 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
We evaluated the higher levels of carcinoembryonic antigen (CEA) secreted by the LoVo human colon carcinoma cells in a medium containing anticancer drugs. Drug-resistant LoVo cells were analyzed by subcutaneously xenotransplanting them into mice. The aim of this study was to evaluate whether the drug-resistant cells isolated in this study were cancer-initiating cells, known also as cancer stem cells (CSCs).
Methods
The production of CEA was investigated in LoVo cells that were cultured with 0–10 mM of anticancer drugs, and we evaluated the increase in CEA production by the LoVo cells that were stimulated by anticancer drug treatment. The expression of several CSC markers in LoVo cells treated with anticancer drugs was also evaluated. Following anticancer drug treatment, LoVo cells were injected subcutaneously into the flanks of severe combined immunodeficiency mice in order to evaluate the CSC fraction.
Results
Production of CEA by LoVo cells was stimulated by the addition of anticancer drugs. Drug-resistant LoVo cells expressed lower levels of CSC markers, and LoVo cells treated with any of the anticancer drugs tested did not generate tumors within 8 weeks from when the cells were injected subcutaneously into severe combined immunodeficiency mice. These results suggest that the drug-resistant LoVo cells have a smaller population of CSCs than the untreated LoVo cells.
Conclusion
Production of CEA by LoVo cells can be stimulated by the addition of anticancer drugs. The drug-resistant subpopulation of LoVo colon cancer cells could stimulate the production of CEA, but these cells did not act as CSCs in in vivo tumor generation experiments.
Introduction
Tumors contain a small subpopulation of cancer-initiating cells, known as cancer stem cells (CSCs), which exhibit a self-renewing capacity and are responsible for tumor generation.Citation1 CSCs are reputed not to be typical cancer cells, and they may persist in tumors as a distinct population, causing relapse and metastasis by giving rise to new tumors. The first evidence for CSCs was reported in 1997 by Bonnet and DickCitation2 in a study in which they isolated a subpopulation of leukemic cells that expressed a specific surface marker, CD34, but lacked the CD38 marker. The authors established that the CD34+/CD38− subpopulation was capable of initiating tumors in non-obese diabetic/severe combined immunodeficiency (SCID) mice and that these tumors were histologically similar to the primary leukemic tumors.
CSCs can form tumors while having stem cell properties such as self-renewal and the ability to differentiate into multiple cell types. It has been suggested that CSCs persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors.Citation3–Citation5 The development of specific therapies that target CSCs could improve the survival and quality of life of cancer patients, especially for those suffering with metastatic disease.
Colon carcinoma was the third most common cause of death in the United States in men and women in 2009.Citation4,Citation6 The hypothesis that stem cells drive tumorigenesis in colon cancer raises the question of whether current anticancer therapies can efficiently target the tumorigenic cell population that is responsible for tumor growth and maintenance.Citation4 Current therapies mostly fail to eradicate CSC clones and instead favor expansion of the CSC pool and/or select for drug-resistant CSC clones, leading to a fatal outcome.Citation7 The isolation and characterization of tumorigenic colon CSCs should enable the development of novel diagnostic and therapeutic procedures.
Specific surface markers for colon CSCs have been reported, and CD133 is the most studied surface marker for colon CSCs.Citation8–Citation11 CD133 is considered an important marker for identifying the subpopulation of CSCs in leukemia, brain tumors, retinoblastoma, renal tumors, pancreatic tumors, colon carcinoma, prostate carcinoma, and hepatocellular carcinoma.Citation8–Citation12 Based on the immunohistochemical findings, Hilbe et alCitation13 suggested that CD133-positive (CD133+) progenitor cells may play a role in the development of tumor vasculature in non-small-cell lung cancer patients. Ricci-Vitiani et alCitation8 reported that CD133 can be used to identify and confirm expansion of human colon CSCs. They injected CD133+ colon cancer cells subcutaneously, which readily generated a tumor in SCID mice, whereas CD133− cells did not form tumors.Citation8 However, their results were controversial.Citation14–Citation22
Shmelkov et alCitation14 prepared a knock-in lacZ reporter mouse (CD133lacz/+) in which the expression of lacZ was driven by the endogenous CD133 promoters. Using these mice, CD133 expression in the colon was found not to be restricted to stem cells alone; CD133 was ubiquitously expressed on differentiated colonic epithelia in both adult mice and humans. An examination of CD133 expression did not reveal the entire population of CSCs in human metastatic colon cancer; both CD133+ and CD133− metastatic tumor subpopulations were capable of long-term tumorigenesis in a non-obese diabetic/SCID xenotransplantation model.Citation14
Several other colon CSC markers have been proposed: epithelial specific antigen (EpCAM, BerEp4; cell adhesion molecule), CD44 (CDW44; cell adhesion molecule, hyaluronic acid receptor), CD166 (ALCAM; cell adhesion molecule), Msi-1 (Musashi-1; RNA-binding protein), CD29 (integrin β1; cell adhesion molecule), CD24 (HSA; cell adhesion molecule), Lgr5 (GPR49; Wnt targeting gene), and ALDH-1 (ALDC; enzyme).Citation4,Citation5,Citation9,Citation22–Citation30 However, exact and reliable surface markers of colon CSCs have not yet been identified. The only reliable method for identifying and quantifying CSCs is to observe tumor formation in a serial xenotransplantation model.
It is generally accepted that CSCs express active transmembrane ATP-binding cassette (ABC) transporter family members, such as the multidrug-resistant transporter 1 and ABC sub-family G member 2 (ABCG2),Citation7 which render them drug resistant.Citation31 In our previous study,Citation32 drug-resistant cells from human colorectal adenocarcinoma tumors produced two orders higher than normal levels of carcinoembryonic antigen (CEA) per cell. Only 1% of cells treated with acetylsalicylic acid (aspirin) in their culture medium survived, compared with cells grown in the normal expansion medium. This experiment raised questions about whether the drug-resistant colorectal cells, which are increased by adding anticancer drugs into the culture medium, might be CSCs; if so, this method might be the simplest isolation method for CSCs. It will also be important to determine which anticancer drugs or chemotherapy treatments can efficiently deplete CSCs when colon cancer cells are subcutaneously xenotransplanted into mice after the cells have been treated with anticancer drugs.
In this study, we evaluated the higher levels of CEA secreted by the LoVo colon carcinoma cell line, which was cultured in serum-free and serum-containing media containing anticancer drugs. We also treated the cells with aspirin because only aspirin enhanced the expression of CEA in colon carcinoma cells in our previous study.Citation32 Drug-resistant LoVo cells were analyzed to determine whether those cells had CSC characteristics, eg, small size of the cells/colonosphere and strong expression of CSC surface markers, as indicated by flow cytometry and immunohistochemistry analysis. Finally, in vivo tumorigenesis was examined by subcutaneously xenotransplanting the isolated drug-resistant LoVo cells into mice. We then evaluated whether the drug-resistant cells isolated in this study were CSCs.
Material and methods
Cell culture
The LoVo human colon cancer cell line purchased from Food Industry Research and Development Institute (BCRC 60148; Hsinchu, Taiwan) was cultured in (a) a serum-containing medium (Ham’s F-12 nutrient mixture medium [catalog # O135-500; Biowest, Nuaillé, France] containing L-glutamine sodium pyruvate and HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], with 20% fetal bovine serum [catalog # 04-001-1A; Biological Industries, Beit Haemek, Israel]) and (b) a serum-free medium (Dulbecco’s Modified Eagle’s Medium/F12 medium containing 10 ng/mL of human recombinant basic fibroblast growth factor and 10 ng/mL of epidermal growth factor) containing 0–10 mM of anticancer drugs. The medium was changed twice a week. The anticancer drugs used in this study were 5-fluorouracil (5-FU) (catalog # F6627; Sigma-Aldrich, St Louis, MO, USA), oxaliplatin, (catalog # O9512; Sigma-Aldrich), cisplatin, (catalog # P4394; Sigma-Aldrich), and acetylsalicylic acid (aspirin) (catalog # A5376; Sigma-Aldrich). A defined amount (0–10 mM) of each anticancer drug was added to the serum-containing or serum-free media. The drugs were dissolved using ultrasonic waves supplied by an ultrasonic cleaner (DC150H; Delta New Instrument, Bangkok, Thailand). The solution was filtered through a disposable 0.22 μm Millex filter (Merck Millipore, Billerica, MA, USA) and adjusted to pH 7.4. Penicillin-Streptomycin Amphotericin B Solution (catalog # 03-033-1B; Biological Industries) was also added to the culture medium, where concentrations of penicillin, streptomycin, and amphotericin B were 250 U/mL, 250 μg/mL, and 0.625 μg/ml, respectively.
The cell survival rate was defined as follows:
where D drug and D 0 represent the density of the cells cultured in the presence or absence, respectively, of the anticancer therapy.
CEA production by LoVo cells
The concentration of CEA in the culture medium was measured using the enzyme-linked immunosorbent assay (ELISA) (catalog # 25-CEAHU-E01; ALPCO Diagnostics, Salem, NH, USA) and an ELISA plate reader.Citation32,Citation33 The concentration of CEA was measured by reading the optical density values obtained at 450 nm. The cell number was estimated by examining the cells on the dishes using an inverted microscope equipped with a charge-coupled devices video camera (MicroPublisher, 3.3RV, Qimaging, Surrey, BC, Canada). CEA production was calculated using the following equation:
where C CEA represents the concentration of CEA in the culture medium, V is the volume of culture medium (2 mL), N is the number of cells in the culture medium, and D is number of days the cells were cultured after the addition of fresh culture medium (2 days).
The CEA production ratio was defined as follows:
where CEA(drug) and CEA(0) are the concentration of CEA produced by LoVo cells in the culture medium in the presence and absence, respectively, of the anticancer drugs.
Flow cytometry and immunostaining
The CD133/2 (293C3)-phycoerythrin (PE) antibody (catalog # 130-090-853, Miltenyi Biotec, Auburn, CA, USA) and immunoglobulin (Ig)G2b-PE antibody (catalog # 130-092-215, Miltenyi Biotec) as an isotype control, were used for flow cytometric analysisCitation34 of LoVo cells. Conventional staining protocolCitation35–Citation39 was used. The expression of CD133, forward scattering intensity, and side scattering intensity of the LoVo cells were analyzed by flow cytometry (Coulter EPICS™ XL; Beckman-Coulter, Brea, CA, USA). Immunohistochemistry was performed after formalin fixation of LoVo cells cultured in tissue culture dishes. The dishes were incubated with antibodies to cell surface markers as follows: CD29 (rabbit anti-human CD29, catalog # NB100-92076; Novus Biologicals, Littleton, CO, USA), CD44 (mouse anti-human CD44, catalog # NBP1-47386; Novus), CD133 (rabbit anti-human CD133, catalog # PAB12663; Abnova, Taipei City, Taiwan), CD166 (rabbit anti-human CD166, catalog # BP1-96579; Novus), ALDH-1 (rabbit anti-human ALDH-1, catalog # PAB3093; Abnova), Lgr5 (goat anti-human Lgr5, catalog # SC-68580; Santa Cruz Biotechnology, Dallas, TX, USA), Msi-1 (mouse anti-human Msi-1, catalog # H00004440-M04, Abnova), as well as secondary antibodies: Alexa Fluor 488 (anti-rabbit IgG, catalog # A21206; Life Technologies, Carlsbad, CA, USA), Alexa Fluor 488 (anti-mouse IgG, catalog # A21202; Life Technologies), and Alexa Fluor 594 (donkey anti-goat IgG, catalog # A11058; Life Technologies). The stained LoVo cells were analyzed using a fluorescence inverted microscope (Eclipse Ti-U; Nikon Instruments, Melville, NY, USA).
MACS sorting
A CD133 micro-bead kit (catalog # 130-050-801, Miltenyi Biotec) was used for the positive and negative selection of LoVo cells using magnetic activated cell sorting (MACS) (MiniMACS™, Miltenyi Biotec). The cells were analyzed using the manufacturer’s protocol.
Xenotransplantation with cancer cells
LoVo cells at concentrations of 105 were treated with either 0, 0.01, 1, or 10 mM concentrations of the anticancer drugs. The cells, unsorted or sorted by CD133 expression using MACS, were then injected subcutaneously into the flanks of SCID mice, with the approval of the Institutional Animal Care and Use Committee of the Cathay General Hospital and National Central University. Six mice were used to evaluate tumor generation on each drug-treated condition. The mice were sacrificed after 8 weeks, and their tumors were extracted, fixed in 10% neutral buffered formalin solution (catalog # HT501128-4L, Sigma-Aldrich), and paraffin embedded.Citation8 The paraffin sectioning and hematoxylin-eosin staining were performed in the Department of Pathology and Medical Laboratory at the Landseed Hospital.
Statistical analysis
All of the quantitative results were obtained from four independent experiments. The data are expressed as the mean ± standard deviation (SD).
Results
The survival of LoVo cells after treatment with anticancer drugs
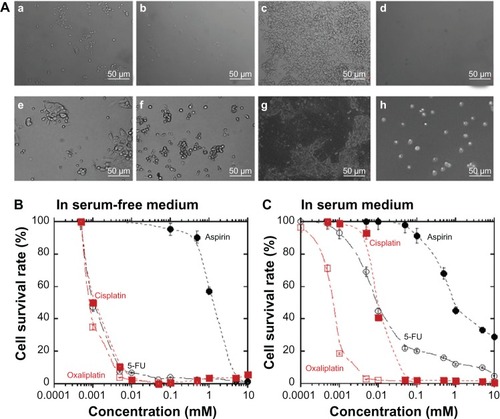
LoVo colon cancer cells were cultured in dishes with serum-free and serum-containing media supplemented with 20% fetal bovine serum (FBS); the cell morphology and density were evaluated for 10 days. The LoVo cell morphology before and after 10 days of treatment with anticancer drugs is shown in . The treated LoVo cells shrank and showed more spherical morphology compared with the cells that did not receive treatment (). The LoVo cell density was lower after treatment with anticancer drugs, which is shown in . The cell survival rate was evaluated for LoVo cells treated with 0–10 mM of several anticancer drugs. The cell survival rate decreased as the concentration of the anticancer drugs increased in both the serum-free () and serum-containing media (). The LoVo cell survival rate in the serum-containing medium was higher than that in the serum-free medium. The LoVo cell survival rate in the serum-containing medium was dependent on the specific anticancer drugs used, whereas the LoVo cells in the serum-free medium treated with 5-FU, oxaliplatin, and cisplatin had similar survival rates with the same concentrations of anticancer drugs. Cell viability, cultured in both the serum-free and serum-containing media, with or without anticancer drugs, was observed to be more than 98% in each case as determined by the trypan blue exclusion method.
Figure 1 Decreased survival of LoVo cells grown in culture medium containing anticancer drugs.
Abbreviation: 5-FU, 5-fluorouracil.
LoVo cell size after treatment with anticancer drugs
The size of the LoVo cells treated with anticancer drugs was evaluated based on the intensity of the forward and side scatter flow cytometry measurements. shows typical examples of forward and side scatter plots of LoVo cells cultured in the serum-free and serum-containing media, each with or without 5-FU. The data were collected using flow cytometry after 10 days of cell culture. We defined the small cell size parameters on the forward and side scatter plots as shown in the green box in , and the frequency of small LoVo cells was evaluated by flow cytometry; the same operation was used for the LoVo cells treated with and without anticancer drugs. Cell size decreased with increasing concentrations of 5-FU in both the serum-free and serum-containing media, as shown in .
Figure 2 Reduction in LoVo cell size in both serum-free medium and serum-containing medium at high concentrations of anticancer drugs.
Abbreviations: 5-FU, 5-fluorouracil; FS, scatter forward; SS, side scatter; SF, serum free.
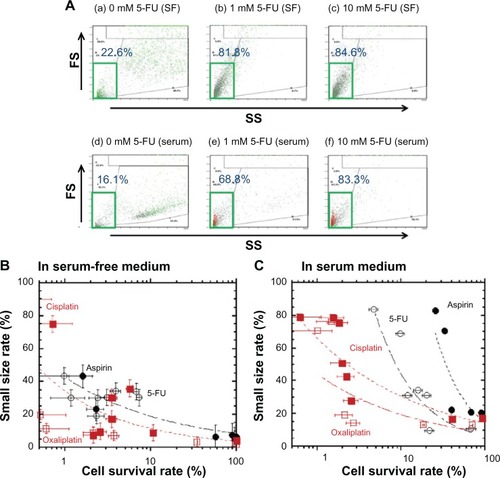
shows the dependence of the frequency of small cell size on the cell survival rate of LoVo cells cultured in the serum-free () and serum-containing media (), containing 5-FU, aspirin, oxaliplatin, and cisplatin. Cell size decreased with increasing concentrations of the anticancer drugs in the serum-free as well as the serum-containing media. LoVo cells in the serum-containing medium were smaller, in parallel with the decrease in the cell survival rate of LoVo cells treated with any of the anticancer drugs used in this study. These results indicate that under conditions where no anticancer drugs are present, drug-resistant LoVo cells are smaller than normal LoVo cells.
Production of CEA by LoVo cells during treatment with anticancer drugs
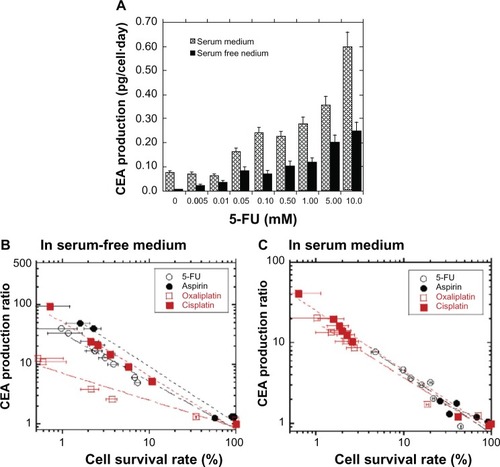
We investigated the production of CEA in LoVo cells when they were cultured with 0–10 mM of anticancer drugs, and we evaluated an increase in CEA production which was stimulated by anticancer drug treatment. shows the dependence of the LoVo cell CEA production on the 5-FU concentration in the culture medium 8–10 days post-treatment. CEA production by LoVo cells increased with increasing 5-FU concentration in both the serum-free and serum-containing media. CEA production by the LoVo cells in the serum-containing medium was higher than that in the serum-free medium for the same concentration of 5-FU. We investigated the dependence of CEA production on cell survival rate in LoVo cells in serum free () and serum-containing media (), containing several anticancer drugs. The CEA production ratio was higher for LoVo cells with lower survival rates. These results suggest that drug-resistant LoVo cells produce CEA with high efficiency when LoVo cells are treated with anticancer drugs. This result is consistent with previous research in which the human colorectal adenocarcinoma tumor cell line expressed higher levels of CEA when cell growth was suppressed by the addition of aspirin to the serum medium.Citation32 In the present study, aspirin and other anticancer drugs tested (5-FU, oxaliplatin, and cisplatin) were found to stimulate CEA production in LoVo cells at a much higher level than in LoVo cells grown in the absence of the anticancer drugs. When LoVo cells were cultured in the serum-free medium containing aspirin, cisplatin, or 5-FU, they produced higher levels of CEA, with the same cell survival rate, than when cultured with oxaliplatin; no significant difference was observed between cells treated with aspirin, cisplatin, or 5-FU (). When LoVo cells were grown in the serum-containing medium, there was no significant difference in the CEA production ratio after treatment with any of the anticancer drugs for the same cell survival rate (). The cell survival rate appears to be an important determinant of the production of CEA by LoVo cells in the serum-containing medium with different anticancer drugs.
Figure 3 Production of CEA by LoVo cells was enhanced by the suppression of cell survival with the addition of anticancer drugs to the culture medium during cell culture for 8–10 days.
Abbreviations: 5-FU, 5-fluorouracil; CEA, carcinoembryonic antigen.
Expression of CSC markers by LoVo cells during treatment with anticancer drugs
We expected that a small fraction of LoVo cells, those that showed drug resistance and produced high levels of CEA, might contain a high proportion of CSCs. Therefore, we evaluated the expression of several CSC markers, including CD29, CD44, CD133, CD166, ALDH-1, Lgr5, and Msi-1 in LoVo cells treated with anticancer drugs.
shows typical flow cytometry analyses of CD133+ LoVo cells that were cultured in the serum-free and serum-containing media, each with or without 5-FU, after 10 days of cell culture. The population of CD133+ cells was dramatically lower after 5-FU treatment in both the serum-free and serum-containing media groups in this study.
Figure 4 CD133 expression on LoVo cells was decreased by the suppression of cell survival with the addition of anticancer drugs to the culture medium after 10 days in cell culture.
Abbreviation: 5-FU, 5-fluorouracil.
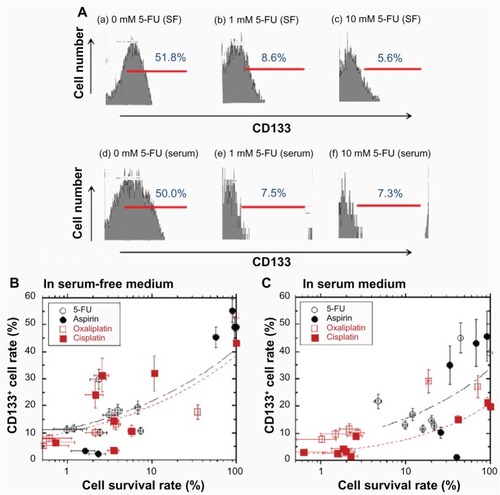
shows the relationship between the percentage of CD133+ cells and the survival rate of LoVo cells treated with several anticancer drugs in the serum-free () and serum-containing media (). The percentage of CD133+ cells decreased in parallel with the decrease in the cell survival rate in the serum-free as well as the serum-containing media. This indicates that the number of CD133+ cells decreased when the LoVo cells were cultured in medium containing higher concentrations of the anticancer drugs. The percentage of CD133+ cells was less than 15% when the LoVo cells were treated with any of the anticancer drugs, and the cell survival rate was less than 2% in both the serum-free and serum-containing media, whereas the rate of CD133+ cells was 50% ± 5% in both the serum-free and serum-containing media with no anticancer drugs. We found that fewer of the drug-resistant LoVo cells were CD133+ compared with the untreated LoVo cells in both the serum-free and serum-containing media.
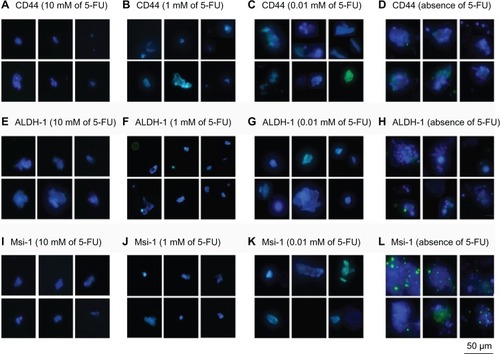
In accordance with CD133, CD29, CD44, CD166, ALDH-1, Lgr5, and Msi-1 having all been reported to be cancer stem cell markers,Citation4–Citation7,Citation9 the expression levels of these molecules were measured by immunohistochemistry on LoVo cells cultured in the serum-free medium with 0, 0.01, 1, and 10 mM of 5-FU, and the results are shown in and . No significant expression of the CSC markers was found on the LoVo cells in the serum-free medium containing and not containing 5-FU. However, the levels of the CSC markers CD29, CD44, CD133, CD166, ALDH-1, Lgr5, and Msi-1 were slightly lower for LoVo cells treated with 5-FU than for the untreated LoVo cells. Thus, the drug-resistant LoVo cells expressed lower levels of the CSC markers in this study.
Figure 5 Immunohistochemical analysis of CSC surface markers on LoVo cells.
Abbreviations: 5-FU, 5-fluorouracil; CSC, cancer-initiating cell; Lgr5, leucine-rich repeat-containing G protein-coupled receptor 5.
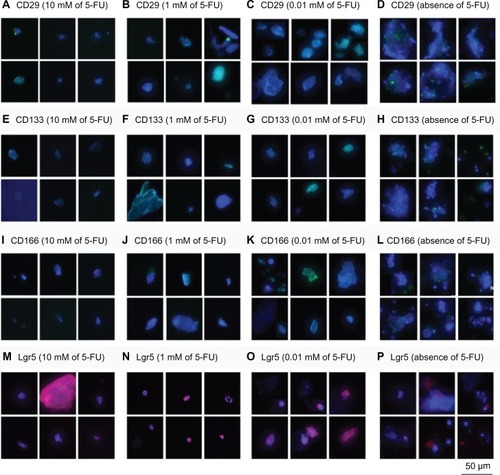
Figure 6 Immunohistochemical analysis of CSC surface markers on LoVo cells.
Abbreviations: 5-FU, 5-fluorouracil; ALDH-1, aldehyde dehydrogenases-1; CSC, cancer-initiating cell; Msi-1, musashi-1.
Tumor generation by LoVo cells receiving anticancer therapy
The induction of tumor generation in vivo by subcutaneously injecting LoVo cells into mice is the most direct method for qualitatively and quantitatively evaluating the tumorigenic potential of CSCs. Therefore, in vivo tumor generation was examined by subcutaneously injecting treated and untreated LoVo cells into SCID mice. shows the tumors generated in mice following the injection of LoVo cells cultured in serum-free and serum-containing media without any anticancer drugs. The size of the tumors in the SCID mice was larger when the cells had previously been cultured in the serum-containing medium than when they had been cultured in the serum-free medium. This indicates that the CSC subpopulation of the LoVo cells cultured in the serum-containing medium was higher than that of the LoVo cells cultured in the serum-free medium in these culture conditions. shows the time dependence of the tumor growth in SCID mice injected with LoVo cells that had been cultured in serum-free and serum-containing media, with or without anticancer drugs. LoVo cells in the serum-free or the serum-containing media, with 0.01, 1, or 10 mM of any anticancer drug (5-FU, aspirin, oxaliplatin, or cisplatin), did not generate tumors within 8 weeks of when the cells were injected subcutaneously into SCID mice. This indicates that 144 mice (3 [different concentration] × 4 [different drugs] × 2 [serum-free and serum medium] × 6 [6 mice on each condition]) generated no tumor within 8 weeks when the drug-treated LoVo cells were injected subcutaneously. These results suggest that the drug-resistant LoVo cells have a smaller population of CSCs than the untreated LoVo cells. Furthermore, all of the anticancer drugs used in this study effectively killed the CSCs within the LoVo cells, as observed in the cells cultured with anticancer drugs in vitro.
Figure 7 The tumor generation potential of LoVo cells in vivo.
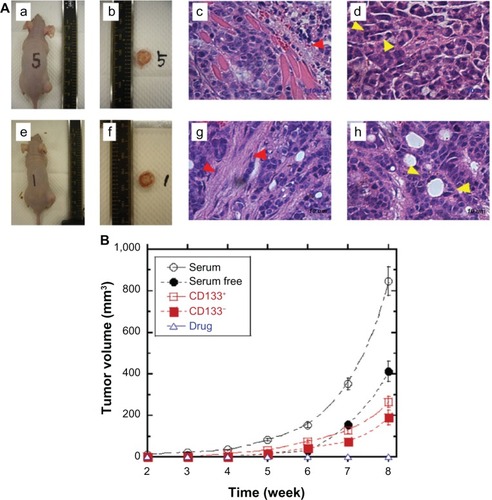
The CD133+ and CD133− cells were sorted using the MACS method, and the tumorigenic potential of those cells was also evaluated (). The CD133+ population of LoVo cells sorted by CD133+ MACS was analyzed and determined to be 45.7% ± 9.2% and CD133− population sorted by CD133− selection was 2.4% ± 1.3%. Tumor generation by the CD133+ cells was slightly higher than that for the CD133− cells (P < 0.05), where the viability of both CD133+ cells and CD133− cells was found to be more than 96%, but tumor generation by both CD133+ and CD133− cells, which were cultured in the serum-containing medium, was lower than that of normal LoVo cells without MACS treatment. This result indicates that LoVo cells are damaged mechanically and/or chemically when they are sorted by MACS treatment, although the cell viability of sorted cells is more than 96%. Treating the LoVo cells with an anticancer drug decreased the CSC population more effectively compared with isolating CD133− LoVo cells in this study.
Discussion
Colon cancer cell lines produced large amounts of CEA when the cell survival rates were decreased by the addition of aspirin and also several anticancer drugs (5-FU, aspirin, oxaliplatin, and cisplatin) in both the serum-free and serum-containing media. In previous studies,Citation32,Citation40,Citation41 only aspirin and 5-FU were reported to stimulate the production of CEA in colorectal cancer cells.
Because CSCs are often reported to be drug-resistant cells,Citation4–Citation7 we hypothesized that the drug-resistant cells selected by adding anticancer drugs to the culture medium might be CSCs. The drug-resistant LoVo cells isolated in this study were smaller than the normal colon cancer cells () but contained fewer CD133+ cells. A future issue for us to evaluate is whether other types of drug-resistant colon cancer cells are smaller than their normal colon cancer cells. The CD133 marker is typically used to identify colon CSCs.Citation8–Citation11 In this study, the presence of several other CSC markers, including CD29, CD44, CD166, ALDH-1, Lgr5, and Msi-1Citation4,Citation5,Citation9,Citation22–Citation25 was also evaluated in the drug-resistant LoVo cells using immunohistochemistry (); however, drug-resistant LoVo cells expressed lower levels of CSC markers.
Currently, the only reliable method of identifying CSCs is by measuring tumor generation in vivo following the subcutaneous xenotransplantation of the cells into mice. LoVo cells cultured in serum-free and serum-containing media, without anticancer drugs, can generate tumors, which implies that the LoVo colon cancer cell line contains a subpopulation of CSCs. However, drug-resistant LoVo cells selected by treatment with any of the tested anticancer drugs (5-FU, oxaliplatin, and cisplatin) or aspirin did not generate tumors in this study. We were unable to perform serial xenotransplantations on mice transplanted with LoVo cells treated with drugs, because these mice did not generate tumors. However, we performed xenotransplation using 144 mice (3 [different concentration] × 4 [different drugs] × 2 [serum-free and serum medium] × 6 [6 mice on each condition]) and no tumor generation was observed on mice transplanted with LoVo cells treated with drugs. These results indicate that LoVo cells treated with drugs contain few or no CSCs in this study. Recently, Yan et alCitation42 reported that Du145 prostate cancer cells treated with drugs (ie, etoposide, paclitaxel, staurosporine, and 2-paclitaxel analogs) exhibited greatly reduced tumorigenicity or were nontumorigenic. This result indicates that drug-resistant Du145 cells are not CSCs, or contain less CSCs compared to Du145 cells without treatment of drugs. Their report also supports our findings in this study.
In the clinic, biomarkers are often measured to evaluate disease progression or responses to a therapeutic intervention such as drug treatment. Predictive biomarkers provide information on the response to a treatment, whereas prognostic biomarkers provide information about the outcome, independent of the treatment effect. CEA is one of the earliest studied biomarkers in colorectal cancer, and the preoperative serum CEA level is an independent negative prognostic factor.Citation43,Citation44 A CEA surge or fare has been observed as an early biochemical phenomenon in metastatic colorectal cancer during chemotherapy in approximately 10% of the patients who experience a clinical benefit.Citation45–Citation47 In light of this clinical evidence, we speculate that drug-resistant cancer cells are not CSCs because patients with CEA surges experience a clinical benefit, which is inconsistent with the CSC theory that predicts that these patients would have a worse prognosis.
Conclusion
Production of CEA by LoVo cells can be stimulated by the addition of anticancer drugs as well as aspirin in both serum-free and serum-containing media. Increased CEA production was also reported in the human colorectal adenocarcinoma tumor cell line, following treatment with aspirin in the serum-containing medium.Citation32 CSCs are believed to be drug resistant cells;Citation4–Citation7 however, although the drug-resistant subpopulation of LoVo colon cancer cells, which were isolated by the addition of anticancer drugs to the culture medium, could stimulate the production of CEA in both serum-free and serum-containing media, these cells did not act as CSCs in in vivo tumor generation experiments.
Acknowledgments
This research was partially supported by the National Science Council of Taiwan under grant numbers NSC100-2120-M-008-004 and NSC101-2120-M-008-003. This work was also supported by the National Central University-Cathay General Hospital Project (CGH-MR-10025, 102NCU-CGH-02, and 101CGH-NCU-A2).
Disclosure
The authors report no conflicts of interest in this work.
References
- Trumpp A Wiestler OD Mechanisms of disease: cancer stem cells – targeting the evil twin Nat Clin Pract Oncol 2008 5 6 337 347 18431377
- Bonnet D Dick JE Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell Nat Med 1997 3 7 730 737 9212098
- Ferlay J Autier P Boniol M Heanue M Colombet M Boyle P Estimates of the cancer incidence and mortality in Europe in 2006 Ann Oncol 2007 18 3 581 592 17287242
- Ricci-Vitiani L Fabrizi E Palio E De Maria R Colon cancer stem cells J Mol Med (Berl) 2009 87 11 1097 1104 19727638
- Todaro M Francipane MG Medema JP Stassi G Colon cancer stem cells: promise of targeted therapy Gastroenterology 2010 138 6 2151 2162 20420952
- Papailiou J Bramis KJ Gazouli M Theodoropoulos G Stem cells in colon cancer. A new era in cancer theory begins Int J Colorectal Dis 2011 26 1 1 11 20680304
- Klonisch T Wiechec E Hombach-Klonisch S Cancer stem cell markers in common cancers – therapeutic implications Trends Mol Med 2008 14 10 450 460 18775674
- Ricci-Vitiani L Lombardi DG Pilozzi E Identification and expansion of human colon-cancer-initiating cells Nature 2007 445 7123 111 115 17122771
- Vermeulen L Todaro M de Sousa Mello F Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity Proc Natl Acad Sci USA 2008 105 36 13427 13432 18765800
- O’Brien CA Pollett A Gallinger S Dick JE A human colon cancer cell capable of initiating tumour growth in immunodeficient mice Nature 2007 445 7123 106 110 17122772
- Ferrand A Sandrin MS Shulkes A Baldwin GS Expression of gastrin precursors by CD133-positive colorectal cancer cells is crucial for tumour growth Biochim Biophys Acta 2009 1793 3 477 488 19321126
- Singh SK Hawkins C Clarke ID Identification of human brain tumour initiating cells Nature 2004 432 7015 396 401 15549107
- Hilbe W Dirnhofer S Oberwasserlechner F CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer J Clin Pathol 2004 57 9 965 969 15333659
- Shmelkov SV Butler JM Hooper AT CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors J Clin Invest 2008 118 6 2111 2120 18497886
- Lugli A Iezzi G Hostettler I Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44 s, EpCAM, and ALDH1 in colorectal cancer Br J Cancer 2010 103 3 382 390 20606680
- Horst D Kriegl L Engel J Kirchner T Jung A CD133 expression is an independent prognostic marker for low survival in colorectal cancer Br J Cancer 2008 99 8 1285 1289 18781171
- Du L Rao G Wang H CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer Cancer Res 2013 73 8 2682 2694 23418321
- Li CY Li BX Liang Y Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB J Transl Med 2009 7 56 63 19583834
- Crea F Fornaro L Masi G Falcone A Danesi R Farrar W Faithful markers of circulating cancer stem cells: is CD133 sufficient for validation in clinics? J Clin Oncol 2011 29 25 3487 3488 21788560
- Chu P Clanton DJ Snipas TS Characterization of a subpopulation of colon cancer cells with stem cell-like properties Int J Cancer 2009 124 6 1312 1321 19072981
- LaBarge MA Bissell MJ Is CD133 a marker of metastatic colon cancer stem cells? J Clin Invest 2008 118 6 2021 2024 18497883
- Du L Wang H He L CD44 is of functional importance for colorectal cancer stem cells Clin Cancer Res 2008 14 21 6751 6760 18980968
- Dalerba P Dylla SJ Park IK Phenotypic characterization of human colorectal cancer stem cells Proc Natl Acad Sci USA 2007 104 24 10158 10163 17548814
- Todaro M Perez Alea M Scopelliti A Medema JP Stassi G IL-4-mediated drug resistance in colon cancer stem cells Cell Cycle 2008 7 3 309 313 18235245
- Huang EH Hynes MJ Zhang T Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis Cancer Res 2009 69 8 3382 3389 19336570
- Lohberger B Rinner B Stuendl N Aldehyde dehydrogenase 1, a potential marker for cancer stem cells in human sarcoma PLoS One 2012 7 8 e43664 22928012
- Garcia VM Batlle JF Casado E Immunohistochemical analysis of tumour regression grade for rectal cancer after neoadjuvant chemoradiotherapy Colorectal Dis 2011 13 9 989 998 20718834
- Fan LF Dong WG Jiang CQ Xia D Liao F Yu QF Expression of putative stem cell genes Musashi-1 and beta1-integrin in human colorectal adenomas and adenocarcinomas Int J Colorectal Dis 2010 25 1 17 23 19714342
- Fan X Ouyang N Teng H Yao H Isolation and characterization of spheroid cells from the HT29 colon cancer cell line Int J Colorectal Dis 2011 26 10 1279 1285 21670985
- Hellsten R Johansson M Dahlman A Sterner O Bjartell A Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells PLoS One 2011 6 7 e22118 21779382
- Singh A Settleman J EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer Oncogene 2010 29 34 4741 4751 20531305
- Higuchi A Uchiyama S Demura M Enhanced CEA production associated with aspirin in a culture of CW-2 cells on some polymeric films Cytotechnology 1999 31 3 233 242 19003147
- Zhang J Li H Wang X Phage-derived fully human antibody scFv fragment directed against human vascular endothelial growth factor receptor 2 blocked its interaction with VEGF Biotechnol Prog 2012 28 4 981 989 22581629
- Alfred R Radford J Fan J Efficient suspension bioreactor expansion of murine embryonic stem cells on microcarriers in serum-free medium Biotechnol Prog 2011 27 3 811 823 21538971
- Higuchi A Huang SC Shen PY Differentiation ability of amniotic fluid-derived stem cells cultured on extracellular matrix-immobilized surface Curr Nanosci 2011 7 6 893 901
- Liu JY Peng HF Gopinath S Tian J Andreadis ST Derivation of functional smooth muscle cells from multipotent human hair follicle mesenchymal stem cells Tissue Eng Part A 2010 16 8 2553 2564 20236033
- Shi B Deng L Shi X The enhancement of neural stem cell survival and growth by coculturing with expanded sertoli cells in vitro Biotechnol Prog 2012 28 1 196 205 22109810
- Mohr JC de Pablo JJ Palecek SP Electroporation of human embryonic stem cells: small and macromolecule loading and DNA transfection Biotechnol Prog 2006 22 3 825 834 16739967
- Malpique R Tostões R Beier AFJ Surface-based cryopreservation strategies for human embryonic stem cells: a comparative study Biotechnol Prog 2012 28 4 1079 1087 22718690
- Aquino A Prete SP Guadagni F Effect of 5-fluorouracil on carcinoembryonic antigen expression and shedding at clonal level in colon cancer cells Anticancer Res 2000 20 5B 3475 3484 11131650
- Prete SP Rossi L Correale PP Combined effects of protein kinase inhibitors and 5-fluorouracil on CEA expression in human colon cancer cells Pharmacol Res 2005 52 2 167 173 15967383
- Yan H Chen X Zhang Q Drug-tolerant cancer cells show reduced tumor-initiating capacity: depletion of CD44 cells and evidence for epigenetic mechanisms PLoS One 2011 6 9 e24397 21935404
- Staab HJ Anderer FA Brümmendorf T Stumpf E Fischer R Prognostic value of preoperative serum CEA level compared to clinical staging. I. colorectal carcinoma Br J Cancer 1981 44 5 652 662 7317270
- Wolmark N Fisher B Wieand HS The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials Ann Surg 1984 199 4 375 382 6370155
- Ailawadhi S Sunga A Rajput A Yang GY Smith J Fakih M Chemotherapy-induced carcinoembryonic antigen surge in patients with metastatic colorectal cancer Oncology 2006 70 1 49 53 16446549
- Fakih MG Padmanabhan A CEA monitoring in colorectal cancer. What you should know Oncology (Williston Park) 2006 20 6 579 587 16773844
- Sørbye H Dahl O Carcinoembryonic antigen surge in metastatic colorectal cancer patients responding to oxaliplatin combination chemotherapy: implications for tumor marker monitoring and guidelines J Clin Oncol 2003 21 23 4466 4467 14645446