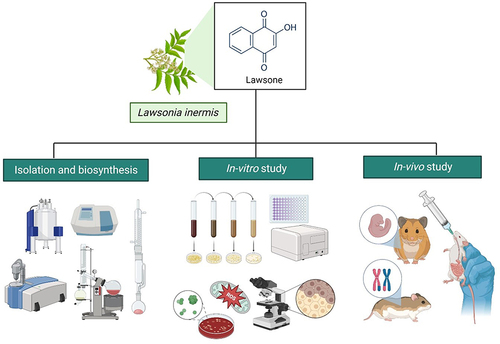
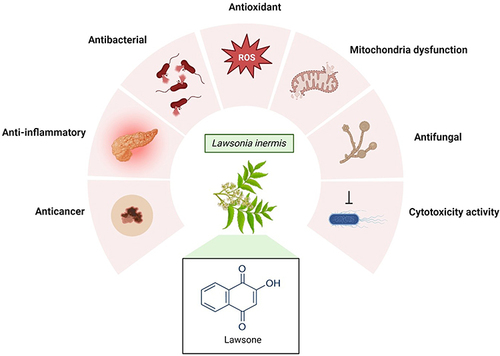
Abstract
Lawsone, a naturally occurring organic compound also called hennotannic acid, obtained mainly from Lawsonia inermis (Henna). It is a potential drug-like molecule with unique chemical and biological characteristics. Traditionally, henna is used in hair and skin coloring and is also a medicinal herb for various diseases. It is also widely used as a starting material for the synthesis of various drug molecules. In this review, we investigate on the chemistry, biosynthesis, physical and biological properties of lawsone. The results showed that lawsone has potential antioxidant, anti-inflammatory, antimicrobial and antitumor properties. It also induces cell cycle inhibition and programmed cell death in cancer, making it a potential chemotherapeutic agent. Additionally, inhibition of pro-inflammatory cytokine production makes it an essential treatment for inflammatory diseases. Exploration of its biosynthetic pathway can pave the way for its development into targets for new drug development. In future, well-thought-out clinical studies should be made to verify its safety and efficacy.
Introduction
Lawsone is chemically called 2-hydroxy-1,4-naphthoquinone, mainly obtained from Lawsonia inermis (L. inermis) is a plant ubiquitous to North Africa, the Middle East and South Asia.Citation1 L. inermis is locally known as Henna, Madurang, Mendi, Manghati, Goranti and Madayantika and, it is one of the most common family of naphthoquinone dyes.Citation2 Lawsone was first isolated in 1950s from the leaves of the henna plantCitation3 and is present at 0.5–1.5%. Its synthesis occurs via the phenylpropanoid pathway in the plant. Henna is a brown-green powder utilized in n cosmetics. It has been extensively used not only in hair coloring products but also as dyes in textiles for coloring of cloth materials like silk, wool and leatherCitation4 since 1400 BC in various parts of the world.
The amino acid tyrosine is converted to p-coumaric acid which is a precursor of lawsone. Lawsone has many pharmacological effects like anti-inflammatory, antioxidant, antibacterial, anti-fungal and anticancer properties (). Traditionally, it was used in the treatment of dermatological infections eczema, psoriasis and fungal infections.Citation5 To date, several derivatives of Lawsone scaffold are being investigated especially against cancer. In this review, we explore the phytochemistry, biosynthesis, physico-chemical properties and also the biological activities of Lawsone. In comparison to other antioxidants, lawsone offers a unique advantage due to its natural origin. While other antioxidants like vitamins C and E, beta-carotene, and flavonoids also provide protection against oxidative stress, lawsone stands out for its efficacy and potential safety profile. By exploring each characteristic of lawsone, it is hoped that a clearer picture can be envisioned to direct the focus of future research areas involving Lawsone.
Methods
Relevant literature was collected from several scientific databases, including PubMed, ScienceDirect, Scopus and Google Scholar. The literature search of the scientific evidence of lawsone published since 1980 was achieved using the following keywords: “Lawsone” OR “Lawsonia inermis” OR “Henna” OR “Benzoquinone” OR “Hennotannic acid”, AND “Chemistry” OR “Biosynthesis” OR “in vitro” OR “in vivo” OR “Biological studies” OR “Pharmacological studies” OR “Molecular mechanisms” OR “Gene expressions” OR “Toxicity studies” OR “Clinical studies” OR “Pharmacokinetics” OR “Pharmacodynamics”. Studies that were not written in English and did not have any abstracts were excluded from the initial screening. There was no restriction to be followed for collecting the studies carried out on lawsone, especially regarding the aspects of dose, route of administration, duration of treatment, or animal versus human studies. The articles were chosen for the final analysis after applying the inclusion and exclusion criteria and removing duplicates from the databases.
Phytochemistry of Lawsone
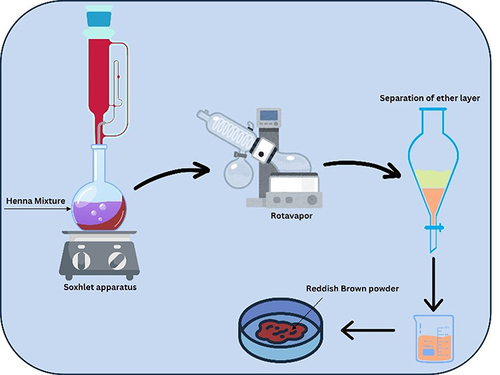
Lawsone is a perennial shrub from L. inermis that belongs to the Lythraceae family.Citation6 It can be isolated from the leaves using a soxhlet extraction method.Citation7
Isolation of Lawsone from Henna Leaves
First, approximately 40 g of powdered henna leaves were added to distilled n-hexane (1 L) with a continuous stirring for 6–7 days. The mixture was then poured into a thimble and transferred to the soxhlet apparatus to be heated for two days. Then, the solvent was evaporated using a rotavap. The residue was dissolved in 100 mL toluene before the content was transferred to a separating funnel. After the addition of 100 mL sodium hydroxide (0.2 M), the solution was shaken well and was kept aside. When two layers are formed, the aqueous layer was separated. The pH was adjusted to 3.0 using hydrochloric acid (HCl) (0.2 M). The filtrate was subjected to extraction using ethyl ether, after which the ether will turn to pale yellow. The ether solution was further extracted with 30 mL water and subsequently dehydrated using magnesium sulfate. The ether was then dried using a vacuum until a reddish-brown solid was formed (). The content was further purified using a thin-layer chromatography with ethanol: ethyl acetate (1:2)Citation8–10 as the solvent mixtures.
Chemistry
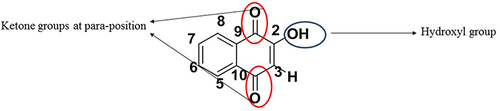
Lawsone is a fused ring system that is composed of two aromatic rings and two ketone groups (). The molecular weight is 174.16 g/mol; molecular formula is C10H6O3.Citation11 It consists of a naphthalene ring with two ketone groups and a hydroxyl group at positions 1, 4 and 2 respectively.Citation12 Some structural characterizations are as stipulated below.
UV-Visible Spectroscopy
When Lawsone is dissolved in a solvent like 0.1 M HCl and is subjected to an ultraviolet-visible (UV-Vis) radiation, it absorbs light at 334 nm to create a distinctive absorption spectraCitation13 due to the presence of its conjugated system of double bonds in the molecule.Citation14 The spectrum, however, also reveals a protracted tail of the band at 334 nm that penetrates well into the visible spectrum which contributes to its yellowish colour.Citation15 Upon removal of an acidic proton, Lawsone is deprotonated to produce an orange solution as the deprotonated form has a more distinctive absorption spectra when compared to the protonated version. The deprotonated form’s absorption maximum occurs at 453 nm which is the visible spectrum’s orange area.Citation16
FTIR Spectroscopy
The Fourier transform infrared (FTIR) spectrum of lawsone indicated a broad band ranging from 3300–3400 cm−1, indicating the OH stretching of the phenolic hydroxyl group. Two bands of high intensities are seen at 1670 cm−1 and 1630 cm−1, which represent the stretching frequencies of the free and chelated (2-hydroxyl hydrogen) carbonyl groups respectively.Citation17 Further splitting in the band at 1630 cm−1 is evident due to the delocalized interaction of the carbonyl with the close-by double bond between the naphthoquinone rings 2 and 3 positions. There are also strong bands at 1583 cm−1, corresponding to the aromatic C-C stretching frequency and 1219 cm−1, corresponding to the 2-hydroxyl group’s C-O stretching frequency.Citation18
NMR Spectroscopy
The proton nuclear magnetic resonance (H1-NMR) spectra revealed the presence of doublets at 8.10 and 8.00 corresponding to H-5 and H-8 protons. There was a multiplet at 7.88, attributable to both H-6 and H-7. At 6.52, the H-3 proton showed up as a singlet while at 11.52, the phenolic proton became visible as a wide singlet.Citation19 The carbon nuclear magnetic resonance C13-NMR spectra indicated the presence of carbonyl peaks at 180.91 and 182.30, which correspond to the C-1 and C-4 carbons, along with a peak at 156.41, representing the C-2 carbon of the hydroxyl group. The C-3 carbon was visible at 110.3, and the other six carbons were visible at 125.2, 125.8, 130.3, 131.6, 133.7 and 134.8.Citation20
Biosynthesis
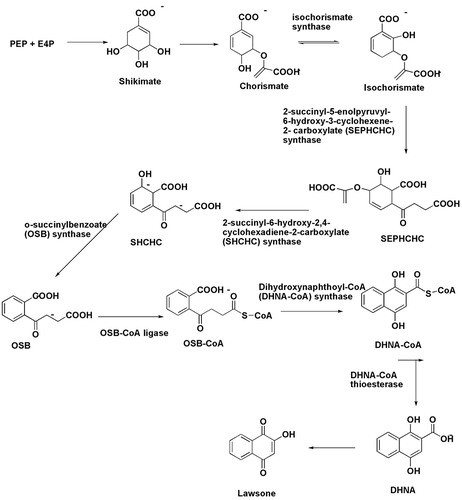
To start the production of lawsone, first, phosphoenolpyruvate (PEP) and D-erythrose 4-phosphate (E4P) are converted to shikimate via the shikimate pathway. The shikimate is subsequently transformed into chorismate, which is a precursor to produce several aromatic compounds including 1, 4-naphthoquinones in the shikimate pathway.Citation21,Citation22 The production of lawsone normally relies heavily on o-succinylbenzoate (OSB). Isochorismate synthase acts via the said route to convert chorismate to 2-succinyl-6-hydroxy-2, 4-cyclohexadiene-1-carboxylate (SHCHC).Citation23 Subsequently, the enzymes, 2-succinylbenzoate synthase and 2-succinylbenzoate-CoA ligase transform SHCHC into OSB. Two other enzymes, OSB-CoA ligase and 1, 4-dihydroxy-2-naphthoate (DHNA) synthase are used to further convert OSB into DHNA. Finally, the DHNA-CoA thioesterase enzyme converts DHNA to lawsone.Citation24,Citation25 The biosynthetic pathway was depicted in .
Biological Activities
Toxicity Profile of Lawsone
Lawsone, which is a natural dye component of henna, is another example of a hair dye chemical with apparent positive in vivo genotoxic activity. Lawsone has complicated toxicity and genetic toxicity profiles. Although it was haematotoxic in an oral sub chronic rodent study and positive/borderline-positive in the mouse lymphoma assay and a chromosome aberration test in Chinese Hamster Ovary (CHO) cells, based on CHO-hypoxanthine phosphoribosyl transferase and Syrian hamster embryo (SHE) assays, it can be negative in Salmonella.Citation26,Citation27 Despite not causing any chromosome aberrations in a series of in vivo genetic toxicity tests including 1) bone marrow chromosome aberrations in hamsters 2) sex-linked recessive Lethal Assay in Drosophila 3) unscheduled DNA synthesis test in rats and 4) chromosome aberrations in peripheral lymphocytes of sub-chronically treated rats, Lawsone causes a weak but statistically significant and reproducible increase in the frequency of micronuclei. Based on these findings, it was concluded that Lawsone is genotoxic in vivoCitation28,Citation29 which supports its external use in humans.
Anti-Bacterial Effects
There are not many effective antibiotics available against Gram-positive bacteria due to the emergence of antibiotic resistance making it a significant problem globally.Citation30 In fact, methicillin resistant staphylococcus aureus (MRSA) is the leading cause of nosocomial infections due to its acquired resistance against a wide range of commercial antibiotics. These antibiotics include β-lactams, tetracyclines, fluoroquinolones, aminoglycosides, lincosamides and even the newly licensed antibiotics, daptomycin and linezolid.Citation31
Maeh et al conducted a study on the combination of α-mangostin-rich extract (AME), lawsone methyl ether (LME) and ampicillin for their synergistic effects on MRSA. In an interaction study against the reference isolate MRSA, the researchers confirmed that there was a synergistic impact between 0.008μg/mL of AME and 0.490μg/mL LME. Additionally, they confirmed in vivo (in patients) that 0.008–0.015 μg/mL of AME and 0.49–0.98 μg/mL of LME confer a synergistic effect against MRSA and that the combination of 1.95–3.90μg/mL of AME and 0.49–1.9μg/mL of LME can synergize with 0.49μg/mL of ampicillin. The researchers also highlighted the fact that LME enhanced the anti-MRSA activity of ampicillin by significantly lowering its minimal inhibitory concentration (MIC) by up to 128-fold. Their findings advocate the potential benefit of using three different antibiotic combinations to treat MRSA: AME + ampicillin, LME + ampicillin, or both.Citation32
Sakunphueak and Panichayupakaranant (2012) compared the effectiveness of 1) lawsone, 2) lawsone methyl ether and 3) methylene-3,30–bilawsone obtained from the leaf extracts of Impatiens balsamina L. (I. balsamina or Rose balsam) (Balsaminaceae). The three compounds were tested for their ability to inhibit the growth of dermatophyte fungi, yeast, aerobic bacteria, facultative anaerobic bacteria and anaerobic bacteria by using a modified agar dilution method to determine the MIC and minimal bactericidal (MBC) or fungicidal concentration (MFC) of each organism. Their result indicated that compound 2 (ie lawsone methyl ether) had 1) the highest antibacterial activity 2) some antibacterial activity against aerobic, facultative anaerobic and anaerobic bacteria 3) some anti-fungal activity against dermatophyte fungi and Candida albicans. On the other hand, compound 1 (lawsone) exhibited only a moderate level of antimicrobial activity against dermatophytes, possessed a low level of potency against aerobic bacteria and did not have any effect on Candida albicans or facultative anaerobic bacteria. The antimicrobial activity of compound 3 (methylene-3,30–bilawsone) was limited to Staphylococcus epidermidis and Bacillus subtilis alone. Thus, the researchers concluded that all tested naphthoquinones from I. balsamina have the ability to kill bacteria and can be a good antimicrobial agent with the highest potential being lawsone methyl etherCitation33 indicating that antibiotic cocktails that incorporated natural products offer a dearth of unexplored potentials.
Rahmoun et al investigated the in vitro activity of lawsone and novel naphthoquinone derivatives against both bacterial and fungal where 100 µL of the compound solution was added into each well. From the eight compounds tested, two compounds (C17H12O2NCl and C17H12O2N) demonstrated excellent antibacterial efficacy against two gram-positive bacteria (Staphylococcus aureus and Enterococcus faecalis)Citation34 again indicating the antimicrobial potential of lawsone.
However, due to their rapid growth, multi-drug resistant (MDR) organisms pose a growing threat. Soliman et al performed a study to test the antimicrobial activities of essential oil from Calli that was extracted from the Calligonum comosum plant by a hydro-steam distillation when administered singly or combined with lawsone. Their findings indicated that the antimicrobial activities of essential oil from Calli were more effective against MDR microbes when combined with lawsone. In fact, they reported that lawsone conferred a significant antibacterial activity against MDR microorganisms between 200 and 300 µg/mL. In addition, Calli oil has a remarkable antibacterial activity against 1) MDR bacteria (between 180 and 200 µg/mL) 2) Candida spp. (between 220 and 240 µg/mL) and 3) spore forming Rhizopus fungus (at 250 µg/mL). The researchers concluded that combining Calli oil with lawsone increased the antibacterial activity of each agent by at least three-fold, while the inclusion of both natural products in a liposome can further lower their toxicities by four to eight times, while preserving the increased efficacy of the combination treatment.Citation35 Overall, their study indicated the potential of antibiotic cocktails consisting of natural products and conventional treatment should be explored further especially for MDR and MRSA organisms. Similarly, in this study, we aim to address the issue of MRSA resistance by utilising antibacterial cocktails composing of α-mangostin-rich extract and lawsone methyl ether, or by combining both with ampicillin.
Anti-Fungal Effects
Fungal infections have been consistently underestimated by the health authorities, despite the fact that they represent a serious threat to humans, animals and plants alike. In terms of humans, fungal pathogens affect more than a billion individuals all over the world, resulting in on average, almost 1.5 million deaths per year.Citation36 It is estimated that Candida, Aspergillus, Cryptococcus and Pneumocystis spp are responsible for approximately 90% of reported deaths.Citation37
Pharmaceutically active substances have long been derived from various natural compounds from plants, animals, marine creatures and even microorganisms.Citation38 Dananjaya et al conducted a study on the antifungal activity of lawsone against Fusarium oxysporum (F. oxysporum) species complex. Although F. oxysporum is known as an ascomycete facultative fungus that affects plants, recently it was identified as an opportunistic pathogen that can infect both humans and other animals. Hence, the development of new antifungal medications is necessary since F. oxysporum is resistant to most current therapies. In their study, the antifungal activity of natural lawsone derived from plants against pathogenic F. oxysporum was investigated. Following plate incubations, significant damage to the mycelium’s cell wall following lawsone treatment (50, 100 and 200 μg/mL) were seen within 24 hours, implying that lawsone may promote membrane permeability and cell disintegration, resulting in cell death. Propidium iodide uptake assays confirmed the dose-dependent manner loss of plasma membrane integrity following lawsone treatment, further confirming cell death.
The use of 2′, 7′-dichlorofluorescin diacetate to measure reactive oxygen species (ROS) has clearly demonstrated that lawsone (100 g/mL) can significantly increase the level of ROS in F. oxysporum filaments. Moreover, autophagy related-1 (ATG-1) and ATG-8 were both upregulated in response to lawsone treatment, indicating that lawsone may be used to activate autophagy-related pathways in F. oxysporum in response to oxidative stress. In summary, overall, the in vitro and in vivo data demonstrate that Lawsone is a promising anti-fusariosis drug and support the idea of employing it as a potential anti-fungal chemotherapeutic treatment.Citation39
Rahmoun et al investigated the antifungal activity of lawsone and six extracts from L. inermis from Algeria against a filamentous fungus. Their findings indicated that lawsone, which was isolated from the leaves of L. inermis, has a considerable antifungal impact against the investigated molds (F. oxysporum, A. niger, A. flavus and Penicillium sp). In fact, the commercial preparation of lawsone exhibited minimum inhibitory concentrations (MICs) against the strains of F. oxysporum (12 g/mL) and Aspergillus flavus (50 g/mL), both of which confer a high potentialCitation34 for further investigation as anti-fungals.
Cytotoxicity Activity
A study by Osman and Noort (2003) showed that lawsone exhibits cytotoxicity activity by inducing oxidative damage profile, though the type of ROS generated was not determined. Nevertheless, although it was hypothesized that lawsone was involved in the production of superoxide anion, there was no evidence of hydrogen peroxide (H2O2) generationCitation40 making some researchers believe that both lawsone and henna are cytotoxic. Their findings indicated that lawsone solution (10μL) can dose-dependently suppress the growth of Escherichia coli strains that express the mammalian form of the catalase gene obtained from both normal catalase (Csa) and catalase-deficient mutant mice (Csb). Moreover, lawsone produced negligible amounts of H2O2 in the phosphate buffer system and did not confer any signs of mutagenicity in the Ames assay regardless of its metabolic activity. Additionally, catalase reduced the zone of inhibition (ZOI) of commercially available henna products, eliminated the ZOI of lawsone in a dose-dependent manner as well as eliminated the toxicity at low concentrations of exogenous manganese-superoxide dismutase and copper- and zinc-containing superoxide dismutase. In this investigation, histidine and diethylenetriaminepentaacetic acid (DTPA), a metal chelator; beta-hydroxyacid and a low dosage of capsaicin, an inducer of NADH-quinone reductase protected Csa and Csb from lawsone. It was proposed that lawsone’s cytotoxicity involves the release of oxygen, H2O2 and OH to a certain extent.Citation41
Anticancer Effects
Globally, cancer is second to cardiovascular disease in terms of increasing trend of incidence.Citation42 It has been reported that more than 50.5 million individuals worldwide are affected with cancer, which is a long-term disease,Citation43 and is regarded as a significant public health issue. In 2020, it contributes to approximately 9.9 million fatalities, accounting for 1 in every 8 deaths (men) and 1 in every 11 deaths (women).Citation44 The global impact of rising cancer cases and prevalence rates, as well as the heterogeneity of the disease, which expresses itself in a multitude of types and specificities and the increased incidence of chemoresistance by tumour cells to antineoplastic treatment, highlight the importance of the search for new bioactive compounds with potential antitumor activity.Citation45
To date, there are only a relatively small number of reports on the anticancer efficacy of lawsone that is derived from naturally occurring sources. The majority of research is done on synthetic derivatives of lawsone, and the findings show that these derivatives have strong anticancer effects.Citation46,Citation47 Wang et al conducted a study on coloured naphthoquinone compounds derived from L. inermis plant (Lythraceae), known for its useful precursor in the production of several anticancer drugs. They also investigated the effect and mechanism of lawsone on chemically induced colon cancerous rats and human colon cancer colorectal adenocarcinoma cell line (DLD-1) cells. First, colon cancer was induced in Kyoto Apc Delta (KAD) rats by injections of azoxymethane (AOM) and instilling dextran sodium sulphate in the drinking water. The rats were orally administered with lawsone (200 mg/mL) for 8 weeks after endoscopic confirmation. To elucidate the mechanism, the researchers exposed human colon DLD-1 cancer cells to lawsone and examined its effect on cell proliferation. Lawsone inhibited abnormal crypt formation without impairing tumour pathogenesis. The histological findings from the colon further revealed a reduction in the number of adenomas and lesions while immunohistochemistry findings revealed the antiproliferative effect in adenocarcinomas while leaving normal colon mucosa cells unaffected. Lawsone retards cell cycle progression in human DLD-1 cells by lowering the expression of cyclin B1 and cyclin-dependent kinase-1 (cdk1) and inactivating NF-kB without causing apoptosis. The researchers concluded that lawsone can reduce cell proliferation in colon cancers in which the suppressive activity was not mediated by apoptosis, but rather via a reduction in the nuclear factor kappa light chain enhancer of activated B cells (NF-kB) activity leading to a reduction in cyclin B1 and cdk1 expressions.Citation48 Another study investigated the effect of lawsone on C6 glioblastoma cell viability, ROS production and mitochondrial function. The researchers reported that lawsone (1 mM) which has strong antioxidant capabilities,Citation49 confer some effects on C6 cell viability and mitochondrial function. Overall, the effectiveness of lawsone in animal studies may be explained by the spontaneous as well as bacteria-mediated breakdown of lawsone in the gastrointestinal tract, resulting in a range of derivatives that potentially can act as anticancer agents.Citation50
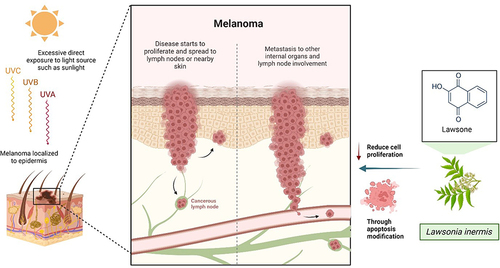
Franca et al conducted a study related to the anti-proliferative and pro-apoptotic activities of glycosidic derivatives of lawsone in melanoma cancer cells. The investigation was conducted to investigate if synthetic derivatives of lawsone have the potential cytotoxic effect against tumour cells. Thus, the researchers aimed to determine the cytotoxic activity of compounds towards three distinct tumour cell lines. Their findings demonstrated that lawsone derivatives gave a significant source of potential chemicals with cytotoxic effect against tumour cells. Compound 9 from lawsone and prepared based on Ottoni et al has remarkable activity in reducing cell proliferation and viability in melanoma cells which was primarily due to the fundamental modifications of apoptosis occurring in melanoma cellCitation51 ().
Figure 5 Anti-proliferative effect of lawsone on melanoma cancer cells. Excessive exposure to ultraviolet (UV) radiation, specifically UVA, UVB, and UVC, is a significant risk factor for the development of melanoma. Melanoma is the most aggressive and rapid-growing form of skin cancer. By modifying apoptosis, lawsone is believed and demonstrated to be able to decrease the proliferation of cancer cells. Created with Biorender.com.
In another study, Royo et al investigated on the metallic complexes of lawsone for their cytotoxic activity. First, a series of metallic complexes of the 1, 4-naphthoquinone lawsone (2–6) were synthesised and tested for their cytotoxicity in a mouse leukemic macrophagic RAW 264.7 cell line. The in vitro cytotoxic activity of all metal complexes produced was evaluated in cancer cell lines, with the copper complex of lawsone exhibiting the maximum cytotoxic activity (compound 4). The said chemical promotes apoptosis in human cancer cells through a mechanism that involves the activation of caspases 3, 8 and 9 since caspase activity is required for the biochemical and morphological changes that occur in apoptotic cells. The extrinsic and intrinsic pathways are the two main ways that initiate apoptosis in mammalian cells.
Caspase-8 is the main executer in the extrinsic pathway, which starts at the plasma membrane by activating cell surface death receptors. Caspase-9 is activated in the intrinsic pathway, which starts inside the cell. Thus, the downstream apoptotic effectors, such as caspase-3 is switched on when both caspases are activated while the regulation of various apoptosis markers such as Bcl-2 associated X protein (Bax), Bcl-2 associated agonist of cell death (Bad) and tumour protein p53 also takes place. Based on the researchers’ findings, copper’s presence in the structure may be a vital element in cell survival. In Conclusion, these findings provide compelling evidence for the apoptotic activity of 1,4-naphthoquinones, namely lawsone derivatives, particularly compound 4, give a valuable starting point for the rational development of novel anticancer medicines.Citation52 In addition, Grandis et al performed a study on ruthenium (II) complexes contained in lawsoneCitation53 which has the potential to serve as a key starting material for the synthesis of additional p-quinones with known or suspected biological action.Citation54 Due to the ease with which naphthoquinone derivatives undergo redox reactions and metal ion chelation, it is likely that the majority of their biological activities are attributed to the said feature.Citation55 Thus, the researchers conducted another study related to new lawsone Mannich bases which can enhance the anticancer activity by preparing substituted lawsone Mannich bases via Mannich reaction of lawsone, 2-pyridylcarboxaldehyde. The corresponding amine was produced following a Mannich reaction. Dodecyl amine for substance “2a”, tetradecyl amine for “2b” and hexadecyl amine for “2c” were used. The substituted novel fatty alkyl for all three substances displayed robust and specific growth inhibitory actions against a panel of human cancer cell lines associated with significant ROS production in the cancer cell.Citation56 Moreover, Grandis et al indicated that ruthenium (II) complexes, phosphine/diamine contained lawsone as bioligand improved cytotoxicity against a wide range of cancer cells and apoptosis induction in prostate cancer cells DU-145. Their findings show that these series of complexes exhibited a remarkable broad spectrum of anticancer activity, with approximately 34-fold higher activity than cisplatin and 5-fold higher than doxorubicin. The compounds inhibit the 1) growth of 3D tumour spheroids and 2) ability of DU-145 cells to maintain their colony survival after being exposed to the compounds. The mechanisms of its anticancer effect potentially include 1) decreasing the production of ROS 2) increasing the BAX/BCL-2 ratio, overall inducing apoptosis. Among these complexes, which are[Ru(law)(N-N)2] PF6 where N-N is 2.2’-bipyridine (1) or 1.10-phenanthroline (2) and [Ru(law)(dppm)(N-N)] PF6, where dppm means bis (diphenylphosphino)methane, N-N is 2.2′-bipyridine (3) or 1.10-phenanthroline (4) and law is lawsone, complex 4 shows antimetastatic potential by inhibiting DU-145 cell adhesion and migration. Hence, the researchers suggested that complex 4 is a promising candidate as a chemotherapeutic agent against prostate cancer. The therapeutic effect may be due to its ability to exhibit massive cytotoxicity effect than metal-free lawsone against many cancer cell lines and can reduce the viability of DU-145 cells at lower concentrations than cisplatin; a known anticancer drug.Citation53 On the other hand, Rani et al conducted a study on structural modification of lawsone to determine the potential activity of cytotoxicity towards two different human cancer cells which are breast cancer (MCF-7) and colon carcinoma cells (HCT-15). The modification is done by combining 2-hydroxy-1, 4-naphthoquinone (lawsone) and isonicotinoyl hydrazine with ultrasonic irradiation to synthesise N’-(1, 4-naphtho-quinone-2-yl) isonicotinohydrazide (NIH). Five different compound concentrations were added to the cancer cells (6.25, 12.50, 25.00, 50.00 and 100.00 μM). The findings yielded by the MTT assay shows that the modified compound had stronger cytotoxic action with a lower 50% minimum inhibition concentration (IC50) value which indicates its ability to kill cancer cells even at low concentration. Yet, it was more cytotoxic than lawsone. Therefore, the researchers suggested that structural alterations on lawsone may be suitable technique for creating a more potent agent.Citation57
Another study investigated on the chemoprevention of skin cancer by henna leaf powder and its pigment, lawsone (Kapadia et al 2013). The aim was to evaluate the suppressive activation of Epstein-Barr virus early antigen (EBV-EA) induced by tumour promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) in Raji cells. Their findings indicated that the chemopreventive effect of henna leaf powder and lawsone on skin cancer is achievable when orally delivered via drinking water containing 0.0025% lawsone. There was a significant reduction in tumour incidence (72%) and multiplicity (50%) as compared to the control. Interestingly, skin cancer in mice generated by 7, 12-dimethylbenz(a)anthracene and enhanced by TPA was also inhibited by topically administered lawsone (15 ug/mL). In fact, lawsone-treated groups had delayed tumour appearance by 1–2 weeks when compared to the control in all three models. Thus, the researchers underline the importance of evaluating the henna-derived green chemopreventive in combination with currently utilized sunscreen medications for their potential to act as a supplementary anticancer agent against UV-induced skin cancer.Citation58
Mitochondria Dysfunction
Xavier et al conducted a study on lawsone’s activity on mitochondrial dysfunction and mitophagy stimulation. The researchers utilised the yeast Saccharomyces cerevisiae to investigate the biological effects of lawsone.Citation59 To date, only a paper has been published on the use of S. cerevisiae for investigation of the toxicity of lawsone and its derivative compounds (Annaisi et al, 2018). After conducting their research, the authors concluded that lawsone was significantly less physiologically active than its derivatives. Furthermore, when evaluated at the concentrations used, lawsone was less toxic to cells that had a deletion in the YAP1 gene as compared to the derivatives.Citation60 In yeast, the YAP1 gene provides instructions for directing the primary oxidative stress response and YAP1 mutant cells are extremely susceptible to oxidant molecules when overexposed to oxidants.Citation61 In the study by Xavier et al based on the BY4741 model strain, the MIC was reported to be 229 mmol/L, while the sub-MIC threshold was 172 mmol/L. Interestingly, YAP1 deletion mutant was sensitive to lawsone, regardless of whether oxygen was present. Lawsone confers an adverse effect on yeast growth in glycerol, indicating that it interfered with the respiratory metabolic process. The presence of thiol groups in the intracellular environment did not imply the presence of extensive oxidative stress as well as the inclusion of the antioxidant N-acetylcysteine (NAC) enhanced toxicity for lawsone. By examining the sensitivity of ATG mutant strains as well as the localization of the GFP-ATG8 fusion protein, it was determined that lawsone largely causes mitochondrial dysfunction, which results in an indirect oxidative stress. Lawsone also activates the autophagic response, which ultimately results in the induction of mitophagy.Citation59
Anti-Inflammatory
The anti-inflammatory cytokines such as tumour necrosis factor-alpha (TNF)-α, interleukin-1β and vascular endothelial growth factor (VEGF) play critical roles in the inflammatory process. Among the pro-inflammatory cytokines, TNF-α, IL-1β and VEGF are important.Citation62 In recent years, anti-inflammatory agents, such as those that reduce the activity of specific cytokines or their receptors (anticytokine therapies) tend to 1) block lymphocyte trafficking into tissues 2) prevent the binding of monocyte-lymphocyte co-stimulatory molecules or 3) deplete B-lymphoblasts have been developed. Anti-inflammatory agents are currently used to treat autoimmune disorders such as rheumatoid arthritis, inflammatory bowel disease, psoriasis and multiple sclerosis.Citation63 Vančo et al conducted a study on copper (II)-lawsone complexes which may have both in vitro and in vivo anti-inflammatory activities. The complexes of copper(II)-lawsone obtained from the general composition of [Cu(Law)2(LN)x(H2O)(2-x)] yH2O; where HLaw= 2-hydroxy-1,4-naphthoquinone, x = 1 when LN = pyridine (1) and 2-aminopyridine (3) and x = 2 when LN = imidazole (2) 3-aminopyridine (4) 4-aminopyridine (5) 3-hydroxypyridine (6) and 3.5-dimethylpyrazole (7). Their findings indicate that complexes 3–7 have the ability to strongly inhibit the activation of nuclear factor B (NF-B) at 100 nM as induced by lipopolysaccharide (LPS) and TNF-α, which was comparable to that of the reference medication prednisone (1 mM). Moreover, following LPS activation of THP-1 cells, all of the complexes 1–7 significantly reduced the levels of secreted TNF-α, demonstrating their anti-inflammatory potential through both NF-B moderation and other mechanisms, such as conferring the influence on TNF-α transcription and translation and/or secretion, respectively. Among these complexes, the most active complexes 1–3 which are administered in a dose equivalent to 40 mol Cu/kg, had a similar effect to the control drug indomethacin (10 mg/kg) and can decrease the likelihood of oedema that was induced by subcutaneous application of λ-carrageenan on the rats’ paw. The acquired results significantly contribute to the understanding of copper (II) complexes’ biological activities, and they may be used as a starting point for the synthesis of new anti-inflammatory active complexes containing 1, 4-naphthoquinones as ligands in the future.Citation64
Biradar and Veeresh (2013) investigated the effectiveness of lawsone in treating L-arginine-induced acute pancreatitis after a period of 24 hours. Serum levels of amylase, lipase and pro-inflammatory cytokines [TNF-α, C-reactive proteins and interleukin (IL)], pancreatic myeloperoxidase (MPO) activity, lipid peroxidation [thiobarbituric acid reactive substances (TBARS)] were measured. Treatment with lawsone and methylprednisolone significantly suppressed the increase in pancreatic wet weight/body weight ratio as induced by L-arginine. These treatments also decreased serum levels of amylase and lipase, as well as TNF-α and IL-6 while significantly lowering the pancreatic levels of MPO, TBARS and nitrate/nitrite. The outcomes of the histoimmunological study further established the amelioration of pancreatic injury by lawsone. Additionally, the data further confirmed that lawsone possesses anti-inflammatory and antioxidant agent properties.Citation65
Side Effects of Lawsone
Henna is frequently utilized in many beauty products for human and is generally regarded as safe for external use. On the other hand, several case reports of adult and child hemolytic crises following the use of henna have also been documented, some of which have led to deaths.Citation66 Notably, vulnerability to henna haemotoxicity appears to be specific to G6PD-deficient individuals.Citation67,Citation68 Henna’s active component, lawsone, is believed to cause haemotoxic effects. When administered, lawsone has been demonstrated to induce a hemolytic response in rats, as revealed by the decreased in hematocrits, lower haemoglobin levels and higher spleen/body weight ratios.Citation69 Additionally, the discovery of circulating erythrocytes carrying Heinz bodies raises the possibility that oxidative damage to the red cell is what causes the hemolytic response to its in vivo administration. Overall, the findings categorised lawsone as an oxidative stress-type hemolytic agent.
Structurally Related Compounds of Lawsone
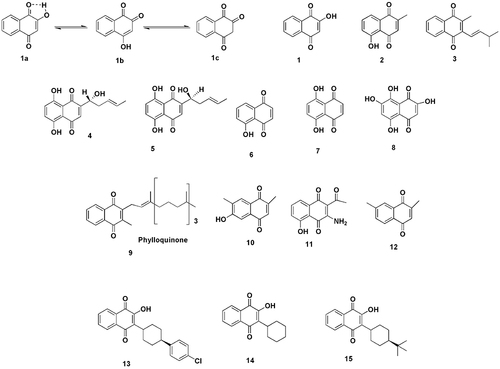
The leaves and stems of henna may be used to produce lawsone (1), a naturally occurring hydroxynaphthoquinoneCitation70,Citation71 and have significant biological effects including anti-malarial, anti-bacterial, anti-fungal and anti-cancer qualities. Lawsone’s chemical formula is C10H6O3 and its melting point is 190°C. It exists in three tautomeric forms; the most stable being the 1, 4-naphthoquinone structure (1a), followed by the 1, 2-naphthoquinone (1b) and the 1, 2, 4-naphthotrione (1c). Despite being the least stable, the trione system is likely to be present in equilibrium in solution with the other two tautomeric forms. The stability of the 1.4 isomer results from the cancellation of carbonyl groups’ dipolar moments, combined with intramolecular hydrogen bonds.Citation72
Some of the related natural hydroxynaphthoquinones such as plumbagin (2) are used to treat leprosy and tuberculosis. They are mostly derived from the roots of plumbago scandens L.Citation73 Shikone (4) is the primary component of the red extracts of lithospermum erythrorhizon plant roots. Lapachole (3) is isolated from the heartwood of plants of the genus Tabebuia spp., Tecoma spp. and tecomella undulata while the alkaline enantiomer (5) is found in the roots of Alkanna tinctoria.Citation74 The roots, leaves, nuts, bark and wood of black walnut (Juglans nigra), European walnut (Juglans regia) and American white walnut (Juglans cinerea) are where juglone (6) is found.Citation75 The wood bark tree species lomatiaobliqua and alkana naturally generate the chemical naphthazarin (7).Citation76 The molecule mompain (8), which has intriguing biological characteristics was discovered from the fungus helicobasidium mompa.Citation77
Further plant derived 1.4-naphthoquinones also consists of a class of specialized metabolites phylloquinone (9), 6-hydroxy-2-isopropyl7-methyl-1, 4-naphthoquinone (10), goniothalaminone (11) and chimaphilin (12) are known to mediate numerous plant–biotic interactions. This class of compounds presents a remarkable case of convergent evolution and spread throughout vascular plants and their production occurs via one of four biochemically distinct pathways.Citation78
Atovaquone (13), a synthetic 2-hydroxy-1, 4-naphthoquinone, works as coenzyme-Q and specifically inhibits P. falciparum by interfering with the parasite’s mitochondrial electron transport, as demonstrated by several investigations. Atovaquone is utilised for the prevention and treatment of simple tropical malaria (42–44). The relevance of this family of compounds is demonstrated by the use of parvaquone (14) and buparvaquone (15), which are 2-hydroxy-1,4-naphthoquinones substituted at position 3 and utilised as medications for the treatment of pneumonia brought on by Pneumocystis pneumonia, toxoplasmosis, malaria and leishmaniasis.Citation79 A series of aminonaphthoquinone derivatives (16–18) were prepared and found to have antibacterial and antimalarial activitiesCitation80 ().
Figure 6 Structurally relevant molecules of lawsone (1a-1c and 1–15). The main body text contains information on the numbered molecules. Created with ChemDraw Ultra 8.0.
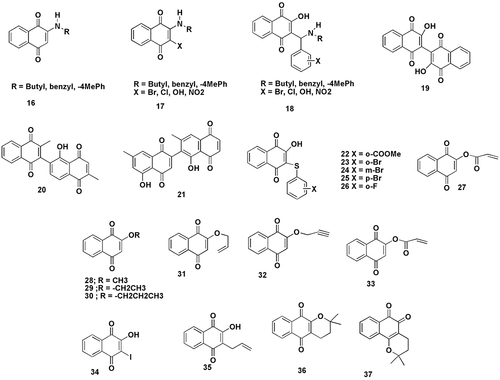
Bislawsone (19) was synthesised by the dimerization of lawsone which binds two lithium atoms, forming a coordination sphere between the 1-carbonyl and the 2-hydroxyl group oxygen atoms at each naphthoquinone ring of the dimer. The electrochemical performance displayed by bislawsone indicates that it is a cost-efficient and environmentally friendly alternative to organic and inorganic intercalation compounds. At the same time, other bis-1,4-naphthoquinone compounds such as chitranone (20) and diospyrin (21) were also synthesised by the acetate polymalonate pathway in the plants.Citation81
A series of non-toxic dyes of lawsone thiophenyl derivatives (22–26) were reported and showed bathochromic shifts when compared to lawsone. Recently, interest was shown in the use of dye-sensitized solar cells.Citation82
Colored polymers of lawsone exhibiting a high degree of thermal stability can be prepared by using the monomer acryloyl lawsone (27) following treatment of lawsone with acryloyl chloride and triethylamine as a moderate base.Citation8
In another study, molecules that were closely related to lawsone; five O-alkyl-lawsone derivatives with alkyl groups of different complexity namely, methyl (28), ethyl (29), isopropyl (30), allyl (31) and propynyl (32)]; an acryloyl ester (33); the iodinated compound (34) and the derivative with an allyl group at C-3 (35) were synthesised and tested for their cytotoxic effect. The O-alkylated active molecules showed full growth inhibition. Esterification, rather than etherification, rendered non-toxic derivatives. The presence of additional functional groups at C-3 of the quinone ring rarely improved toxicity.Citation83 Additionally, related compounds of lawsone like α–lapachone (36) and β-lapachone (37) were also synthesised by multicomponent reactionsCitation84 ().
Figure 7 Structurally relevant molecules of lawsone (16-37). The main body text contains information on the numbered molecules. Created with ChemDraw Ultra 8.0.
Further O-acyl (38–49) and O-alkyl derivatives (38a-49a) of lawsone with different linear chain lengths were reported (). These compounds were tested on etiolated wheat coleoptiles, standard Target Species (STS) and four weeds, namely Echinochloa crus-galli L., Lolium rigidum Gaud., Lolium perenne L. and Aveda fatua L. The findings showed a strong influence of lipophilicity, and, in most cases, the data fitted a log P-dependent quadratic mathematical model. The effects produced were mostly stunting, with necrosis caused by growth inhibition.Citation85
Figure 8 Structurally relevant molecules of lawsone (38-54). The main body text contains information on the numbered molecules. Created with ChemDraw Ultra 8.0.
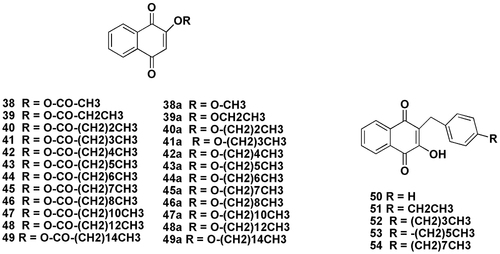
Apart from the above, lawsone (2-hydroxy-1, 4-naphthoquinone)-based compounds (50–54) have also been reported to confer some antimicrobial effects against methicillin-resistant Staphylococcus aureus (MRSA). The therapeutic efficacy of the compound was validated using murine models of wound infection as well as non-lethal systemic infection as induced by MRSA.Citation86
Future Perspectives of Lawsone
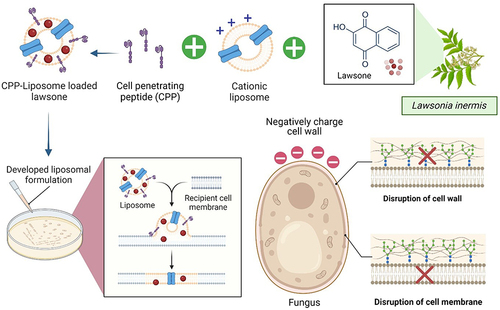
As a result of their instability and constrained solubility, bioactive compounds such as lawsone have certain drawbacks. Despite the numerous benefits of lawsone, such as its antifungal, antibacterial, and anticancer properties, its hydrophobic nature results in poor solubility and low bioavailability in biological systems, thereby reducing its efficacy. The application of nanotechnology can enhance the stability and therapeutic efficacy of phytochemicals,Citation86 drugs,Citation87,Citation88 theranostic agents,Citation89 and herbal extracts.Citation90,Citation91 Liposomes are typically constituted of naturally or synthetically derived phospholipids with cholesterol; once formed, the lipid bilayer is capable of encapsulating hydrophilic compounds while the inner core may enclose hydrophobic molecules.Citation92 The surface of the liposomes can be designed to avoid recognition by the mononuclear phagocyte system,Citation93,Citation94 thereby extending their circulation duration and improving cell uptake. As an alternative to facilitating drug delivery to target sites and improving therapeutic efficacy, in-depth research on the potential delivery design should be conducted in the future. Based on this study, we propose to encapsulate lawsone into cell-penetrating peptide (CPP)-conjugated cationic liposomes against fungal infections (). Due to the electrostatic interaction between the positively charged cationic liposome formulation and the negatively charged fungal cell wall, the formulation will be specifically targeted against the fungus, and once deposited on the surface, the presence of a cell-penetrating peptide on the liposomes’ surface enables the developed formulation to penetrate the fungal cell,Citation95 and subsequently release lawsone. The membrane disruption caused by lawsone will result in the death of fungi.Citation96
Figure 9 Future perspectives of encapsulation lawsone into cell-penetrating peptide (CPP)-conjugated cationic liposomes against fungal infections. The liposomes will be attracted to the fungus via electrostatic attraction and proceed to penetrate the cell membrane of the fungus via endocytosis with the aid of CPP before releasing the bioactive compound lawsone. Lawsone will disrupt the cell wall and cell membrane, leading to further cell lysis and ultimately fungal death. Created with Biorender.com.
Conclusion
The Henna (L. inermis) plant contains lawsone, a colorant component used in hair and textile dyeing. It has been utilised for centuries to treat or prevent a wide range of diseases and to enhance overall health. Scientists have recently focused more on the potential therapeutic benefits and mechanisms of action of this plant, particularly to lawsone, its primary constituent. Lawsone has antibacterial, anti-inflammatory, anticancer, and antioxidant properties. Derivatives of lawsone that also have strong antifungal and anticancer properties. Further investigation is warranted to ascertain the relevant mechanism and identify the molecular targets that mediate lawsone’s beneficial effects on health. In addition, the protective benefits of lawsone have not yet been confirmed in clinical trials, and more safety evaluations are required to discover any possible side effects of lawsone for long-term usage in humans. Therefore, more research is required to confirm their clinical efficacy and safety profile.
Disclosure
Graphical abstract was created with BioRender.com. The authors report no conflicts of interest in this work.
Data Sharing Statement
All data provided in the manuscript are from cited published studies.
Additional information
Funding
References
- Semwal RB, Semwal DK, Combrinck S, Cartwright-Jones C, Viljoen A. Lawsonia inermis L.(henna): ethnobotanical, phytochemical and pharmacological aspects. J Ethnopharmacol. 2014;155(1):80–103. doi:10.1016/j.jep.2014.05.042
- Bhattacharyya S, Ghosh H, Covarrubias-Zambrano O, et al. Anticancer activity of novel difluorinated curcumin analog and its inclusion complex with 2-hydroxypropyl-β-cyclodextrin against pancreatic cancer. Int J Mol Sci. 2023;24(7):6336. doi:10.3390/ijms24076336
- Fraser CM, Chapple C. The phenylpropanoid pathway in Arabidopsis. Am Soc Plant Biol. 2011;9:2
- Kasiri MB, Safapour S. Natural dyes and antimicrobials for green treatment of textiles. Environ. Chem. Lett. 2014;12(1):1–13. doi:10.1007/s10311-013-0426-2
- Barani M, Mirzaei M, Torkzadeh-Mahani M, Nematollahi MH. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: a Nano-herbal treatment for Cancer. DARU J Pharma Sci. 2018;26(1):11–17. doi:10.1007/s40199-018-0207-3
- Al-Snafi AE. A review on Lawsonia inermis: a potential medicinal plant. Int J Curr Pharma Res. 2019;11(5):1–13.
- Ashnagar A, Shiri A. Isolation and characterization of 2-hydroxy-1, 4-naphthoquinone (lawsone) from the powdered leaves of henna plant marketed in Ahwaz city of Iran. Int J ChemTech Res. 2011;3(4):1941–1944.
- Mahkam M, Nabati M, Rahbar Kafshboran H. Isolation, identification and characterization of lawsone from henna leaves powder with soxhlet technique. Quart J Iranian Chem Commun. 2014;2(1):1–81.
- Alia B, Bashir A, Tanira M. Anti-inflammatory, antipyretic, and analgesic effects of Lawsonia inermis L.(henna) in rats. Pharmacology. 1995;51(6):356–363. doi:10.1159/000139347
- Hasan MM, Abu Nayem K, Anwarul Azim AYM, Ghosh NC. Application of purified lawsone as natural dye on cotton and silk fabric. J Text. 2015;2:201511.
- Olyaei A, Sadeghpour M. A review on lawsone-based benzo [a] phenazin-5-ol: synthetic approaches and reactions. RSC Adv. 2022;12(22):13837–13895. doi:10.1039/D2RA02139K
- Salunke-Gawali S, Kathawate L, Shinde Y, Puranik VG, Weyhermüller T. Single crystal X-ray structure of Lawsone anion: evidence for coordination of alkali metal ions and formation of naphthosemiquinone radical in basic media. J Mol Struct. 2012;1010:38–45. doi:10.1016/j.molstruc.2011.11.015
- Ikhmal W, Yasmin M, Maria M, et al. Evaluating the performance of andrographis paniculata leaves extract as additive for corrosion protection of stainless steel 316l in seawater. Int J Corros Scale Inhib. 2020;9(1):118–133.
- Singh N, Aazam ES, Riaz U. Synthesis and characterization of lawsone incorporated singlet oxygen generating conjugated polymers: experimental and computational studies. J Mol Struct. 2021;1240:130533. doi:10.1016/j.molstruc.2021.130533
- Khadtare SS, Ware AP, Salunke-Gawali S, Jadkar SR, Pingale SS, Pathan HM. Dye sensitized solar cell with lawsone dye using a ZnO photoanode: experimental and TD-DFT study. RSC Adv. 2015;5(23):17647–17652. doi:10.1039/C4RA14620D
- Babula P, Mikelova R, Potesil D, et al. Simultaneous determination of 1, 4-naphtoquinone, lawsone, juglone and plumbagin by liquid chromatography with UV detection. Biomed Papers. 2005;149(1):25–28.
- Singh RP, Ramakant N. Preparation and evaluation of phytosome of lawsone. Int J Pharm Sci Res. 2015;6(12):5217–5226.
- Salunke-Gawali S, Pereira E, Dar UA, Bhand S. Metal complexes of hydroxynaphthoquinones: lawsone, bis-lawsone, lapachol, plumbagin and juglone. J Mol Struct. 2017;1148:435–458. doi:10.1016/j.molstruc.2017.06.130
- Olyaei A, Mohamadi A, Rahmani N. Green synthesis of new lawsone enaminones and their Z/E (C [double bond, length as m-dash] C)-isomerization induced by organic solvent. RSC Adv. 2021;11(21):12990–12994. doi:10.1039/D1RA01858B
- Nariya P, Shukla F, Vyas H, Devkar R, Thakore S. Synthesis and characterization of Mannich bases of lawsone and their anticancer activity. Synth Commun. 2020;50(11):1724–1735. doi:10.1080/00397911.2020.1755440
- Bakkali A, Jaziri M, Foriers A, Vander Heyden Y, Vanhaelen M, Homes J. Lawsone accumulation in normal and transformed cultures of henna, Lawsonia inermis. Plant Cell Tissue Organ Culture. 1997;51(2):83–87. doi:10.1023/A:1005926228301
- Foong LC, Chai JY, Ho ASH, Yeo BPH, Lim YM, Tam SM. Comparative transcriptome analysis to identify candidate genes involved in 2-methoxy-1, 4-naphthoquinone (MNQ) Biosynthesis in Impatiens balsamina L. Sci Rep. 2020;10(1):16123. doi:10.1038/s41598-020-72997-2
- Floss HG. The shikimate pathway. Biochem Plant Phenolics. 1979;2:59–89.
- Samanta D, Das D, Sinha S, et al. Transcriptome analysis reveals upregulated secondary metabolite pathways in micropropagated Lawsonia inermis L. Vegetos. 2023;3:1–9.
- Derksen GC, Van Beek TA. Studies in natural products chemistry; 2002:629–684.
- Kirkland D, Marzin D. An assessment of the genotoxicity of 2-hydroxy-1, 4-naphthoquinone, the natural dye ingredient of Henna. Mutat Res Genet Toxicol Environ Mutagen. 2003;537(2):183–199. doi:10.1016/S1383-5718(03)00077-9
- Narayanan SV, Kumar M, Gnanaraj VR, Rajan SC, Selvaraj V, Ananthakumar S. 2‐Hydroxy 1, 4, naphthoquinone [Lawsone] integrated rare‐earth hybrid materials as biocompatible UV/IR filter agents. MedComm Biomat Appl. 2023;2(1):e29. doi:10.1002/mba2.29
- Nohynek GJ, Fautz R, Benech-Kieffer F, Toutain H. Toxicity and human health risk of hair dyes. Food and Chemical Toxicology. 2004;2(4):517–543. doi:10.1016/j.fct.2003.11.003
- Muheyuddeen G, Divya SR, Verma S, Gautam SK, Gupta SK. Lawsonia inermis Linnaeus: pharmacological peculiarity and modern progression. Res J Pharma Phytochem. 2023;15(1):63–76. doi:10.52711/0975-4385.2023.00010
- Dabhade A, Patel P, Patil U. Proteinaceous protease inhibitor from Lawsonia inermis: purification, characterization and antibacterial activity. Nat Prod Commun. 2013;8(10):193457. doi:10.1177/1934578X1300801033
- Panichayupakaranant P, Septama AW, Sinviratpong A. Synergistic activity of lawsone methyl ether in combination with some antibiotics and artocarpin against methicillin-resistant Staphylococcus aureus, Candida albicans, and Trychophyton rubrum. Chin Herb Med. 2019;11(3):321–325. doi:10.1016/j.chmed.2019.06.001
- Charoensup R, Duangyod T, Palanuvej C, Ruangrungsi N. Pharmacognostic specifications and lawsone content of Lawsonia inermis leaves. Pharmacogn Res. 2017;9(1):60. doi:10.4103/0974-8490.199775
- Sakunphueak A, Panichayupakaranant P. Comparison of antimicrobial activities of naphthoquinones from Impatiens balsamina. Nat Prod Res. 2012;26(12):1119–1124. doi:10.1080/14786419.2010.551297
- Rahmoun N, Boucherit-Otmani Z, Boucherit K, Benabdallah M, Choukchou-Braham N. Antifungal activity of the Algerian Lawsonia inermis (henna). Pharm Biol. 2013;51(1):131–135. doi:10.3109/13880209.2012.715166
- Soliman SS, Alsaadi AI, Youssef EG, et al. Calli essential oils synergize with lawsone against multidrug resistant pathogens. Molecules. 2017;22(12):2223. doi:10.3390/molecules22122223
- Almeida F, Rodrigues ML, Coelho C. The still underestimated problem of fungal diseases worldwide. Front Microbiol. 2019;10:214. doi:10.3389/fmicb.2019.00214
- Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi. 2017;3(4):57. doi:10.3390/jof3040057
- Pettit RK. Mixed fermentation for natural product drug discovery. Appl. Microbiol. Biotechnol. 2009;83(1):19–25. doi:10.1007/s00253-009-1916-9
- Dananjaya S, Udayangani R, Shin SY, et al. In vitro and in vivo antifungal efficacy of plant based lawsone against Fusarium oxysporum species complex. Microbiol Res. 2017;201:21–29. doi:10.1016/j.micres.2017.04.011
- Osman A, Van Noort P. Evidence for redox cycling of lawsone (2‐hydroxy‐1, 4‐naphthoquinone) in the presence of the hypoxanthine/xanthine oxidase system. J Appl Toxicol. 2003;23(4):209–212. doi:10.1002/jat.908
- Sauriasari R, Wang D-H, Takemura Y, et al. Cytotoxicity of lawsone and cytoprotective activity of antioxidants in catalase mutant Escherichia coli. Toxicology. 2007;235(1–2):103–111. doi:10.1016/j.tox.2007.03.019
- Hoyert DL, Xu J. National vital statistics reports. Cent Dis Control Prevent. 2012;21:2
- International Agency for Research on Cancer W. Estimated number of new cases in 2020, worldwide, both sexes, all ages. Cancer Today; 2021. Available from: https://gco.iarc.fr/today/online-analysis-table. Accessed July 3, 2024.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. doi:10.3322/caac.21660
- Yeldag G, Rice A, Del Río Hernández A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers. 2018;10(12):471. doi:10.3390/cancers10120471
- Hamdoun S, Fleischer E, Klinger A, Efferth T. Lawsone derivatives target the Wnt/β-catenin signaling pathway in multidrug-resistant acute lymphoblastic leukemia cells. Biochem Pharmacol. 2017;146:63–73. doi:10.1016/j.bcp.2017.10.008
- Oliveira KM, Liany L-D, Corrêa RS, Deflon VM, Cominetti MR, Batista AA. Selective Ru (II)/lawsone complexes inhibiting tumor cell growth by apoptosis. J Inorg Biochem. 2017;176:66–76. doi:10.1016/j.jinorgbio.2017.08.019
- Wang S-B, Tao Z, Li P. Lawsone suppresses azoxymethane mediated colon cancer in rats and reduces proliferation of DLD-1 cells via NF-κB pathway. Biomed. Pharmacother. 2017;89:152–161. doi:10.1016/j.biopha.2017.01.169
- Majiene D, Kuseliauskyte J, Stimbirys A, Jekabsone A. Comparison of the effect of native 1, 4-naphthoquinones plumbagin, menadione, and lawsone on viability, redox status, and mitochondrial functions of C6 glioblastoma cells. Nutrients. 2019;11(6):1294. doi:10.3390/nu11061294
- Yang L, Cai T, Ding D, et al. Biodegradation of 2-hydroxyl-1, 4 naphthoquinone (lawsone) by Pseudomonas taiwanensis LH-3 isolated from activated sludge. Sci Rep. 2017;7(1):6795. doi:10.1038/s41598-017-06338-1
- de Franca MNF, Isidório RG, Bonifacio JHO, et al. Anti-proliferative and pro-apoptotic activity of glycosidic derivatives of lawsone in melanoma cancer cell. BMC Cancer. 2021;21(1):1–13. doi:10.1186/s12885-021-08404-4
- Oramas-Royo S, Torrejon C, Cuadrado I, et al. Synthesis and cytotoxic activity of metallic complexes of lawsone. Bioorg. Med. Chem. 2013;21(9):2471–2477. doi:10.1016/j.bmc.2013.03.002
- De Grandis RA, Santos PWdS D, de Oliveira KM, et al. Novel lawsone-containing ruthenium (II) complexes: synthesis, characterization and anticancer activity on 2D and 3D spheroid models of prostate cancer cells. Bioorg. Chem. 2019;85:455–468. doi:10.1016/j.bioorg.2019.02.010
- Borade AS, Kale BN, Shete RV. A phytopharmacological review on Lawsonia inermis (Linn.). Int J Pharm Life Sci. 2011;2(1):536–541.
- Pradhan R, Dandawate P, Vyas A, et al. From body art to anticancer activities: perspectives on medicinal properties of henna. Curr Drug Targ. 2012;13(14):1777–1798. doi:10.2174/138945012804545588
- Mahal K, Ahmad A, Schmitt F, et al. Improved anticancer and antiparasitic activity of new lawsone Mannich bases. Eur J Med Chem. 2017;126:421–431. doi:10.1016/j.ejmech.2016.11.043
- Rani PK, Fernandez A, George A, et al. Synthesis, spectral characterization, molecular structure and pharmacological studies of N’-(1, 4-naphtho-quinone-2yl) isonicotinohyWdrazide. Spectrochimica Acta Part A. 2015;135:1156–1161. doi:10.1016/j.saa.2014.07.092
- Kapadia GJ, Rao GS, Sridhar R, et al. Chemoprevention of skin cancer: effect of Lawsonia inermis L.(Henna) leaf powder and its pigment artifact, lawsone in the Epstein-Barr virus early antigen activation assay and in two-stage mouse skin carcinogenesis models. Anti Cancer Agent Med Chem. 2013;13(10):1500–1507. doi:10.2174/18715206113139990096
- Xavier MR, Santos MMS, Queiroz MG, de Lima Silva MS, Goes AJS, De Morais MA. Lawsone, a 2-hydroxy-1, 4-naphthoquinone from Lawsonia inermis (henna), produces mitochondrial dysfunctions and triggers mitophagy in Saccharomyces cerevisiae. Mole Biol Rep. 2020;47:1173–1185. doi:10.1007/s11033-019-05218-3
- Sadhukhan P, Saha S, Sinha K, Brahmachari G, Sil PC. Selective pro-apoptotic activity of novel 3, 3′-(aryl/alkyl-methylene) bis (2-hydroxynaphthalene-1, 4-dione) derivatives on human cancer cells via the induction reactive oxygen species. PLoS One. 2016;11(7):e0158694. doi:10.1371/journal.pone.0158694
- Kuge S, Jones N, Nomoto A. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 1997;16(7):1710–1720. doi:10.1093/emboj/16.7.1710
- Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Med Inflam. 2014;2014:1. doi:10.1155/2014/561459
- Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140(6):935–950. doi:10.1016/j.cell.2010.02.043
- Vančo J, Trávníček Z, Hošek J, Suchý P, Shahid M. In vitro and in vivo anti-inflammatory active copper (II)-lawsone complexes. PLoS One. 2017;12(7):e0181822. doi:10.1371/journal.pone.0181822
- Biradar S, Veeresh B Protective effect of lawsone on L-Arginine induced acute pancreatitis in rats; 2013.
- Valaes T. Severe neonatal jaundice associated with glucose‐6‐phosphate dehydrogenase deficiency: pathogenesis and global epidemiology. Acta Paediatrica. 1994;83(s394):58–76. doi:10.1111/j.1651-2227.1994.tb13216.x
- Kandil HH, Al-Ghanem MM, Sarwat MA, Al-Thallab FS. Henna (Lawsonia inermis Linn.) inducing haemolysis among G6PD-deficient newborns. A new clinical observation. Ann Trop Paediatrics. 1996;16(4):287–291. doi:10.1080/02724936.1996.11747840
- Raupp P, Hassan JA, Varughese M, Kristiansson B. Henna causes life threatening haemolysis in glucose-6-phosphate dehydrogenase deficiency. Arch Dis childhood. 2001;85(5):411–412. doi:10.1136/adc.85.5.411
- Munday R, Smith B, Fowke E. Haemolytic activity and nephrotoxicity of 2‐hydroxy‐1, 4‐naphthoquinone in rats. J Appl Toxicol. 1991;11(2):85–90. doi:10.1002/jat.2550110203
- Saeed SMG, Sayeed SA, Ashraf S, et al. A new method for the isolation and purification of lawsone from Lawsonia inermis and its ROS inhibitory activity. Pak J Bot. 2013;45(4):1431–1436.
- Yusuf M, Ahmad A, Shahid M, et al. Assessment of colorimetric, antibacterial and antifungal properties of woollen yarn dyed with the extract of the leaves of henna (Lawsonia inermis). J Cleaner Prod. 2012;27:42–50. doi:10.1016/j.jclepro.2012.01.005
- Lamoureux G, Perez AL, Araya M, Agüero C. Reactivity and structure of derivatives of 2‐hydroxy‐1, 4‐naphthoquinone (lawsone). J Phys Organic Chem. 2008;21(12):1022–1028. doi:10.1002/poc.1435
- Paiva SR, Lima LA, Figueiredo MR, Kaplan MAC. Plumbagin quantification in roots of Plumbago scandens L. obtained by different extraction techniques. Anais da Academia Brasileira de Ciências. 2004;76(3):499–504. doi:10.1590/S0001-37652004000300004
- Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2012;32(6):1131–1158. doi:10.1002/med.20235
- Aithal BK, Kumar MS, Rao BN, Udupa N, Rao BS. Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol. Int. 2009;33(10):1039–1049. doi:10.1016/j.cellbi.2009.06.018
- Pekin G, Ganzera M, Senol S, Bedir E, Korkmaz KS, Stuppner H. Determination of naphthazarin derivatives in endemic Turkish Alkanna species by reversed phase high performance liquid chromatography. Planta med. 2007;73(03):267–272. doi:10.1055/s-2007-967110
- Yakubovskaya AY, Pokhilo N, Anufriev V, Anisimov M. Synthesis and antimicrobial and antifungal activities of compounds of the naphthazarin series. Pharm Chem J. 2009;43(7). doi:10.1007/s11094-009-0322-z
- Meyer GW, Bahamon Naranjo MA, Widhalm JR. Convergent evolution of plant specialized 1, 4-naphthoquinones: metabolism, trafficking, and resistance to their allelopathic effects. J Exper Bota. 2021;72(2):167–176. doi:10.1093/jxb/eraa462
- Fisher N, Abd Majid R, Antoine T, et al. Cytochrome b mutation Y268S conferring atovaquone resistance phenotype in malaria parasite results in reduced parasite bc1 catalytic turnover and protein expression. J Biol Chem. 2012;287(13):9731–9741. doi:10.1074/jbc.M111.324319
- López López LI, Nery Flores SD, Silva Belmares SY, Sáenz Galindo A. Naphthoquinones: biological properties and synthesis of lawsone and derivatives-a structured review. Vitae. 2014;21(3):248–258. doi:10.17533/udea.vitae.17322
- Miroshnikov M, Kato K, Babu G, et al. A common tattoo chemical for energy storage: henna plant-derived naphthoquinone dimer as a green and sustainable cathode material for Li-ion batteries. RSC Adv. 2018;8(3):1576–1582. doi:10.1039/C7RA12357D
- Monroy-Cárdenas M, Forero-Doria O, Araya-Maturana R, Martínez-Cifuentes M. An Experimental and Theoretical Study of Dye Properties of Thiophenyl Derivatives of 2-Hydroxy-1, 4-naphthoquinone (Lawsone). Materials. 2021;14(19):5587. doi:10.3390/ma14195587
- Anaissi-Afonso L, Oramas-Royo S, Ayra-Plasencia J, et al. Lawsone, juglone, and β-lapachone derivatives with enhanced mitochondrial-based toxicity. ACS Chem. Biol. 2018;13(8):1950–1957. doi:10.1021/acschembio.8b00306
- Cardoso MF, Forezi LS, Cavalcante VG, et al. Synthesis of new xanthenes based on lawsone and coumarin via a tandem three-component reaction. J Braz Chem Soc. 2017;28:1926–1936.
- Durán AG, Chinchilla N, Molinillo JM, Macías FA. Influence of lipophilicity in O‐acyl and O‐alkyl derivatives of juglone and lawsone: a structure–activity relationship study in the search for natural herbicide models. Pest Manage Sci. 2018;74(3):682–694. doi:10.1002/ps.4764
- Song R, Yu B, Friedrich D, et al. Naphthoquinone-derivative as a synthetic compound to overcome the antibiotic resistance of methicillin-resistant S. aureus. Commun Biol. 2020;3(1):529. doi:10.1038/s42003-020-01261-0
- Kumar P, Yadav N, Chaudhary B, et al. Promises of phytochemical based nano drug delivery systems in the management of cancer. Chem Biol Interact. 2022;351:109745. doi:10.1016/j.cbi.2021.109745
- Li Y, Tan X, Liu X, et al. Enhanced anticancer effect of doxorubicin by TPGS-coated liposomes with Bcl-2 siRNA-Corona for dual suppression of drug resistance. Asian J Pharm Sci. 2020;15(5):646–660. doi:10.1016/j.ajps.2019.10.003
- Fouladi F, Steffen KJ, Mallik S. Enzyme-responsive liposomes for the delivery of anticancer drugs. Bioconjugate Chem. 2017;28(4):857–868. doi:10.1021/acs.bioconjchem.6b00736
- Imlimthan S, Khng YC, Keinänen O, et al. A theranostic cellulose nanocrystal‐based drug delivery system with enhanced retention in pulmonary metastasis of melanoma. Small. 2021;17(18):2007705. doi:10.1002/smll.202007705
- Rahman HS, Othman HH, Hammadi NI, et al. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int J Nanomed. 2020;15:2439–2483. doi:10.2147/IJN.S227805
- Ansari SA, Qadir A, Warsi MH, et al. Ethosomes-based gel formulation of karanjin for treatment of acne vulgaris: in vitro investigations and preclinical assessment. Biotech. 2021;11:1–14.
- Gonzalez Gomez A, Syed S, Marshall K, Hosseinidoust Z. Liposomal nanovesicles for efficient encapsulation of staphylococcal antibiotics. ACS omega. 2019;4(6):10866–10876. doi:10.1021/acsomega.9b00825
- Juhairiyah F, de Lange EC. Understanding drug delivery to the brain using liposome-based strategies: studies that provide mechanistic insights are essential. AAPS J. 2021;23(6):1–16. doi:10.1208/s12248-021-00648-z
- van der Koog L, Gandek TB, Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthcare Mater. 2022;11(5):2100639. doi:10.1002/adhm.202100639
- Yang J, Firdaus F, Azuar A, et al. Cell-penetrating peptides-based liposomal delivery system enhanced immunogenicity of peptide-based vaccine against Group A Streptococcus. Vaccines. 2021;9(5):499. doi:10.3390/vaccines9050499