Abstract
Purpose
Odatroltide (LT3001), a novel small synthetic peptide molecule designed to recanalize occluded blood vessels and reduce reperfusion injury, is safe and efficacious in multiple embolic stroke animal models. This study aimed to investigate the safety and tolerability of intravenous administration of odatroltide in patients with acute ischemic stroke within 24 hours of onset.
Patients and Methods
Patients with National Institutes of Health Stroke Scale (NIHSS 4–30) who were untreated with intravenous thrombolysis or endovascular thrombectomy were randomized (2:1) to receive a single dose of odatroltide (0.025 mg/kg) or placebo within 24 hours of stroke symptom onset. The primary safety outcome was symptomatic intracranial hemorrhage (sICH) occurrence within 36 hours.
Results
Twenty-four patients were enrolled and randomized; of these 16 and 8 received intravenous odatroltide infusion and placebo, respectively. sICH did not occur in both groups, and other safety measures were comparable between the groups. The rate of excellent functional outcome (modified Rankin Scale score, 0–1, at 90 days) was 21% and 14% in the odatroltide and placebo groups, respectively. Furthermore, 47% and 14% of patients in the odatroltide and placebo groups, respectively, showed major neurological improvement (NIHSS improvement ≥4 points from baseline to 30 days). Among the 9 odatroltide-treated patients with baseline NIHSS ≥6, 78% showed major neurological improvement.
Conclusion
Compared with placebo, treatment with intravenous odatroltide within 24 hours following onset of ischemic stroke appears to be safe and may be associated with better neurological and functional outcomes. However, the efficacy and safety of odatroltide requires further confirmation in the next phase of clinical trials.
Clinical Trial Registration
Clinicaltrials.gov identifier: NCT04091945.
Keywords:
Introduction
Stroke is the primary cause of disability and the second most common cause of global deaths for individuals older than 60 years.Citation1 Traditionally, it is defined as a neurological deficit caused by an immediate focused insult to the central nervous system from a vascular source.Citation2 Based on the pathophysiology of the condition, the two primary goals of ischemic stroke therapy techniques are restoring cerebral blood flow and reducing the detrimental effects of ischemia on neurons.Citation3 Alteplase (Activase®), a recombinant tissue-type plasminogen activator (rtPA), was the first approved medication for treatment of ischemic stroke by the US Food and Drug Administration in 1996. Alteplase has been shown to improve the outcome of patients with acute ischemic stroke (AIS); however, its use has been limited owing to the low eligibility rate for this medication. Only approximately 5% of stroke patients are administered alteplase.Citation4 Therefore, the development of treatments that can offer similar efficacy to alteplase is required, but with a lower increase of bleeding and/or extended therapeutic time window.
Odatroltide is a novel multifunctional synthetic small molecule comprising a tripeptide Pro–Ala–Lys (PAK) and an (S)-6,7-dihydroxy-1,1-dimethyl-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid domain. It can restore cerebral blood flow, scavenge free radicals, and inhibit leukocyte migration. In 1978, P6A, an active pentapeptide with the sequence Ala–Arg–Pro–Ala–Lys, was isolated from human fibrin and fibrinogen and was shown to inhibit thrombus formation and angiotensin-converting enzyme.Citation5 In 1989, Mehta et al demonstrated the thrombolytic effect of P6A in a canine coronary embolism model generated by electrical stimulation of the endothelium.Citation6 Zhao reported that PAK exhibited improved thrombolytic activity than the original P6A in a rat arteriovenous bypass intubation model.Citation7 PAK was combined with (S)-6,7-dihydroxy-1,1-dimethyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid to target the thrombus by downregulating P-selectin expression through computer-assisted screening and mesoscale simulation.Citation8,Citation9
Odatroltide lyophilized powder for injection, known as LT3001 (Lumosa Therapeutics, Taiwan), has been developed for the treatment of AIS and characterized in various in vitro and in vivo models.Citation10 In vitro studies have reported that odatroltide exhibits substantial antioxidant activity and can inhibit leukocyte chemotaxis and platelet aggregation. In animal studies, odatroltide with a once daily administration could restore cerebral blood flow in the rodent embolic stroke model and reduce cerebral infarct and swelling volume while improving neurological outcomes in rodent and nonhuman primate stroke models after 24 hours of onset of stroke.Citation11,Citation12 Moreover, odatroltide showcased a wider therapeutic time window and a better safety profile than those reported for rtPA. The effect of odatroltide on bleeding time was evaluated in male ICR mice using an amputation tail model. The tail bleeding time of mice treated with odatroltide was significantly shorter than that of mice treated with rtPA.
In a previous first in-human study, a single dose of 0.025 mg/kg odatroltide was safe and well-tolerated in healthy adults.Citation13 Thus, we are conducting this prospective, multicenter, randomized, double-blind trial to investigate the safety and tolerability of single-dose odatroltide in patients with AIS within 24 hours of onset of symptoms.
Materials and Methods
Study Design and Participants
This is a phase 2, double-blind, single-dose, randomized, placebo-controlled study designed to examine the safety and tolerability of odatroltide in patients with AIS. Between January 2020 and February 2021, 24 patients were enrolled from 11 centers in the United States and Taiwan and randomized to receive a single dose of either odatroltide (0.025 mg/kg) or placebo via intravenous infusion for 15 min at a 2:1 ratio. The Institutional Review Board / Ethics Committee and clinical trial number were summarized in the supplementary material. The study was conducted in compliance with the International Council for Harmonization, the Declaration of Helsinki, and Good Clinical Practice guidelines. The local ethics review committees approved the protocol at all study sites. All participants or their legal representatives provided written informed consent before the study-specific procedures were performed.
We included eligible patients aged 18–90 years with AIS within 24 hours of stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 4–30 at enrollment and were screened using computed tomography (CT) or magnetic resonance imaging (MRI) to exclude hemorrhagic stroke. Patients were excluded if they received rtPA or endovascular thrombectomy or had prestroke disability (mRS ≥ 2), imaging evidence of acute hemorrhage, Alberta Stroke Program Early CT Score (ASPECTS) of ≤4, generalized seizure, uncontrolled blood pressure, abnormal activated partial thromboplastin time (aPTT) or platelet count, and pregnancy. Furthermore, we excluded those with a history of intracranial hemorrhage, prior AIS, myocardial infarction, or serious head trauma, and any major surgery within 90 days of screening. All patients or their legal representatives provided full consent prior to receiving any treatment in the study.
Procedures
A single dose of 0.025 mg/kg odatroltide or placebo (Lumosa Therapeutics, Taiwan) was administered with 15 min of intravenous infusion following randomization. While the first 4 patients had completed the study procedures 7 days after dosing, the data safety monitoring board reviewed the safety data and checked every sequential 8 patients thereafter. All participants received standard treatment for secondary stroke prevention from the investigational drug dosing until the study completed. The overall safety review was performed when the study procedures were completed on day 90.
The occurrence of symptomatic intracranial hemorrhage (sICH) was measured using NIHSS of ≥4 points and CT/ MRI within 36 hours and 7 days after dosing. Furthermore, the occurrence of asymptomatic ICH (aICH) was measured using the Heidelberg Bleeding Classification at 24 hours and 7 days after dosing. Moreover, to study the relationship between treatment and severity, mortality caused by intracranial bleeding complications or any other reason and adverse events (AEs) was evaluated and recorded.
Functional outcomes were assessed using the modified Rankin Scale (mRS) at baseline and 7, 30, and 90 days after dosing. Neurological outcomes were assessed using the NIHSS at baseline, 1 hour, 24 hours, 7 days, and 30 days after dosing.
Outcomes
The primary outcome was the occurrence of sICH within 36 hours of treatment. Secondary safety events included symptomatic and asymptomatic ICH, bleeding, mortality, and recurrent stroke. Tolerability was assessed using AE rate, and specifically serious treatment-emergent adverse events (TEAEs) occurring on or after odatroltide treatment. Efficacy outcomes were excellent functional outcome (90-day mRS of 0–1), good functional outcome (90-day mRS of 0–2), and major neurological improvement (NIHSS improvement ≥4 points) from baseline to 30 days after dosing. The mRS scale is a 7-point scale ranging from 0 (no symptom) to 6 (death). The NIHSS has 11 elements with a total score ranging from 0 (no stroke symptom) to 42 (severe stroke).
Statistical Analysis
Because this was the first in-patient study, no formal sample size calculations or statistical hypotheses were conducted. In this study, 24 patients were planned to be enrolled based on the recommendations of the investigator meeting to observe the minimum risk of hemorrhagic stroke within 24 hours of onset. Safety events were assessed in all randomized patients who received at least one dose of the investigational product. Efficacy was explored in all randomized patients irrespective of any deviation from the protocol or premature discontinuation. The estimated difference, Chi-squared test, Fisher exact test, t-test, and relative risk were used to compare the baseline characteristics, primary and secondary outcomes, and safety outcomes of the trial groups. Treatment effects for secondary outcomes were presented as relative risk (RR) with 95% confidence intervals (CIs). The data were represented as numbers with percentage (%), median with interquartile range (IQR), and mean ± SD. Statistical significance was determined at P < 0.05. SAS® software was used for statistical tests.
Results
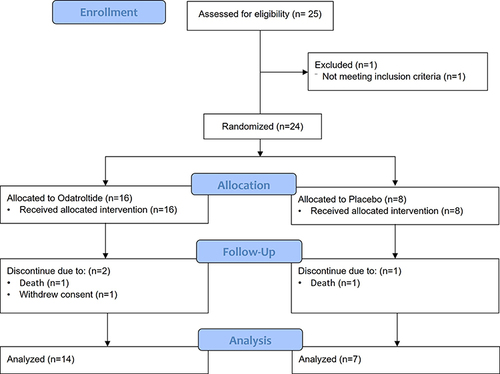
In total, 25 patients were screened; of these, 24 met the inclusion criteria and were randomly assigned to the odatroltide (16 patients) or placebo (8 patients) group (). Eventually, 21 patients completed the study, and 3 patients discontinued, including one in the odatroltide group who withdrew consent and two patients belonging to either group who experienced serious AEs (sAEs), including severe pneumonia and respiratory arrest because of coronavirus disease 2019 (COVID-19), which resulted in death. The baseline characteristics are shown in . Most of the patients were Asian (18 patients, 75%) and male (19 patients, 79.1%). The patients in the odatroltide and placebo groups had a median age of 61.5 (IQR: 53.5–70.3) and 69 (IQR: 65–73) years, respectively. The majority of poststroke mRS in the odatroltide and placebo groups was 4 points, 56.3% and 37.5%, respectively. The majority of baseline NIHSS in the odatroltide and placebo groups was 5–15 points, 62.5% and 50.0%, respectively. The median time from stroke onset to treatment was 19.4 hours (IQR: 12.4–20.9) and 18.0 hours (IQR: 14.8–21.7) for odatroltide and placebo, respectively. All participants received antithrombotic agents/ anticoagulants after the investigational drug dosing and covered the further study period. The most common concomitant medication was acetylsalicylic acid and then clopidogrel.
Table 1 Baseline Characteristics
Safety Outcomes
Both groups did not develop sICH within 36 hours and 7 days of stroke onset (RR: 0.53, 95% CI: 0.01–24.53). The aICH rates within 24 hours of stroke onset were 6.3% (1/16) and 12.5% (1/8) in the odatroltide and placebo groups, respectively (RR: 0.5, 95% CI: 0.04–7.00), whereas those within 7 days of stroke onset were 18.8% (3/16) and 12.5% (1/8) in the odatroltide and placebo groups, respectively (RR: 1.5, 95% CI: 0.2–12.2). No patient died because of intracerebral or other major bleeding complications within 90 days (). Two patients died: one in the odatroltide group had aspiration pneumonia on day 35 and one in the placebo group experienced COVID-19 with respiratory arrest on day 34 (RR: 0.5, 95% CI: 0.04–7.00). Four patients reported SAEs in the odatroltide group (25%, including aspiration pneumonia, cerebral infarction, cerebral artery occlusion, and bronchial neoplasm), and two patients in the placebo group (25%, COVID-19 respiratory arrest and severe aspiration pneumonia). All serious TEAEs were considered unrelated or unlikely to be associated with the study drug. The most common AEs reported were constipation and hypertension, with 62.5% (10/16) and 50% (4/8) experiencing constipation and 31.3% (5/16) and 25% (2/8) experiencing hypertension in the odatroltide and placebo groups, respectively.
Table 2 Summary of Safety Outcomes
Efficacy Outcomes
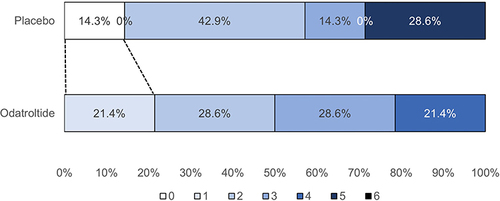
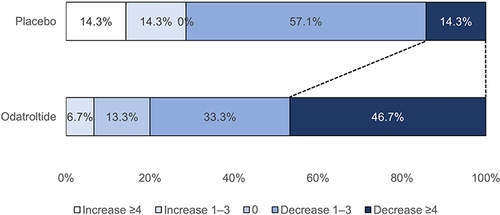
summarizes the efficacy outcomes and subgroup analysis. Excellent functional outcome (mRS of 0–1) was seen in 21.4% (3/14) and 14.3% (1/7) of the odatroltide and placebo groups, respectively, with no significant difference between the groups (RR: 1.5, 95% CI: 0.19–11.93). A good functional outcome (mRS of 0–2) was observed in 50.0% (7/14) and 57.2% (4/7) of the odatroltide and placebo groups, respectively (RR: 0.9, 95% CI: 0.38–2.00). shows the distribution of the mRS score at 90 days. Major neurological improvement (≥4 points NIHSS improvement) from baseline to 30 days was observed in 46.7% (7/14) and 14.3% (1/7) of patients in the odatroltide and placebo groups, respectively (RR: 3.27, 95% CI: 0.49–21.70). presents the proportion of NIHSS changes from baseline to 30 days.
Table 3 Summary of Efficacy Outcomes
When stratified by baseline NIHSS score, a posthoc analysis of NIHSS and mRS revealed a trend toward odatroltide in patients with NIHSS ≥6. When the analysis excluded patients with mild stroke (NIHSS of 0–5), 78% (7/9) patients showed major NIHSS improvement in the odatroltide group compared with none in the placebo group. More patients had a good or excellent functional outcome in the odatroltide group in this subset.
Discussion
This pilot, first in-patient, randomized, double-blinded study demonstrated that single-dose odatroltide administration was safe and well-tolerated in patients with AIS within 24 hours of symptom onset and not associated with sICH or major bleeding complications within 90 days. Moreover, odatroltide was associated with excellent functional outcomes at 90 days and improved early neurological outcomes at 30 days.
Cerebral bleeding complications in AIS trials can compromise therapeutic effects. Delayed rtPA treatment enhances the risk of edema, hemorrhagic transformation, and ICH in animals and patients with AIS.Citation14,Citation15 In the EXTEND study, delayed rtPA treatment was associated with a 6.2% sICH rate compared with 0.9% with placebo.Citation16 Furthermore, another study reported a higher sICH rate in the rtPA treatment group in patients with AIS with unknown onset time.Citation17 In vitro studies have shown that odatroltide has thrombolytic properties.Citation8 In the embolic stroke rat model, odatroltide had significantly improved therapeutic effects and less hemorrhagic transformation than rtPA. However, the risk of bleeding with odatroltide, especially when patients are treated >4.5 h from stroke onset, remains to be elucidated.Citation10 Because sICH did not occur, this study reported that odatroltide exhibited a favorable safety profile in patients with AIS, even when administered beyond 24 hours from stroke onset.
This is the first drug having a significantly broader therapeutic window to treat patients with AIS within 24 hours of stroke onset, providing an alternative for patients not meeting or exceeding the treatment window for receiving tPA. Recently, numerous studies have reported different cut-off values or prognostic factors in predicting favorable functional outcomes.Citation18–21 Our study indicated that odatroltide may provide a more favorable functional outcome in patients with AIS with an initial NIHSS score ≥6. However, further validation through large and randomized trials is required.
The major limitation of this study was its small sample size, which might require more data to prove its therapeutic impact on patients with AIS within 24 hours of symptom onset. Moreover, no stratified randomization was conducted for the region or baseline stroke severity, which might interfere with the determination of functional and neurological outcomes. Compared with the placebo group, more patients in the odatroltide group achieved an excellent functional outcome, specifically those with baseline NIHSS ≥6 or recruited from Taiwan sites. Finally, a less neuroimaging strategy was used to select a specific population to demonstrate reperfusion before and after the intravenous thrombolysis intervention.
The safety and efficacy of odatroltide, either in combination with mechanical endovascular treatment or imaging assessment at enrollment will be further investigated through a series of ongoing Phase II trials. Currently, a trial using multiple odatroltide doses is investigating the feasibility of endovascular thrombectomy for AIS (odatroltide versus placebo for stroke thrombolysis evaluation, NCT05198323). To investigate the safety of administering odatroltide to AIS within 24 hours of the onset of stroke symptoms, another multiple odatroltide dose study (BRIGHT, late window patients to assess the safety and efficacy of odatroltide, NCT05403866) is being conducted.
Conclusion
In conclusion, single-dose intravenous odatroltide administered within 24 hours of symptom onset is safe and well-tolerated and potentially provides benefits to patients with AIS. The data revealed that odatroltide might provide better functional outcomes at 90 days, notably in patients with an initial NIHSS ≥6. Overall, our results indicated that odatroltide may be potentially favorable for AIS to improve functional outcomes. More randomized studies are required to determine whether odatroltide provides significant benefits in patients with AIS with an extended treatment time window or conjugates with endovascular thrombectomy.
Abbreviations
AE, Adverse events; AIS, Acute ischemic stroke; ASPECTS, Alberta Stroke Program Early CT Score; CI, Confidence intervals; CT, Computed tomography; MRI, Magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; RR, Relative risk; SAE, Serious adverse event; SD, Standard deviation; TEAE, Treatment-emergent adverse events.
Ethics Approval and Informed Consent
The ethics committee of all participating centers approved the study protocol.
Author Contributions
All authors contributed to the study conception and design, execution, data collection, analysis, and interpretation. Each version of the submitted article was drafted, revised, and critically reviewed by all authors. All authors approved the article and any subsequent revisions for submission, and they are responsible for the entire content of this article.
Disclosure
Dr Luther Pettigrew reports grants from Lumosa Therapeutics Co., Ltd., during the conduct of the study; grants from NIH/NINDS, United States, outside the submitted work. Dr Yousef Hannawi reports grants from Lumosa Therapeutics Co, Ltd, during the conduct of the study. Dr Thomas Devlin reports personal fees from Medtronic, Viz.ai, NeuraSignal, and Neuro Trauma Sciences, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
We are thankful to the patients and their families for their participation, trust, and partnership. Furthermore, we are grateful to Dr. Pooja Khatri for editing the manuscript. The abstract of this paper was presented at the 2021 World Stroke Organization Congress as a poster presentation with the number: WSC21-570. The poster’s abstract was published in “WSO Congress supplement” in the International Journal of Stroke 2021, Vol. 16(2S) 3–170: Hyperlink with DOI 10.1177/17474930211041949.
Data Sharing Statement
The dataset used and/or analyzed in this study will be available through de-identification from the corresponding author upon reasonable request within one week.
Additional information
Funding
References
- Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–211. doi:10.1055/s-0038-1649503
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi:10.1161/STR.0b013e318296aeca
- Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17(3):197–218. doi:10.1016/j.pathophys.2009.12.001
- Fassbender K, Balucani C, Walter S, Levine SR, Haass A, Grotta J. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol. 2013;12(6):585–596. doi:10.1016/S1474-4422(13)70100-5
- Belew M, Gerdin B, Porath J, Saldeen T. Isolation of vasoactive peptides from human fibrin and fibrinogen degraded by plasmin. Thromb Res. 1978;13(6):983–994. doi:10.1016/0049-3848(78)90227-X
- Mehta JL, Nichols WW, Saldeen TG. Effects of peptide 6A on coronary blood flow dynamics in canine coronary thrombosis. J Cardiovasc Pharmacol. 1989;13(6):803–811. doi:10.1097/00005344-198906000-00001
- Zhao M, Wang C, Yang J, et al. Identification, synthesis and bioassay for the metabolites of P6A. Bioorg Med Chem. 2003;11(23):4913–4920. doi:10.1016/j.bmc.2003.09.021
- Feng Q, Zhao M, Gan T, et al. DHDMIQK(KAP): a novel nano-delivery system of dihydroxyl-tetrahydro-isoquinoline-3-carboxylic acid and KPAK towards the thrombus. J Mater Chem B. 2016;4(36):5991–6003. doi:10.1039/C6TB00874G
- Myers DD, Hawley AE, Farris DM, et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38(5):1075–1089. doi:10.1016/S0741-5214(03)01033-4
- Jiang Y, Ji Y, Zhou IY, et al. Effects of the new thrombolytic compound LT3001 on acute brain tissue damage after focal embolic stroke in Rats. Transl Stroke Res. 2022;15(1):30–40.
- Preclinical efficacy of Repetitive Dose of LT3001 in rat embolic stroke model once a day for 6 days. [ Unpublished data]. Lumosa Therapeutics; 2018.
- Preclinical efficacy of Repetitive Dose of LT3001 in rat embolic stroke model three times a day for 6 days. [ Unpublished data]. Lumosa Therapeutics; 2019.
- Safety, tolerability, and pharmacokinetics of single dose of LT3001 drug product in healthy subjects. [ Unpublished data]. Lumosa Therapeutics; 2019.
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(2):581–641. doi:10.1161/STR.0000000000000086
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi:10.1056/NEJM199512143332401
- Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795–1803. doi:10.1056/NEJMoa1813046
- Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611–622. doi:10.1056/NEJMoa1804355
- Brunser AM, Ouyang M, Arima H, et al. No benefit of flat head positioning in early moderate–severe acute ischaemic stroke: a HeadPoST study subgroup analysis. Stroke Vasc Neurol. 2020;5(4):406–409. doi:10.1136/svn-2020-000387
- Asuzu D, Nystrom K, Amin H, et al. Modest association between the discharge modified Rankin scale score and symptomatic intracerebral hemorrhage after intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2015;24(3):548–553. doi:10.1016/j.jstrokecerebrovasdis.2014.09.034
- Brown C, Terrell K, Goodwin R, Nathaniel T. Stroke severity in ischemic stroke patients with a history of diastolic blood pressure treated in a telestroke network. J Cardiovasc Dev Dis. 2022;9(10):345. doi:10.3390/jcdd9100345
- Poupore N, Okon M, Mackey T, Nathaniel TI. Pre-stroke factors (morbitities, diet, medication, demograhics) that affect the severity of a stroke. Thromb Update. 2021;5:100073. doi:10.1016/j.tru.2021.100073