. doi:10.1097/01.anes.0000281916.65365.4e
- Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur j Neurosci. 2008;27(12):3182–3190. doi:10.1111/j.1460-9568.2008.06260.x
- El Sherif FA, Abdel-Ghaffar H, Othman A, et al. Pharmacokinetics and Pharmacodynamics of Dexmedetomidine Administered as an Adjunct to Bupivacaine for Transversus Abdominis Plane Block in Patients Undergoing Lower Abdominal Cancer Surgery. J Pain Res. 2022;15:1–12. doi:10.2147/jpr.S335806
- Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med. 2014;39(4):289–298. doi:10.1097/aap.0000000000000113
- El-Boghdadly K, Brull R, Sehmbi H, Abdallah FW. Perineural Dexmedetomidine Is More Effective Than Clonidine When Added to Local Anesthetic for Supraclavicular Brachial Plexus Block: a Systematic Review and Meta-analysis. Anesthesia Analg. 2017;124(6):2008–2020. doi:10.1213/ane.0000000000002014
- Kirksey MA, Haskins SC, Cheng J, Liu SS. Local Anesthetic Peripheral Nerve Block Adjuvants for Prolongation of Analgesia: a Systematic Qualitative Review. PLoS One. 2015;10(9):e0137312. doi:10.1371/journal.pone.0137312
- Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–181.