Abstract
Objective
To quantitatively assess all dosage forms of three active vitamin D and its analogs, namely, calcitriol, alfacalcidol, and eldecalcitol, to provide a basis for the selection of active vitamin D and its analogs in hospitals.
Methods
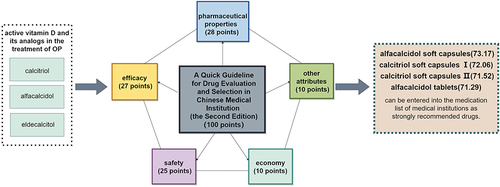
In this study, three active vitamin D and its analogs were evaluated by quantitative scoring in five dimensions, including pharmaceutical properties (28 points), efficacy (27 points), safety (25 points), economy (10 points), and other attributes (10 points).
Results
The final scores of quantitative assessment for the selection of alfacalcidol soft capsules, calcitriol soft capsules I, calcitriol soft capsules II, alfacalcidol tablets, alfacalcidol capsules, alfacalcidol oral drops, calcitriol injection, and eldecalcitol soft capsules were 73.17, 72.06, 71.52, 71.29, 69.62, 68.86, 65.60, 64.05 points.
Conclusion
Based on the scoring results, alfacalcidol soft capsules, calcitriol soft capsules I, calcitriol soft capsules II, alfacalcidol tablets can be entered into the medication list of medical institutions as strongly recommended drugs. This study offers guidance on selecting and using active vitamin D and its analogs in hospitals, with consideration for the patient’s needs.
Introduction
Osteoporosis (OP) is a systemic bone disease characterized by low bone mass and damage to the microstructure of bone tissue, leading to increased bone fragility and susceptibility to fracture,Citation1 Like osteoarthritis, the incidence of osteoporosis increases with age, and it is more common in women than in men, causing a burden to society.Citation2,Citation3 Currently, active vitamin D and its analogs (hereinafter referred to as active vitamin D) are marketed in China for treating OP, including calcitriol, alfacalcidol, and eldecalcitol. Active vitamin D has been shown to increase bone mineral density and reduce the risk of falls and lower fractures.Citation4–7 Evidence-based pharmacology has shown differences in safety and efficacy of active vitamin D with different molecular structures. Select the appropriate active vitamin D can play a vital role in the condition of patients with OP and reduce the economic burden. But how to scientifically evaluate and select active vitamin D is an urgent issue for medical institutions. In 2023, China issued “A Quick Guideline for Drug Evaluation and Selection in Chinese Medical Institution (the Second Edition)” (hereinafter referred to as “The Second Edition”).Citation8 Compared with the first edition, the guidelines made scientific and reasonable adjustments to the weight of the five dimensions of rapid selection of drugs for hospitals. Based on “The Second Edition”, this study quantitatively evaluates the active vitamin D through five dimensions, aiming to provide a basis for the selection of drugs for hospitals and can be a reference for medical decision-making in other regions.
Materials and Methods
Evaluation Basis
This study is based on “The Second Edition”, which adopts the Mini-health technology assessment (Mini-HTA) combined with the system of objectified judgment analysis(SOJA). The evaluation dimensions and weights were determined by the guideline guidance group and the expert group through the Delphi method.Citation8 In addition, compared with the first edition of this guideline, “The Second Edition” has revised and refined the evaluation indicators, so that the quantitative scoring can better reflect the priority of drugs in medical institutions, and the scoring items are more detailed, clear, and easy to operate. “The Second Edition” is a set of quantitative scoring system for drug evaluation and selection in Chinese medical institutions, which is widely used in China.
Evaluation of Drugs
Because of the existence of several different manufacturing units for calcitriol, alfacalcidol, and eldecalcitol, drugs evaluated in this study are preferentially selected that have been listed in China and meet one of the following conditions: 1. Original research drug; 2. Included in the national centralized procurement list; 3. Included in the national essential drug list. It should be noted that all existed dosage forms were considered in the assessment and the researchers are medical institution workers independent from the product manufacturers.
The final drugs included in the evaluation are shown in .
Table 1 Drugs Included in the Evaluation
Detailed Criteria for Quantitative Assessment of Drug Selection in Hospitals
A health technology assessment of the 8 OP drugs included in the study was performed according to “The Second Edition” and graded by a hundred mark system. The evaluation included five dimensions: Pharmaceutical properties, efficacy, safety, economy, and other attributes. The specific index system and weighting coefficients were as follows: Pharmaceutical properties included pharmacological effects (5 points), in vivo processes (5 points), pharmacy and methods of use (12 points), storage conditions (4 points), and expiry date (2 points), totaling 28 points; efficacy included indications (5 points), guideline recommendations (12 points), and clinical efficacy (10 points), totaling 27 points; safety included adverse reactions (8 points), special populations (11 points), adverse reactions due to drug interactions (3 points), and others (3 points), totaling 25 points; economy included drugs with the same generic name (3 points) and alternative drugs for the main indications (7 points), totaling 10 points; and other attributes included the national health insurance (3 points), the national essential medicines (3 points), the national centralized purchasing of medicines (1 point), the original research drug / reference listed drug /consistency evaluation (1 point), the status of the manufacturing company (1 point), and the global use of the drug (1 point), totaling 10 points.
Quantitative Scoring Results and Recommendations
The scoring results have two primary uses according to “The Second Edition”: drug introduction and drug transfer, and the specific recommendations are detailed in .
Table 2 Quantitative Scoring Results and Recommendation
Results
Pharmaceutical Properties
The information was obtained from drug instructions, guidelines, Chinese and English databases, etc. The goal is to score the selected drugs on five aspects: pharmacological effects, in vivo processes, pharmacy and method of use, storage conditions, and expiry date.
Pharmacological Effects
The clinical efficacy and mechanism of action of the 8 drugs are apparent, and all of them scored 4 points;
In vivo Processes
The in vivo processes of the 8 drugs are clear, and the pharmacokinetic parameter is complete. All scored 5 points;
Pharmacy and Method of Use
The main components and excipients of alfacalcidol tablets are not identified, scored 1 point, and the other drugs are scored 2 points; in terms of specification and packaging, the 8 drugs are all suitable for clinical application or dose adjustment, all scored 2 points; in terms of dosage form, except for calcitriol injection, the other 7 drugs are oral dosage form, so calcitriol injection scored 1 point, and the other drugs scored 2 points; in terms of dosage administration, the 8 drugs need to formulate the optimal dosage according to the patient’s blood calcium level, all scored 1.5 points; in terms of the frequency of dosage administration, the recommended dosage for treating OP is twice daily for both calcitriol soft capsules I and II, scored 1.5 points. The recommended dose for calcitriol injection is three times weekly, every other day, scored 2 points. The rest of the drugs are once daily, scored 2 points; in terms of ease of use, except for calcitriol injection, which needs to be administered by medical personnel, scoring 1 point, the rest of the drugs can be self-administered, scored 2 points;
Storage Conditions
Oral drops need to be refrigerated and protected from light, scored 1 point; alfacalcidol capsules, alfacalcidol soft capsules, eldecalcitol soft capsules need to be stored in the shade and protected from light, scored 2 points, while the other drugs need to be stored in room temperature and protected from light, scored 3 points.
Expiry Date
Calcitriol injection and alfacalcidol tablets only have 24 months, scored 1 point, while the other drugs have 36 months, scored 1.5 points.
The pharmaceutical properties score Results are shown in .
Table 3 Pharmaceutical Properties Score Results
Efficacy
Indications
The Indications of calcitriol, alfacalcidol, and eldecalcitol are all for the treatment of OP; the former two have been listed in the Chinese domestic market for many years, with rich experience in the clinical use of the drugs and more complete indications, while eldecalcitol was approved for the domestic market in 2022, and is currently only approved for the treatment of postmenopausal women with OP. Regarding indications, the eight drugs have more clinically available drugs, and all scored 1 point.
Guideline Recommendations
Checking the guideline query websites such as Yimaitong, Medication Assistant, Yaozhi Data, and Up to date database. Calcitriol and alfacalcidol were recommended in several guidelines. The highest recommendation grade for both was IA, and they both scored a guideline recommendation score of 12; Eldecalcitol did not have a high recommendation grade due to its late introduction to the Chinese market. It is recommended in the Guidelines for the Diagnosis and Treatment of Osteoporosis in Men,Citation9 etc., so it scored 7 points; domestic and international guidelines or consensus recommendations are shown in .
Table 4 Recommendations from National and International Guidelines/Consensus
Clinical Efficacy
Check related drugs’ RCT and meta-analysis literature to determine the clinical Efficacy.Citation15–22 To determine the clinical efficacy, the degree of bone pain and itching of the patients after treatment was used as the primary efficacy endpoint indicators: reduction of the degree of pain and improvement of bone mineral density (BMD) after treatment was judged to be effective; no change or aggravation of the degree of pain, and no change or decrease in BMD was judged to be ineffective. In 2 studies,Citation23,Citation24 it was noted that in terms of BMD, eldecalcitol was more effective than alfacalcidol on BMD. A meta-analysis indicated that eldecalcitol was more effective than alfacalcidol in reducing bone turnover markers (BTM).Citation25 The primary efficacy endpoint indicator score was 6 for eldecalcitol and 5 for calcitriol and alfacalcidol. The degree of improvement in bone metabolism marker levels such as bone-specific alkaline phosphatase, blood calcium, and blood phosphorus after treatment was used as secondary efficacy endpoint indicators; the improvement in bone metabolism marker levels after treatment with eldecalcitol was more favorableCitation21 so the score was 4 for eldecalcitol and 3 for both calcitriol and alfacalcidol.
The efficacy score results are shown in .
Table 5 Efficacy Score Results
Safety
The Safety assessment of the selected drugs was based on the drug instructions, drug registration information, information published on government websites such as the Food and Drug Administration (FDA) and the National Medical Protection Administration (NMPA), and relevant literature in both Chinese and English.Citation26–30 The safety assessment of the drugs to be selected was based on the following four aspects: adverse drug reactions, use in special populations, drug interactions, and other aspects of safety.
Adverse Reactions
Refer to the CTCAE-V5.0 grading to determine the severity of adverse reactions of the drugs to be selected. Calcitriol, alfacalcidol, and eldecalcitol, as active vitamin D, produce adverse reactions similar to those of vitamin D, mainly including hypercalcemia and hypercalciuria,Citation31 and other adverse reactions include loss of appetite, headache, nausea, vomiting, abdominal pain, and constipation. However, post-marketing clinical use of calcitriol in soft capsules I and II showed a low incidence of adverse reactions (less than 0.001%). Post-marketing adverse reactions to calcitriol injection were mainly hypersensitivity (2.3%). Still, they were primarily mild pain and localized redness in the injected portion of the injection and were not considered moderate adverse reactions. Therefore, this item scored 8 points. Alfacalcidol soft capsules and oral drops are both products of Leo Pharmaceuticals (Denmark) Ltd. In the bioavailability study conducted by the company, it was suggested that the adverse reactions were mainly caused by overdose, and the related symptoms were mostly mild adverse reactions such as itching, headache, nausea, excessive thirst, constipation, etc. Therefore, both of them scored 8 points in this item. Alfacalcidol tablets and alfacalcidol capsules in treating OP adverse reactions are mainly loss of appetite, vomiting, and other mild adverse reactions, so both scored 8 points. Eldecalcitol as a new type of active vitamin D, post-marketing adverse reaction data are limited, but in the clinical trials conducted in Japan, in addition to the frequency of acute renal failure is not known, the incidence of hypercalcemia and urinary stones was 1.5%, 0.9%. Therefore, 2 points were awarded for ”severe adverse reactions”, and the total score for this item was 5 points.
Special Populations
(1) Children: The safety of calcitriol and eldecalcitol in children has not been adequately studied, and there is no reliable literature, so both have no point in this item. Alfacalcidol soft capsules and oral drops instructions for children with different body weights to use the dose precise instructions, so both scored 2 points in this item. The rest of the drugs did not score. (2) Elderly: Elderly patients do not need particular dosages for calcitriol soft capsules I and soft capsules II, so this item scored 1 point. When using calcitriol injection, the dosage selection for elderly patients should be cautiously treated, so this item scores 0.5 points. Caution should be exercised when elderly patients use alfacalcidol tablets and eldecalcitol soft capsules, so both score 0.5 points. The rest of the drugs do not have specific use experience in the elderly, and no point should be scored. (3) Pregnant women: There are no appropriate and well-controlled trials to study the effects of calcitriol on pregnant women. Therefore, all forms of calcitriol are not scored. Based on the package insert, alfacalcidol should be used with caution in pregnant women. To differentiate from situations such as prohibition or absence of relevant studies, alfacalcidol in all dosage forms scored 0.5 points for this. Eldecalcitol is contraindicated in pregnant women, so eldecalcitol soft capsules are not scored. (4) Lactating women: Domestic instructions for calcitriol capsules II state that mothers can breastfeed while taking the product if they monitor calcium levels in both the mother and the baby. Therefore, the calcitriol soft capsules II will receive 0.5 points for this item, and no points will be awarded for the remaining dosage forms of calcitriol. If the risks have been weighing, alfacalcidol soft capsules, oral drops, and capsules should only be used on medical advice. Therefore, scored 0.5 points. Alfacalcidol tablets should preferably be avoided during breastfeeding but are not strictly prohibited, so this item scored 0.5 points. The use of eldecalcitol soft capsules is prohibited during lactation, so are not scored. (5) Hepatic dysfunction: Only the eldecalcitol soft capsules have been studied on the pharmacokinetics of patients with hepatic dysfunction, suggesting that the safety of patients with severe hepatic dysfunction has not been established and should be cautiously administered. Therefore, the eldecalcitol soft capsules scored 2 points, and the rest of the drugs scored 0 points. (6) Renal dysfunction: refer to the Consensus on the Clinical Use of Vitamin D and its Analogs.Citation32 Calcitriol and its analogs may be considered in CKD patients with combined OP and/or high fracture risk. Therefore, all drugs included in the study scored 3 points for this item.
Drug Interactions
Calcitriol, alfacalcidol, and eldecalcitol are active vitamin D. Therefore, pharmacologic doses of vitamin D and its derivatives should be suspended when taking them. Its combination with magnesium-containing drugs (such as antacids) may cause an increase in the concentration of magnesium ions in the blood, which can lead to hypermagnesemia, so long-term dialysis patients are prohibited from taking it at the same time. In addition, when taking active vitamin D, thiazides and digitalis should be avoided as much as possible, so all drugs included in the study received a score of 1 for this item.
Other Aspects of Safety
All adverse reactions of the drugs included in the study were reversible and scored 1 point. No carcinogenicity was seen with all the drugs included in the study. Calcitriol soft capsules I and II showed no adverse effects on reproductive toxicity studies. The rest suggest that high doses may cause fetal malformations, so calcitriol soft capsules I and II scored 1 point, and the rest did not score any points. The instructions for all drugs have no special medication warning, and all scored 1 point. In conclusion, calcitriol soft capsules I and II scored 3 points, and the rest scored 2 points.
The safety score results are shown in .
Table 6 Safety Score Results
Economy
The economic study was conducted by comparing the difference in average daily treatment costs between the selected drugs and those with the same generic name and substitutable drugs for the main indications. The information was obtained from drug pricing information released by the Yaozhi Data, the National Centralized Purchasing Platform for Drugs, the Sunshine Purchasing Platform, information on corporate websites, NMPA, the National Medical Protection Administration, and other information on governmental websites. All information counted as of July 1, 2023.
Drugs with the Same Generic Name
The lowest average daily cost of treatment for the same generic name is 3 points, and the evaluation drug score = the lowest average daily cost of treatment / average daily cost of treatment for the evaluated drug *3.Calcitriol injection, alfacalcidol capsules, and alfacalcidol oral drops are currently listed in the domestic market by only one manufacturer; all scored 3 points: Calcitriol soft capsules II (In China, its generic name is different from other calcitriol soft capsules), alfacalcidol tablets have the lowest average daily cost of treatment among the same generic name, all scored 3 points; calcitriol soft capsules (Sichuan Guowei Pharmaceutical Co. Ltd), with an average daily cost of 0.63 yuan, according to the evaluation method, calcitriol soft capsules I score of 1.82 points. The price of the cheapest alfacalcidol soft capsules (CP Pharmaceutical Qingdao Co. Ltd). is 3.76 yuan, so the evaluated alfacalcidol soft capsules score of 2.64 points; eldecalcitol soft capsules (Wenzhou Haihe Pharmaceutical Co., Ltd). is the cheapest daily treatment option with the same generic name, priced at 3.6 yuan. According to the evaluation method, the eldecalcitol soft capsule score is 2.95 points. See and for details.
Table 7 Basic Economy Information
Table 8 Economy Score Results
Substitutable Medicines for Main Indications
The drug with the lowest average daily treatment cost of the same kind of drug is scored 7 points, and the evaluation drug score = the lowest average daily treatment cost/the average daily treatment cost of the evaluated drug * 7. Among the leading drugs for OP, the drug with the lowest average daily treatment cost is calcitriol soft capsules (Sichuan Guowei Pharmaceutical Co. Ltd), with an average daily cost of 0.63 yuan; the corresponding scores were calculated according to the evaluation method, and the score of the calcitriol soft capsules I, calcitriol soft capsules II, calcitriol injection, alfacalcidol capsules, alfacalcidol soft capsules, alfacalcidol oral drops, alfacalcidol tablets, and eldecalcitol soft capsules were scored as 4.24, 0.52, 0.60, 1.62, 1.03, 0.36, 1.79, and 1.20 respectively. See and for details.
Other Attributes
Relevant information from National Essential Drug List (2018 Edition), National Medical Insurance List (2023 Edition), National Centralized Purchasing Drug List; drug packaging/instructions, China List of Listed Drugs Collection, NMPA Center For Drug Evaluation, FDA, European Medicines Agency (EMA) and Pharmaceuticals and Medical Devices Agency (PMDA) ; status of manufacturers from world sales of the top 50 pharmaceutical manufacturers published by US Pharmaceutical Managers, and the list of the top 100 pharmaceutical industry published by Ministry of Industry and Information Technology (MIIT).
Calcitriol injection and eldecalcitol soft capsules are in National Medical Insurance Category B with payment restrictions, which scored 1.5 points, other evaluated drugs are in National Medical Insurance Category B without payment restrictions, scored 2 points. All dosage forms of calcitriol and eldecalcitol soft capsules are not in the National Essential Drugs Catalog (2018 Edition), and all of them scored 1 point; all dosage forms of alfacalcidol belong to the National Essential Drugs, of which oral drops have Δ requirements, scored 2 points, and other dosage forms scored 3 points. Calcitriol soft capsules I, alfacalcidol soft capsules, and alfacalcidol tablets are in the National Centralized Purchasing Drug Catalogue, which scored 1 point, and the other drugs are not, which scored no points. Calcitriol soft capsules II, alfacalcidol soft capsules, and eldecalcitol soft capsules all belong to the original research drug, and scored 1 point; calcitriol soft capsules I and alfacalcidol tablets passed the consistency evaluation, all scored 0.5 points, the others do not score. Calcitriol capsules II belong to Roche Group, calcitriol injection belongs to AbbVie Group, and both belong to the world’s top 10 pharmaceutical manufacturers in terms of sales in 2022, scored 1 point. Eldecalcitol soft capsules belong to Chugai Group, which is in the 35th position of the world sales ranking in 2022 scored 0.4 points. The manufacturers of Calcitriol soft capsules I and all dosage forms of alfacalcidol are not in the 2022 World Sales Top 50/ MIIT ‘s Top 100 Pharmaceutical Industry list, and all are not scored. Calcitriol soft capsules I, alfacalcidol capsules and alfacalcidol tablets are only listed and sold within China, with no score. Calcitriol soft capsules II listed in all of the United States, Europe, and Japan, scored 1 point. and others are sold domestically and internationally but are not listed in all of the United States, Europe, and Japan scored 0.5 points.
The other attribute score results are shown in .
Table 9 Other Attribute Score Results
Discussion
Significance of the Assessment
Currently, there are no unified standards for the admission and transfer of medicines to and from medical institutions in China. To establish a sound drug supply security system, “The Second Edition” improved drug evaluation in medical institutions for better prioritization and compliance with national policy. And at the same time, the scoring entries are more detailed, clearer, and easier to operate.
Active vitamin D is a commonly used preventive and curative drug for OP. This study provides a rapid health assessment of 2 clinically typical active vitamin Ds (calcitriol, alfacalcidol) and a new active vitamin D (eldecalcitol) for each dosage form through “The Second Edition”, guide medical institutions in selecting drugs that align with patient needs.
Description of the Assessment and Analysis of Results
The drugs included in this study in descending order of quantitative final scores were alfacalcidol soft capsules, calcitriol soft capsules I, calcitriol soft capsules II, alfacalcidol tablets, alfacalcidol capsules, alfacalcidol oral drops, calcitriol injection, eldecalcitol soft capsules. The final total scores shown in , were 73.17, 72.06, 71.52, 71.29, 69.62, 68.86, 65.6, and 64.05 points respectively.
Table 10 Final Total Score Results
In the final score, the top four drugs with the highest scores were alfacalcidol soft capsules, calcitriol soft capsules I, calcitriol soft capsules II, alfacalcidol tablets. All are recommended for treating OP, with scores over 70. Among them, calcitriol soft capsules I have a more significant economic advantage compared with the other 7 drugs; alfacalcidol soft capsules, alfacalcidol tablets are national essential drugs, and in the national centralized purchasing in the selection, have a more significant policy advantage; calcitriol soft capsules II are a well-established drug with positive clinical experience and recommended for treating OP.
The scores of other drugs (calcitriol injection, alfacalcidol capsules, alfacalcidol oral drops, and eldecalcitol soft capsules) are located between 60 and 70, which are weakly recommended, and medical institutions can choose according to the actual situation. It is worth mentioning that the lowest-scoring eldecalcitol soft capsule, as a new type of active vitamin D, has been on the market for a shorter period in China, with limited clinical data, and is less often recommended by the guidelines. However, its effectiveness is better than the other 7 drugs, and it is the only drug in this study that has been studied in the population with hepatic insufficiency.
Shortcomings and Limitations
“The Second Edition” has been refined based on the former, but in the actual evaluation, we found that some of the scoring rules are still imperfect. For example, when evaluating pharmaceutical properties, the main ingredient and excipients have the same weight, but in practice, they should be distinguished in the light of their clinical importance.
In the process of evaluation, subjectivity is inevitable. For example, in the assessment of the efficacy of drugs, there are many primary and secondary efficacy endpoints in the literature, and they are not uniform; in the evaluation of the safety of drugs, it is hard to accurately categorize them into any of the available cases because the instructions or some of the literature do not indicate the stage of pregnancy in which pregnant women in special populations can use the drug, or only indicate that it should be used with caution.
The final score derived from this selection guideline is time-sensitive. As evidence-based evidence such as guidelines, expert consensus, and adverse reaction reports are updated, the final score based on the selection guidelines may change. Due to the differences in policies and hospital management models in different regions, it is necessary to keep an eye on this information when applying it in clinical practice, discuss the actual situation of each medical institution in each region, and reevaluate the drugs when necessary.
Conclusion
Alfacalcidol soft capsules, calcitriol soft capsules I, calcitriol soft capsules II, alfacalcidol tablets can be entered into the medication list of medical institutions as strongly recommended drugs. This health technology assessment can provide evidence-based evidence for the selection and rational use of active vitamin D, and also serve as a reference for hospitals in other countries to select drugs.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to thank all of the authors that participated in the present study.
Additional information
Funding
References
- WA P. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–650. doi:10.1016/0002-9343(93)90218-e
- Zhang H, Hu Y, Chen X, et al. Expert consensus on the bone repair strategy for osteoporotic fractures in China. Front Endocrinol. 2022;13:989648. doi:10.3389/fendo.2022.989648
- Xi X, Mehmood A, Niu P, et al. Association of X-linked TLR-7 gene polymorphism with the risk of knee osteoarthritis: a case-control study. Sci Rep. 2022;12(1):7243. doi:10.1038/s41598-022-11296-4
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomized controlled trials. BMJ. 2009;339:b3692. doi:10.1136/bmj.b3692
- de Freitas PH, Hasegawa T, Takeda S, et al. Eldecalcitol, a second-generation vitamin D analog, drives bone mini modeling and reduces osteoclastic number in trabecular bone of ovariectomized rats. Bone. 2011;49(3):335–342. doi:10.1016/j.bone.2011.05.022
- Matsumoto T, Takano T, Saito H, Takahashi F. Vitamin D analogs and bone: preclinical and clinical studies with eldecalcitol. Bonekey Rep. 2014;3:513. doi:10.1038/bonekey.2014.8
- Matsumoto T, Ito M, Hayashi Y, et al. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures--a randomized, active comparator, double-blind study. Bone. 2011;49(4):605–612. doi:10.1016/j.bone.2011.07.011
- Zhao Z, Dong Z, Liu J. A quick guideline for drug evaluation and selection in Chinese medical institutions(the Second Edition). Herald Med. 2023;(04):447–456. doi:10.3870/j.issn.1004-0781.2023.04.001
- Chinese Society of Osteoporosis and Bone Mineral Research. Guidelines for the diagnosis and treatment of osteoporosis in men. Chin J Osteop Bone Min Res. 2020;5:381–395. doi:10.3969/j.issn.1674-2591.2020.05.001
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(Suppl 1):3–36. doi:10.1007/s10195-017-0474-7
- Compston J, Cooper A, Cooper C, et al.; National Osteoporosis Guideline Group (NOGG). UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteop. 2017;12(1):43. doi:10.1007/s11657-017-0324-5
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):1–59. doi:10.1016/j.kisu.2017.04.001
- Chinese Society of Osteoporosis and Bone Mineral Research. Guidelines for the diagnosis and treatment of primary osteoporosis(2022). Chin J Osteop Bone Min Res. 2022;06:573–611. doi:10.3969/j.issn.1674-2591.2022.06.001
- Chinese Medical Association, Chinese Society of Clinical Pharmacy, Chinese Medical Journals Publishing House, et al. Guideline for rational medication of osteoporosis in primary care. Chin J Gen Practit. 2021;5:523–529. doi:10.3760/cma.j.cn114798-20210318-00258
- Zhao J, Mou A, Mao J, Zhu X. Clinical efficacy of different doses of intravenous calcitriol in the treatment of secondary hyperparathyroidism for hemodialysis patients. Chin J Coal Ind Med. 2018;1:53–57.
- Gui G, Han Z, Xu Y, Wang F, Xia X. Clinical efficacy of calcitriol injection and oral shock therapy in patients with secondary hyperparathyroidism. West Med. 2019;2:282–286+290.
- Pang XP. Clinical efficacy of calcitriol combined with Telmisartan in the treatment of diabetic nephropathy. Chin Mod Doctor. 2013;20:52–54.
- Liu LH. Clinical effect of calcitriol on 40 cases of uremia complicated with renal bone disease. Mod Diagn Treat. 2014;09:1983–1984.
- Tang H, Wang Z. Effect of Alfacalcidol soft capsules combined with bone peptide injection on osteoporosis. Med Theor Pract. 2019;19:3113–3115. doi:10.19381/j.issn.1001-7585.2019.19.037
- Shen L, Liu P, Zhu S. Alfacalcidol soft capsules combined with raloxifene in the treatment of postmenopausal osteoporosis. Modern Med Clin. 2017;(07):1328–1332. doi:10.7501/j.issn.1674-5515.2017.07.038
- Cui L, Xia W, Yu C, Dong S, Pei Y. Overview of the clinical efficacy and safety of eldecalcitol for the treatment of osteoporosis. Arch Osteop. 2022;17(1):74. doi:10.1007/s11657-022-01071-3
- Lin Q, Tan R. Meta-analysis of the effects of calcitriol on microinflammation and calcium and phosphorus metabolism in maintenance hemodialysis patients. Chin Contemp Med. 2022;36:13–17. doi:10.3969/j.issn.1674-4721.2022.36.005
- Liu H, Wang G, Wu T, Mu Y, Gu W. Efficacy and safety of eldecalcitol for osteoporosis: a meta-analysis of randomized controlled trials. Front Endocrinol. 2022;13:854439. doi:10.3389/fendo.2022.854439
- Nakamura T, Takano T, Fukunaga M, Shiraki M, Matsumoto T. Eldecalcitol is more effective for the prevention of osteoporotic fractures than alfacalcidol. J Bone Miner Metab. 2013;31(4):417–422. doi:10.1007/s00774-012-0418-5
- Xu Z, Fan C, Zhao X, Tao H. Treatment of osteoporosis with eldecalcitol, a new vitamin D analog: a comprehensive review and meta-analysis of randomized clinical trials. Drug Des Devel Ther. 2016;10:509–517. doi:10.2147/DDDT.S84264
- Jiang Y, Xing P, Li M, Wang O, Xia W. Efficacy and safety of alfacalcidol in osteoporosis: a meta-analysis. Chin J Osteop Bone Min Res. 2021;03:221–229. doi:10.3969/j.issn.1674-2591.2021.03.001
- Takeuchi Y, Saito H, Makishima M, et al. Long-term safety of eldecalcitol in Japanese patients with osteoporosis: a retrospective, large-scale database study. J Bone Miner Metab. 2022;40(2):275–291. doi:10.1007/s00774-021-01276-5
- Kawahara T, Suzuki G, Mizuno S, et al. Effect of active vitamin D treatment on development of type 2 diabetes: DPVD randomised controlled trial in Japanese population. BMJ. 2022;377:e066222. doi:10.1136/bmj-2021-066222
- Laskou F, Fuggle NR, Patel HP, Jameson K, Cooper C, Dennison E. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire cohort study. J Cach Sarcop Mus. 2022;13(1):220–229. doi:10.1002/jcsm.12870
- Maeda K, Imatani J, Moritani S, Kondo H. Effects of eldecalcitol alone or a bone resorption inhibitor with eldecalcitol on bone mineral density, muscle mass, and exercise capacity for postmenopausal women with distal radius fractures. J Orthop Sci. 2022;27(1):139–145. doi:10.1016/j.jos.2020.11.009
- Ringe JD. Plain vitamin D or active vitamin D in the treatment of osteoporosis: where do we stand today? Arch Osteop. 2020;15(1):182. doi:10.1007/s11657-020-00842-0
- Xia W, Zhang Z, Lin H, Jin X, Yu W, Fu Q. the consensus on the clinical use of vitamin D and its analogs. Chin J Osteop Bone Min Res. 2018;01:1–19. doi:10.3969/j.issn.1674-2591.2018.01.001