Abstract
Purpose
We aimed to evaluate the effect of intravenous esketamine combined with dexmedetomidine as supplemental analgesia in reducing intraoperative visceral pain during elective cesarean section under combined spinal-epidural anesthesia (CSEA).
Patients and Methods
A total of 269 parturients scheduled for elective cesarean section under CSEA between May 2023 and August 2023 were assessed. The parturients were randomly allocated to receiving either intravenous infusion of 0.3-mg/kg esketamine combined with 0.5-μg/kg dexmedetomidine (group ED, n=76), 0.5-μg/kg dexmedetomidine (group D, n=76), or normal saline (group C, n=76) after umbilical cord clamping. The primary outcome was intraoperative visceral pain. Secondary outcomes included the visual analog scale (VAS) score for pain evaluation and other intraoperative complications.
Results
The incidence of visceral pain was lower in group ED [9 (12.7%)] than in group D [32 (43.8%)] and group C [36 (48.6%), P <0.0001]. The VAS score was also lower in group ED when exploring abdominal cavity [0 (0), P <0.0001] and suturing the muscle layer [0 (0), P =0.036]. The mean arterial pressure was higher in group D [83 (9) mmHg] and group ED [81 (11) mmHg] than in group C [75 (10) mmHg, P <0.0001] after solution infusion. The heart rate after infusion of the solution was lower in group D [80 (12) bpm] than in group C [86 (14) bpm] and group ED [85 (12) bpm, P = 0.016]. The incidence of transient neurologic or mental symptoms was higher in group ED compared to group C and group D (76.1% vs 18.9% vs 23.3%, P<0.0001).
Conclusion
During cesarean section, 0.3-mg/kg esketamine combined with 0.5-μg/kg dexmedetomidine can alleviate visceral traction pain and provide stable hemodynamics. Parturients receiving this regimen may experience transient neurologic or mental symptoms that can spontaneously resolve at the end of the surgery.
Plain language summary
Some parturients endure experience indescribable pain and discomfort during fetal delivery. Esketamine combined with dexmedetomidine can alleviate this pain during cesarean section under combined spinal-epidural anesthesia. However, after intravenous injection of esketamine and dexmedetomidine, the parturients may experience nightmares, dizziness, hallucinations, and drowsiness, etc.
Introduction
Neuraxial anesthesia (spinal or epidural anesthesia) is the gold standard mode for cesarean section to balance the risks and benefits to both the parturient and her fetus.Citation1,Citation2 However, due to partial blockage of the splanchnic nerve, some parturients experience intraoperative visceral pain, nausea, and vomiting related to peritoneal traction during fetal delivery or handling of intraperitoneal organs.Citation3,Citation4 Visceral pain not only negatively affects parturients but also impacts the surgical procedure. In severe cases, it can result in adverse psychological sequelae that may lead to legal disputes.Citation5 Approximately one out of every 1750 parturients undergoing a cesarean section needs to be converted to general anesthesia because of insufficient neuraxial anesthesia. Additionally, 14.6% of women need additional analgesia or anesthesia.Citation6 Consequently, supplementary analgesics are often necessary during cesarean section performed under neuraxial anesthesia. Intrathecal or epidural opioids have been reported to be effective in preventing visceral pain during cesarean sections.Citation7–9 However, adverse reactions like respiratory depression, urinary retention, nausea, vomiting, and itching limit their extensive use.Citation10
Esketamine, a dextrorotatory isomer of ketamine, is roughly twice as potent as ketamine.Citation11 It has recently received widespread attention for its therapeutic effect on depression.Citation12,Citation13 A randomized controlled trial has found it can reduce postpartum depression and does not cause postoperative adverse reactions.Citation14,Citation15 In fact, ketamine and esketamine have been used to prevent hypotension,Citation11 insufficient analgesia,Citation4 and shiversCitation16 during cesarean section. Although ketamine can easily pass through the placental barrier, it is safe for newborns within a certain dose range.Citation4,Citation17 However, ketamine and its isoforms may result in tachycardia and hypertension, and may increase the incidence of dizziness, hallucination, nausea, and other adverse reactions.Citation14 Combining ketamine with dexmedetomidine can reduce these adverse effects.Citation18 Dexmedetomidine is a selective drug α-2 adrenergic agonist drug that reportedly has intrathecal administration safety in reducing shiver and stress reactions during cesarean section while prolonging sensory and motor block duration.Citation19–21 Nevertheless, intravenous administration of dexmedetomidine can also effectively reduce shivering and cardiovascular reactions during cesarean section without affecting the Apgar score of newborns.Citation22,Citation23 However, dexmedetomidine has limited analgesic effect during invasive procedures and takes effect slowly; occasionally causing bradycardia and hypotension at high doses.Citation24 Therefore, dexmedetomidine may reduce tachycardia, hypertension, salivation, and other symptoms caused by esketamine, and esketamine may reduce bradycardia and hypotension caused by dexmedetomidine.
Although the safety of intravenous administration of either esketamine or dexmedetomidine in parturients and their fetuses during cesarean section has been reported,Citation4,Citation17,Citation22,Citation23 the efficacy and safety of their combination in this population are unknown. In this study, we investigated the analgesic effects and safety of intravenous esketamine combined with dexmedetomidine after umbilical cord clamping during cesarean section under combined spinal epidural anesthesia (CSEA). We hypothesize that low-dose esketamine combined with dexmedetomidine could reduce visceral pain during cesarean section under CSEA.
Materials and Methods
Ethics
In this double-blinded, randomized controlled study, we recruited and screened 269 parturients in the Third Affiliated Hospital of Sun Yat-sen University between May 2023 and August 2023 for eligibility. The trial protocol was authorized by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (NO. II2023-095-01) and was registered on www.chictr.org.cn (ChiCTR2300071350). The registration of our clinical trial occurred prior to the start of the trial and any patient enrollment undertaken. This study was conducted in accordance with the Declaration of Helsinki. All participating parturients gave written informed consent. This report adheres to the Consolidated Standards of Reporting Trials reporting guidelines for randomized trials.
Patient Recruitment
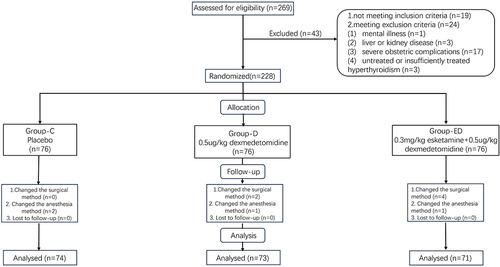
Parturients who met the following criteria were included in this trial: American Society of Anesthesiologists physical status II, age between 18 and 40 years, ongoing gestational week ≥ 37 weeks, and scheduled for elective conventional cesarean section under CSEA. The exclusion criteria were as follows: (1) previous mental illness (eg schizophrenia, cognitive dysfunction, hysteria, etc), liver disease (eg acute hepatitis, liver failure, and cirrhosis, etc), or kidney disease (eg acute renal failure, chronic renal insufficiency, etc); (2) severe obstetric complications like uterine rupture, umbilical cord prolapse, pregnancy-induced hypertension, preeclampsia, and eclampsia; (3) untreated or insufficiently treated hyperthyroidism; and (4) contraindications to intraspinal anesthesia, such as coagulation dysfunction, hemodynamic instability, or shock.
Randomization and Blinding
Random allocations were generated utilizing SPSS software, version 25.0 (IBM Corp) in a 1:1:1 proportion. The parturients were randomly and equally allocated to three groups: group C (normal saline placebo), group D (0.5-μg/kg dexmedetomidine), and group ED (0.3-mg/kg esketamine and 0.5-μg/kg dexmedetomidine). Assignments were concealed in sequentially numbered opaque envelopes. Prior to anesthesia, study coordinators who were not otherwise involved in the trial opened the envelopes in accordance with the recruitment sequence, prepared the designated solutions (each with a total volume of 20 mL), and handed them over the study drugs to the attending anesthesiologists. The attending anesthesiologist, who was blinded to the grouping, administered anesthesia and collected data. All participating parturients, attending anesthesiologists, obstetricians, other medical team members, and researchers responsible for data collection and follow-up were blinded to the grouping.
Implementation Methods
No pre-anesthesia medication was given. After entering the operating room, blood pressure readings were noninvasively taken every 3 min. Similarly, the heart rate and oxygen saturation were continuously monitored. Subarachnoid block and epidural catheterization were performed with the parturients in the left lateral decubitus position. We used AS-E needles for epidural puncture in the L3-L4 or L2-L3 intervertebral space. After determining the epidural space via the loss-of-resistance technique to air or saline with a glass syringe, the S II needle was inserted into the subarachnoid space. Once the outflow of the cerebrospinal fluid was noted, 1.7–2.2 mL of 0.75% isobaric ropivacaine was given (the parturients’ height <155cm: 1.7mL; 155–165cm: 1.8–1.9mL; 165–175cm: 2.0–2.1mL; and >175cm: 2.2cm). Finally, the anesthesiologist withdrew the spinal anesthesia needle and inserted an epidural catheter 3–4 cm in a cephalad direction. Parturients were then returned to the supine position and tilted 15° to the left. They were given oxygen at a flow rate of 5 L/min through a mask. A 3-mL test dose of 1.5% lidocaine was administered via the epidural catheter. The anesthesia plane was then assessed by inquiring whether the patient experiences a sensation akin to pinprick. After observing for 5 min, 2% lidocaine was added according to reach a sensation blockade plane between T4 and T6 levels before incision. Study drugs were administered intravenously within 10 minutes after fetal delivery and umbilical cord clamping. Specifically, group ED received 0.3-mg/kg esketamine and 0.5-μg/kg dexmedetomidine, whereas group D received 0.5-μg/kg dexmedetomidine, and group C received a normal saline injection. Fluid infusion was given as usual throughout anesthesia and surgery. The anesthesiologist will pre administer 6 mL/kg of colloid before anesthesia to prevent and treat hypotension. Subsequently, crystalloids and the remaining colloid were used to replace the amount of fluid lost due to fasting, hourly intraoperative physiological requirements, fluid redistribution during anesthesia and surgery, and intraoperative blood loss. Blood pressure was maintained within 20% from baseline and higher than 90 mmHg. When the decrease in systolic blood pressure of the parturient is greater than 20% of the baseline or lower than 90 mmHg, 50–100 ug phenylephrine is injected intravenously. When the heart rate of the parturient is less than 50 beats per minute, 0.3–0.5mg atropine is injected intravenously. For the first two postoperative days, a patient-controlled epidural analgesic pump was connected to the epidural catheter for postoperative analgesia; it had a 100-mL solution containing 135 mg ropivacaine and 6 mg morphine and was continuously infused at 2 mL/h according to the procedure.
Data Collection and Outcome Assessment
The baseline data included demographic characteristics, gestational week, pregnancy history, and times of cesarean section. Intraoperative data included surgical duration, infusion volume, use of uterotonic drug use, and vital signs. Vital signs were recorded at the following time points: when entering the operating room (t1), before the administration of study drug (t2), and after the infusion of study drug was completed (t3). Our primary outcome was the incidence of visceral traction pain during cesarean section. All patients were asked about unpleasant feelings, pain, or both at the following time points Citation25 placenta delivery (T1), exploring the abdominal cavity (T2), and suturing the muscle layer (T3). If these feelings or pains are dull, aching, ill-defined, badly localized, sometimes referred to remote areas of the body, and may be accompanied by strong motor and autonomic responses,Citation26 we determined that the parturients had experienced visceral pain. Subsequently, we assessed the pain intensity using an 11-point visual analog scale (VAS) score (0: no pain, 10: the worst pain). The incidence and intensity of pain were similarly recorded at 2 h after surgery (T4), 6 h after surgery (T5), and 24 h after surgery (T6).
Our secondary outcomes were the incidence of adverse events, including nausea, vomiting, stomach spasms, chest tightness, and chest pain caused by peritoneal traction. We also documented the occurrence of contractive pain, headache, shivers, dyspnea, hypotension, hypertension, bradycardia, tachycardia, and neurologic or mental symptoms (eg, dizziness, nystagmus, daymare, hallucination, diplopia, and somnolence). Before the patient was allowed to leave the operating room, we used the Ramsay score to assess their level of sedation (a six-point scale defined as follows: 1: awake; agitated or restless or both; 2: awake; cooperative, oriented, and tranquil; 3: awake but responds to commands only; 4: asleep; brisk response to light glabellar tap or loud auditory stimulus; 5: asleep; sluggish response to light glabellar tap or loud auditory stimulus; and 6: asleep; no response to glabellar tap or loud auditory stimulus). Adverse events were handled according to the standard procedures. The trial was terminated if the patient experienced any of the following situations: (1) conversion to general anesthesia (unable to perform VAS scoring); (2) change of surgical technique (no pelvic or abdominal exploration was performed during extraperitoneal cesarean section compared to conventional cesarean section); (3) requests from the participant to withdraw consent during the trial; and (4) lost to follow up.
Statistical Analysis
Based on our initial investigation, we hypothesized that the incidence of traction pain during cesarean section is 75% in Group C, 50% in Group D, and 27% in Group ED. To detect significant differences using multiple comparisons of proportions for treatments versus a control, with a two-sided test, an α level of 0.05 and a power of 80%, each group requires a sample size of 64 patients. Considering a potential dropout rate of approximately 20%, we have accordingly designed our study to enroll 76 patients per group. The sample size was calculated using the PASS software (version 15.0 NCSS).
The Shapiro–Wilk test was used to assess the distribution of continuous variables (Table S1). Analysis of variance (ANOVA) test was used to compare intergroup differences of variables with a normal distribution. Levene test was used for homogeneity of variance. The Kruskal–Wallis test was used for intergroup comparisons, and medians (IQR) were reported for variables with non-normal distribution. Bonferroni method was used for post - hoc tests for ANOVA and Kruskal–Wallis test. Categorical variables were examined using the Fisher’s exact test or the Pearson chi-square test and are represented by count (%). All statistical analyses were performed using SPSS for Windows, version 25.0 (IBM Corp).
Results
Patients
269 parturients were assessed for eligibility, and finally, 228 parturients were enrolled and randomized. During the study period, the surgical technique of 6 parturients were changed and 4 parturients required conversion to general anesthesia. Therefore, after excluding these individuals, 218 parturients were included in the final analysis [mean (SD) age, 32.7 (4.3) years; mean (SD) BMI, 27.1 (3.1) kg/m2; mean (SD) gestational weeks, 38.3 (0.97) weeks] (). The baseline characteristics among the three groups were comparable ().
Table 1 Baseline Data
Efficacy Outcomes
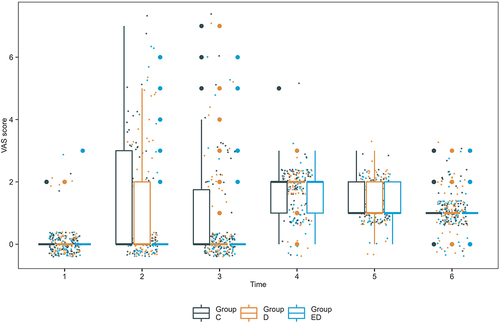
During surgery, the total incidence of visceral traction pain [presented as number (%)] was significantly lower in group ED [9 (12.7%)] than in group C [36 (48.6%)] and group D [32 (43.8%)] (P < 0.0001). However, there was no significant difference in visceral traction pain during placental delivery among the three groups. The VAS score during exploration the abdominal cavity was lower in group ED [median (IQR), 0 (0, 0)] than in group C [median (IQR), 0 (0, 3)] and group D [median (IQR), 0 (0, 2)] (P < 0.0001). In addition, the VAS score while suturing the muscle layer was also lower in group ED [median (IQR), 0 (0, 0)] than in group C [median (IQR), 0 (0, 2)] and group D [median (IQR), 0 (0, 1)](P = 0.036, , ).
Table 2 Efficacy Outcomes
Secondary Outcomes and Safety Outcomes
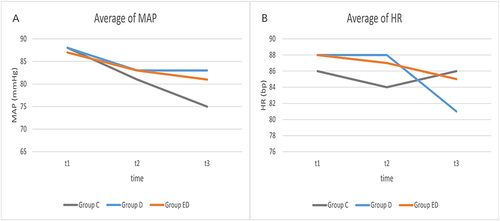
Among secondary and other outcomes, group ED showed significantly lower incidence [all P < 0.05, presented as number (%)] of stomach spasms [4 (5.6%)], contractive pain [4 (5.6%)], shivers [3 (4.2%)], hypotension [5 (7.0%)], and bradycardia [0 (0%)]. However, the incidence [presented as number (%)] of neurologic or mental symptoms [54 (76.1%)], including dizziness [52 (73.2%)], diplopia [20 (28.2%)], nystagmus [4 (5.6%)], hallucination [6 (8.5%)], and somnolence [52 (73.2%)], was higher in group ED (all P < 0.05). Group ED also showed significantly deeper sedation compared to the other two groups at the end of the surgery (P < 0.05). Among the other adverse events, the mean arterial pressure [presented as mean (SD)] was higher in group D [83 (9) mmHg] and group ED [81 (11) mmHg] than in group C [75 (10) mmHg] after infusion of the study drugs. The heart rate [presented as mean (SD)] was lower in group D [80 (12) bpm] than in group C [mean (SD), 86 (14) bpm] and group ED [85 (12) bpm] after infusion of the designated solution (, ). During the first postoperative day, the VAS scores at 2 h and 24 h after surgery were significantly different among the three groups (). Lactation time and postoperative hospitalization days were comparable among the three groups ().
Table 3 Intraoperative and Postoperative Data
Discussion
The findings of this randomized clinical study demonstrated that a single subanesthetic dose of esketamine combined with dexmedetomidine, given after fetal delivery and umbilical cord clamping, significantly reduced the incidence and intensity of visceral traction pain. This therapy could also suppress shivering without increasing the incidence of hypotension, bradycardia, nausea, and vomiting in parturients undergoing elective cesarean delivery under CSEA. However, it is worth noting that the addition of esketamine to dexmedetomidine, comparing to dexmedetomidine alone, resulted in more common neurologic and mental symptoms; however, these adverse effects were only present during the intraoperative period.
Just like other studies on cesarean section anesthesia,Citation27–29 we administered medication only after the fetus is delivered to avoid contact with these substances and reduce potential risks for the fetus. As is well known, esketamine and dexmedetomidine can easily penetrate the placenta.Citation30 Ketamine can reach the maternal venous blood level as quickly as 1 minute and 37 seconds,Citation31 producing sedative and analgesic effects on the fetus. Current researches suggest that the subanesthetic doses of ketamine do not affect the UABGA, Apgar score, and total length of hospital stay of newborns,Citation17 but may be rapidly metabolized or redistributed in the fetus.Citation4 Moreover, some studies have raised concerns that early exposure to ketamine before and after childbirth may have harmful effects on the development of the fetal immature brain.Citation32,Citation33 And there are no studies reported the long-term effects of fetal exposure to esketamine on the central nervous system. Importantly, there is no consensus or relevant guidelines on the effects of subanesthetic doses of esketamine and dexmedetomidine on the fetus. The medication instructions also do not recommend the use of these two drugs for pregnant women. Based on the above concerns, we choose to administer the medication after clamping the umbilical cord. But some of the greatest visceral stimulation and discomfort may occur during manual efforts to assist with delivery of the fetus. Further research is needed to confirm the effectiveness and safety of the drugs for pain relief before delivery.
Previous studiesCitation4,Citation11,Citation15 have shown that the commonly used dosage of esketamine during cesarean section to prevent intraoperative hypotension, shiver, and postpartum depression is 0.15–0.25 mg/kg. However, in our study we used a dosage of 0.3 mg/kg for esketamine because preliminary experiments revealed that a dosage of 0.25 mg/kg did not significantly reduce the incidence of traction pain during cesarean section. Increasing the esketamine dosage from 0.25 mg/kg to 0.3 mg/kg without changing the dexmedetomidine dosage could reduce the incidence of intraoperative traction pain by 43%.
In this study, rapid intravenous infusion of low-dose esketamine (0.3 mg/kg) and dexmedetomidine (0.5 µg/kg) after umbilical cord clamping significantly reduced the incidence of visceral traction pain during cesarean section. Although, dexmedetomidine alone cannot significantly reduce the incidence of the visceral pain, it can decrease the pain intensity during surgery steps involving peritoneal traction. Notably, group ED showed a more significant reduction in both the intensity and the incidence compared to group D regarding intraoperative visceral pain indicating that the combination of esketamine and dexmedetomidine produces better effect. In particular, this combination significantly alleviates the pain caused by visceral and peritoneal traction during intraoperative exploration of the abdominal cavity and suturing of the muscular layer. Although a large-sample randomized controlled study showed that esketamine alone at a dose of 0.25 mg/kg could not significantly alleviate intraoperative visceral pain, our study showed different Results,Citation4 possibly because we used higher dose of esketamine and combined it with low dose of dexmedetomidine. Consistent with previous studies on esketamine, our findings show clinically significant improvements in postoperative pain relief within 24 hours. For example, a previous study indicated that the use of low-dose ketamine during cesarean section resulted in lower postoperative analgesic consumption and lower VAS scores, indicating a preemptive analgesic effect.Citation34
Hypotension often occurs after neuraxial anesthesia during cesarean section and may lead to poor prognosis for both the parturients and fetus in severe cases.Citation35 In theory, esketamine has a sympathetic nerve stimulating effect,Citation36 which can increase the heart rate and blood pressure. Therefore, it may not be suitable for Patients with gestational hypertension, preeclampsia, or eclampsia. However, for pregnant women at risk of hypotension, esketamine is a recommended as an analgesic and sedative. A study demonstrated that a single intravenous injection of 0.15 mg/kg ketamine can significantly reduce the risk of hypotension during cesarean section.Citation11 Our results revealed that group ED patients had significantly higher blood pressure than controls and significantly faster heart rate than group D patients after drug injection. No patients from group ED experienced hypotension or bradycardia. A comparison of heart rate and blood pressure among the three groups showed that the combined use of the two medications offset each other’s individual adverse effect on heart rate and blood pressure. That is, the combination of esketamine with dexmedetomidine can prevent hypotension and bradycardia, and can also reduce the incidence of well-known adverse reactions to esketamine, such as hypertension, tachycardia, and headache. Moreover, our study indicated that both dexmedetomidine and esketamine can significantly reduce shivering during cesarean section, which is consistent with the finding of previous studies.Citation16,Citation37
However, it is notable that more parturients in group ED experienced neurologic or mental symptoms like dizziness, diplopia, nystagmus, hallucination, and somnolence during cesarean section. These are common adverse reactions after rapid intravenous infusion of esketamine.Citation38,Citation39 According to our observations, these reactions can spontaneously resolve within 10–20 minutes after drug infusion. Moreover, due to the separation anesthesia effect of esketamine, most parturients simply presented with exhibited dizziness and drowsiness, which did not interfere with the ability to interact with their baby after delivery. Dexmedetomidine, as a sedative drug, has an antagonizing effect on the excitatory neurologic or mental symptoms of esketamine. Notably, some parturients in the groups C and D also experienced neurologic or mental symptoms. Importantly, by utilizing dexmedetomidine to antagonize the excitatory neurologic or mental symptoms of esketamine, none of the parturients in group ED experienced excitatory neurologic or mental symptom such as irritability, multilingualism, and mania. Compared with the use of 0.25mg/kg esketamine alone, our protocol reduced the incidence of intraoperative neurologic or mental symptoms from 97.7% to 76.1%.Citation4 All these findings suggest that these neurologic or mental symptoms are mild complications such as dizzy, diplopia and somnolence. They will not have a serious or long-term impact on the parturients. In group C, 14 parturients experienced neurologic or mental symptoms, all of which were characterized by dizziness, possibly due to hypotension. Similarly, 17 parturients in group D showed neurologic or mental symptoms primarily manifested as dizziness and somnolence. These symptoms could possibly be attributed to medication effects of dexmedetomidine or hypotension. At the end of surgery, the parturients can recover to a level of consciousness comparable to preoperative status (Ramsay score = 2), and although a few patients may experience slight sedation, excessive sedation is unlikely to occur among the parturients we observed. Further clarification is needed regarding the indications for this protocol and optimal dosage of esketamine. It should only be considered when parturients need supplementary analgesia or when the benefits to the parturients outweigh the potential risks.
Limitations
This study has some Limitations. First, this was a single-center study which may limit the generalizability of the results to other populations. Caution should be exercised when extrapolating these findings to different populations. Second, we did not design a group that used esketamine alone. The purpose of designing a dexmedetomidine-alone group was to confirm whether esketamine is the main analgesic component in the mixture. While there may not be a statistically significant difference in the effect on visceral pain between using esketamine alone and using the mixture, further clarification is needed regarding the advantages of combining esketamine with dexmedetomidine compared to using esketamine alone. Third, we only tested the effect of 0.3-mg/kg esketamine combined with 0.5-μg/kg dexmedetomidine. Other dosage combination may also be useful. Further research is needed to clarify the indications, contraindications, and effects of supplementing esketamine in parturients. Fourthly, it remains unclear why patients using dexmedetomidine had a lower incidence of intraoperative hypotension compared to those in the control group; larger studies are needed to elucidate both effect and mechanism of dexmedetomidine or its combination with esketamine on hemodynamics during elective cesarean sections in parturients. Finally, since we did not conduct long-term follow-up on the parturients, the long-term impact of the medication on patients remains unknown.
Conclusion
The results of this randomized clinical trial study showed that a subanesthetic dose of esketamine and dexmedetomidine administered after clamping the umbilical cord can significantly alleviate visceral traction pain in parturients undergoing cesarean delivery under CSEA. Considering the high incidence of transient and mild reactions in the nervous system, this approach may be more suitable for parturients with severe pain.
Ethics Approval and Informed Consent
The trial protocol was authorized by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (NO. II2023-095-01) and registered under www.chictr.org.cn (ChiCTR2300071350). This study was conducted in accordance with the Declaration of Helsinki. All participating parturients gave written informed consent.
Consent for Publication
Authors confirm that the details of any images, videos, recordings, etc can be published, and that the person(s) providing consent have been shown the article contents to be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We are grateful to the obstetricians and nurses for their help to us. Thank you to Dr. Zhang Yihan for her suggestions on improving the language quality of our manuscript.
Data Sharing Statement
Under existing ethical approvals, the availability of these data is limited and therefore cannot be publicly obtained. However, upon reasonable request and with the permission of the author and the Third Affiliated Hospital of Sun Yat-sen University, data may be provided.
Additional information
Funding
References
- Klimek M, Rossaint R, van de Velde M, Heesen M. Combined spinal-epidural vs. spinal anaesthesia for caesarean section: meta-analysis and trial-sequential analysis. Anaesthesia. 2018;73(7):875–888. doi:10.1111/anae.14210
- Ng KW, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev. 2004;2012(4). doi:10.1002/14651858.CD003765.pub2
- Liu M, Wang B, Prudence B, Chen X. Effect of different doses of epidural dexmedetomidine on reducing visceral traction reaction for cesarean section: a double-blind randomized controlled trial. J Anes. 2023;37(3):371–378. doi:10.1007/s00540-023-03166-8
- L-L X, Wang C, Deng C-M, et al. Efficacy and safety of esketamine for supplemental analgesia during elective cesarean delivery. JAMA Network Open. 2023;6(4). doi:10.1001/jamanetworkopen.2023.9321
- Plaat F, Stanford SER, Lucas DN, et al. Prevention and management of intra‐operative pain during caesarean section under neuraxial anaesthesia: a technical and interpersonal approach. Anaesthesia. 2022;77(5):588–597. doi:10.1111/anae.15717
- Patel R, Kua J, Sharawi N, et al. Inadequate neuraxial anaesthesia in patients undergoing elective caesarean section: a systematic review. Anaesthesia. 2022;77(5):598–604. doi:10.1111/anae.15657
- Chen X-R, Gao T, Zhang Y, Peng M-Q. Addition of low-dose sufentanil to ropivacaine for reducing shivering and visceral traction pain during cesarean section. J Int Med Res. 2021;49(5). doi:10.1177/03000605211017000
- Lu Q, Dong C-S, J-M Y, et al. The dose response of sufentanil as an adjuvant to ropivacaine in cesarean section for relief from somato-visceral pain under epidural anesthesia in parturients with scarred uterus. Medicine. 2018;97(38):e12404. doi:10.1097/md.0000000000012404
- Ishiyama T, Yamaguchi T, Kashimoto S, Kumazawa T. Effects of epidural fentanyl and intravenous flurbiprofen for visceral pain during cesarean section under spinal anesthesia. J Anes. 2001;15(2):69–73. doi:10.1007/s005400170029
- Smith LA, Burns E, Cuthbert A. Parenteral opioids for maternal pain management in labour. Cochrane Database Syst Rev. 2018;2018(6). doi:10.1002/14651858.CD007396.pub3
- Zhang X, Wang J, X-H A, et al. Optimum dose of spinal ropivacaine with or without single intravenous bolus of S-ketamine during elective cesarean delivery: a randomized, double-blind, sequential dose-finding study. BMC Pregnancy Childbirth. 2021;21(1). doi:10.1186/s12884-021-04229-y
- McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178(5):383–399. doi:10.1176/appi.ajp.2020.20081251
- Kaur U, Pathak BK, Singh A, Chakrabarti SS. Esketamine: a glimmer of hope in treatment-resistant depression. European Arch Psy Clini Neuro. 2019;271(3):417–429. doi:10.1007/s00406-019-01084-z
- Shen J, Song C, Lu X, et al. The effect of low-dose esketamine on pain and post-partum depression after cesarean section: a prospective, randomized, double-blind clinical trial. Frontiers in Psychiatry. 2023:13. 10.3389/fpsyt.2022.1038379.
- Yang SQ, Zhou YY, Yang ST, et al. Effects of different doses of esketamine intervention on postpartum depressive symptoms in cesarean section women: a randomized, double-blind, controlled clinical study. J Affective Disorders. 2023;339:333–341. doi:10.1016/j.jad.2023.07.007
- Lema GF, Gebremedhn EG, Gebregzi AH, Desta YT, Kassa AA. Efficacy of intravenous tramadol and low-dose ketamine in the prevention of post-spinal anesthesia shivering following cesarean section: a double-blinded, randomized control trial. Int J Women’s Health. 2017;Volume 9:681–688. doi:10.2147/ijwh.S139655
- Liang Z, Zhou T, Wang M, Li Y. Neonatal outcomes when intravenous esketamine is added to the parturients transferred from labor analgesia to emergency cesarean section: a retrospective analysis report. BMC Anesthesiology. 2023;23(1). doi:10.1186/s12871-023-02132-x
- Riccardi A, Serra S, De Iaco F, Fabbri A, Shiffer D, Voza A. Uncovering the Benefits of the Ketamine–Dexmedetomidine Combination for Procedural Sedation during the Italian COVID-19 Pandemic. J Clin Med. 2023;12(9):3124. doi:10.3390/jcm12093124
- Zhang Q, L-y X, Liang W-D, et al. Intrathecal dexmedetomidine combined with ropivacaine in cesarean section: a prospective randomized double-blind controlled study. Front Med. 2022:9. 10.3389/fmed.2022.922611.
- Miao S, Shi M, Zou L, Wang G. Effect of intrathecal dexmedetomidine on preventing shivering in cesarean section after spinal anesthesia: a meta-analysis and trial sequential analysis. Drug Des Devel Ther. 2018;12:3775–3783. doi:10.2147/DDDT.S178665
- Wang Y-Q, Zhang X-J, Wang Y. Effect of intrathecal dexmedetomidine on cesarean section during spinal anesthesia: a meta-analysis of randomized trials. Drug Des Devel Ther. 2019;13:2933–2939. doi:10.2147/DDDT.S207812
- Lamontagne C, Lesage S, Villeneuve E, Lidzborski E, Derstenfeld A, Crochetière C. Intravenous dexmedetomidine for the treatment of shivering during cesarean delivery under neuraxial anesthesia: a randomized-controlled trial. Can J Anaesth. 2019;66(7):762–771. doi:10.1007/s12630-019-01354-3
- Ao L, Shi J, Bai Y, Zheng Y, Gan J. Effectiveness and safety of intravenous application of dexmedetomidine for cesarean section under general anesthesia: a meta-analysis of randomized trials. Drug Des Devel Ther. 2019;13:965–974. doi:10.2147/DDDT.S197165
- Tobias JD. Dexmedetomidine and ketamine. Pediatr Crit Care Med. 2012;13(4):423–427. doi:10.1097/PCC.0b013e318238b81c
- Hirabayashi Y, Saitoh K, Fukuda H, Shimizu R. Visceral pain during caesarean section: effect of varying dose of spinal amethocaine. Br J Anaesth. 1995;75(3):266–268. doi:10.1093/bja/75.3.266
- Cervero F. Mechanisms of acute visceral pain. Br Med Bull. 1991;47(3):549–560. doi:10.1093/oxfordjournals.bmb.a072492
- Liu S, Peng P, Hu Y, et al. The effectiveness and safety of intravenous dexmedetomidine of different concentrations combined with butorphanol for post-caesarean section analgesia: a randomized controlled trial. Drug Des Devel Ther. 2021;15:689–698. doi:10.2147/DDDT.S287512
- Liu Q-R, Zong Q-K, Ding -L-L, et al. Effects of perioperative use of esketamine on postpartum depression risk in patients undergoing cesarean section: a randomized controlled trial. J Affective Disorders. 2023;339:815–822. doi:10.1016/j.jad.2023.07.103
- H-Y Y, Wang S-Y, Quan C-X, et al. Dexmedetomidine alleviates postpartum depressive symptoms following cesarean section in Chinese women: a randomized placebo-controlled study. Pharmacotherapy. 2019;39(10):994–1004. doi:10.1002/phar.2320
- Musk GC, Netto JD, Maker GL, Trengove RD. Transplacental transfer of medetomidine and ketamine in pregnant ewes. Lab Anim. 2012;46(1):46–50. doi:10.1258/la.2011.010179
- Ellingson A, Haram K, Sagen N, Solheim E. Transplacental passage of ketamine after intravenous administration. Acta Anaesthesiol Scand. 1977;21(1):41–44. doi:10.1111/j.1399-6576.1977.tb01191.x
- Cheung HM, Yew DTW. Effects of perinatal exposure to ketamine on the developing brain. Front Neurosci. 2019;13:138. doi:10.3389/fnins.2019.00138
- Brambrink AM, Evers AS, Avidan MS, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116(2):372–384. doi:10.1097/ALN.0b013e318242b2cd
- Sen S, Ozmert G, Aydin ON, Baran N, Caliskan E. The persisting analgesic effect of low-dose intravenous ketamine after spinal anaesthesia for caesarean section. Eur J Anaesthesiol. 2005;22(7):518–523. doi:10.1017/S026502150500089X
- Gong R-S, Liu X-W, W-X L, Zhao J. Effects of colloid preload on the incidence of hypotension in spinal anesthesia for cesarean section: a systematic review and meta-analysis. Chinese Med J. 2021;134(9):1043–1051. doi:10.1097/cm9.0000000000001477
- Kienbaum P, Heuter T, Pavlakovic G, Michel MC, Peters J. S(+)-ketamine increases muscle sympathetic activity and maintains the neural response to hypotensive challenges in humans. Anesthesiology. 2001;94(2):252–258. doi:10.1097/00000542-200102000-00014
- Liu J, Wang Y, Ma W. Shivering prevention and treatment during cesarean delivery under neuraxial anesthesia: a systematic review. Minerva Anestes. 2018;84(12). doi:10.23736/s0375-9393.18.12478-3
- Gastaldon C, Raschi E, Kane John M, Barbui C, Schoretsanitis G. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Psychothe Psycho. 2021;90(1):41–48. doi:10.1159/000510703
- Feeney A, Papakostas GI. Pharmacotherapy. Psychiat Clin North Am. 2023;46(2):277–290. doi:10.1016/j.psc.2023.02.003