?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A number of 2-((4-ethylphenoxy)methyl)-N-(substituted-phenylcarbamothioyl) benzamides (1a–h) were synthesized via reaction of 2-((4-ethylphenoxy)methyl)benzoyl isothiocyanate (2) as a key intermediate with several substituted primary aromatic amines. The new compounds were characterized by proton nuclear magnetic resonance (1H-NMR), carbon-13 nuclear magnetic resonance (13C-NMR), infrared spectrometry (IR), mass spectrometry (MS), and elemental analysis. The anti-inflammatory activity of 1a–h was investigated by acute carrageenan-induced paw edema in mice using the reference drug indomethacin. The results obtained indicated that, of the derivatives developed, 1a and 1d–h exhibited significantly higher anti-inflammatory activity (26.81%–61.45%) when compared with the reference drug indomethacin (22.43%) (P = 0.0490 for 1a, 0.0015 for 1d, 0.0330 for 1f, and P < 0.001 for 1e and 1h). Moreover, the ulcer incidence of 20% for 1e and 1h was clearly lower when compared with the indomethacin group (in which the ulcer incidence was 80%). Of particular note, the ulcer index of 0.2 for 1e was significantly less than that in the indomethacin group (0.6, P = 0.014). Additionally, prostaglandin E2 (PGE2) inhibitory properties were found to be high with 1e (68.32 pg/mL), significantly different from those of the placebo group (530.13 pg/mL, P < 0.001), and equipotent to the effect observed in the indomethacin-pretreated group (96.13 pg/mL, P > 0.05). Moreover, the PGE2 level of 54.15 pg/mL with 1h was also significantly different from that of the placebo group (P < 0.001) and of the indomethacin group (P < 0.05). The significant inhibition of PGE2 observed with 1e (68.32 pg/mL) and 1h (54.15 pg/mL) agree with their observed ulcer incidences. Our overall findings for N-phenylcarbamothioylbenzamides 1a–h clearly suggest that the compounds exhibit an anti-inflammatory effect, potently inhibit PGE2 synthesis, and markedly demonstrate low ulcer incidence.
Introduction
Thiourea and its derivatives have a broad range of applications in medicine, chemistry, industry, and agriculture. They have been found to demonstrate various biological activities such as antimicrobial,Citation1–Citation3 anthelmintic,Citation4,Citation5 antitumor,Citation6 antiviral,Citation7 and are also suitable as insecticides,Citation8 fungicides,Citation9 herbicides,Citation10 and plant-growth regulators.Citation11 In addition, mounting evidence suggests that the moiety of thiourea also possesses analgesic and anti-inflammatory properties. A study of the structure–activity relationship of thiourea derivatives resulted in evaluating some of them as vanilloid-receptor agonists with potential analgesic and anti-inflammatory effects.Citation12 In that study, the analgesic activity, anti-inflammatory activity, and pungency of thiourea derivatives were investigated in animal models via the writhing test, ear edema assay, and eye-wiping test, respectively.Citation12
Moreover, thiourea derivatives containing partial cyproheptadine structure have been developed and screened for antitumor and anti-inflammatory activity, and were found to show stronger anti-inflammatory activity than ibuprofen in vivo in a xylene-induced ear swelling assay in mice.Citation13 Different chemical classes of thiourea have also been synthesized and found to exhibit good anti-inflammatory activity in carrageenan-induced paw edema (CPE) in a rat model.Citation14,Citation15
The constant medical use of nonsteroidal anti-inflammatory drugs to treat inflammation is limited since gastrointestinal adverse effects impair gastric ulcer healing.Citation16,Citation17 These gastrointestinal side effects are associated with prostaglandin E2 (PGE2) deficiency, which is caused by nonsteroidal anti-inflammatory drugs due to the inhibition of cyclooxygenase (COX) that exists in two isoenzymes: the constitutively expressed COX-1 and the inducible COX-2.Citation18 Based on the aforementioned studies and as a part of our ongoing research, the pharmacological profile of thiourea derivatives drew our interest with a view to synthesizing several new compounds belonging to the thiourea class. The newly synthesized N-phenylcarbamothioylbenzamides (which we called 1a–h) were screened for anti-inflammatory and ulcerogenic side-effect properties. Additionally, PGE2 inhibitory properties of the compounds were evaluated, since the inhibition of PGE2 synthesis strongly correlates with anti-inflammatory and gastric ulcerogenic properties.Citation18
Materials and methods
General
To chemically prepare the new compounds, all starter compounds used for the synthesis were variously procured from Merck & Co (Whitehouse Station, NJ, USA) and Sigma-Aldrich (St Louis, MO, USA). Freshly distilled 4-Ethylphenol was used and acetone was dried using potassium carbonate and then distilled. Dried ammonium thiocyanate treated by heating at 100°C was used in the reactions. The necessary liquid amines were dried with potassium hydroxide and afterwards distilled. Melting points were obtained with the aid of an Electrothermal 9100 capillary melting point apparatus in open capillary tubes (Bibby Scientific Ltd, Stone, UK); the values reported here are uncorrected. Elemental analysis was performed on a PerkinElmer 2400 Series II CHNS/O Analyzer (Waltham, MA, USA) and the results were within ±0.4% of the theoretical values. The Fourier-transform infrared (FT-IR) spectra of the all synthesized compounds were run on a Bruker Vertex 70 FT-IR spectrometer (Bruker Corporation, Billerica, MA, USA). Nuclear magnetic resonance (NMR) spectra were recorded employing a Varian Inova 400 instrument (Varian Medical Systems, Palo Alto, CA, USA) operating at 400.0 MHz for proton (1H)-NMR, 100.0 MHz for carbon-13 (13C)-NMR, and 376.3 MHz for fluorine-19 (19F)-NMR. Mass spectrometry (MS) measurements were carried out on an Agilent 6540 Ultra High Definition Q-TOF instrument (Agilent Technologies, Santa Clara, CA, USA).
Chemistry
General procedure for the preparation of compounds titled 1a–h
The synthesis of the 2-(4-ethylphenoxymethyl)benzoic acid (3) and 2-(4-ethyl-phenoxymethyl)benzoyl chloride (4) have been previously reported.Citation19,Citation20 The compounds 1a–h were prepared by adding a solution of 2-(4-ethylphenoxymethyl)-benzoyl chloride (4) (0.01 mol) in acetone (15 mL) to a solution of ammonium thiocyanate (0.01 mol) in acetone (5 mL) to correct arylisothiocyanate 2 in situ. The reaction mixture was heated under reflux for 1 hour then cooled at room temperature. A solution of primary amine (0.01 mol) in acetone (2 mL) was added to the mixture and the mixture was again heated under reflux for 1 hour. After the cooled reaction mixture was poured into 500 mL water, the respective acylthiourea was precipitated out. The solid product was purified by recrystallization from isopropanol with active carbon. The data of 1H-NMR, 13C-NMR, IR, MS, and elemental analysis for titled compounds 1a-h can be found in the Supplementary materials.
Pharmacology
Animals
All experiments were performed in adult male Tuck Ordinary (TO) mice (Harlan UK Ltd, Blackthorn, UK).Citation21 Mice were group housed in the central animal facility of the College of Medicine and Health Sciences (United Arab Emirates University) on a 12-hour light–dark cycle (lights on at 6 am). Food and water were available ad libitum. Experiments were conducted in agreement with the United Arab Emirates University Institutional Animal Care and Use Committee guidelines (protocol no A7-13).
Anti-inflammatory activity: acute CPE
Male TO mice weighing 22–25 g were randomly divided into ten groups of five animals each. Freshly prepared 1% carrageenan in dimethyl sulfoxide (DDDT; 0.01 mL) was injected in the plantar site of the right hind paw of each mouse to induce acute inflammation. The first group remained the placebo control groups and received DDDT (1.5%) only, while the second group received the reference drug indomethacin (0.028 mmol/kg).Citation22 The remaining eight groups received test compounds 1a–h (0.028 mmol/kg, orally) dissolved in DDDT (1.5%) before induction of acute inflammation. The control and test compounds were administered orally by intubation gauge 1 hour before carrageenan injection. Right hind paw thicknesses were measured before and 3 hours after induction of inflammation by a vernier caliper to estimate the anti-edematous effects of the reference drug indomethacin and test compounds 1a–h. Percent inhibition of test compounds was calculated according to the following equation:
in which “n” and “n′” indicate the difference in thickness between the first and second measurements of hind paws in the placebo control and test groups, respectivelyCitation23–Citation27 The results were expressed as mean ± standard error of the mean, and the significant difference between groups was tested by using one-way analysis of variance followed by Tukey’s honestly significant difference test at P < 0.05.
Ulcerogenicity
Male TO mice weighing 22–25 g were divided into five groups of five animals each. The animals were fasted 18 hours before drug administration. The reference compound indomethacin (0.028 mmol/kg) and the test compounds 1a, 1e, and 1h (0.028 mmol/kg) were suspended in saline solution with the aid of few drops of Tween®80 (Sigma Aldrich) and were administered orally for 3 successive days (single dose/day) to the fasted animals. The placebo control group was given saline with few drops of Tween 80. Four hours after the last dose, the animals were sacrificed and the stomachs were examined for the presence of lesions and erosions. The degree of ulcerogenicity was expressed in terms of: average number of ulcers per stomach, percentage incidence of ulcers, and ulcer index.Citation28
Measurement of PGE2 level
PGE2 level measurement was undertaken using the previously described 6-day-old air-pouch standard method in mice.Citation29–Citation31 Male TO mice weighing 22–25 g were divided into five groups of five animals each. The air pouch was induced as follows: on the first day of the experiment, 5 mL of air was injected subcutaneously in the back of each animal. Three days later, the air pouch was reinforced with 2.5 mL of air. Then, on Day 6, and before injecting the pouch with carrageenan (1 mL of 1% solution in saline), three groups of animals were pretreated orally with test compounds 1a, 1e, and 1h at a dose of 0.028 mmol/kg body weight, the positive control group was pretreated with the reference drug indomethacin (0.028 mmol/kg) suspended in saline solution with the aid of few drops of Tween 80, and the placebo control group was pretreated with sterile saline. All injections were conducted under light ether anesthesia. Then, 1 hour after the carrageenan injection, animals were lightly anaesthetized with ether and the contents of the pouch were aspirated using a Pasteur pipette and transferred into graduated plastic tubes kept in ice. The bulk of the exudate was frozen and stored at −20°C until required for PGE2 assay. PGE2 was measured using an enzyme-linked immunosorbent assay (ELISA) technique using a PGE2 assay kit (Prostaglandin E2 EIA Kit-Monoclonal, Cayman Chemical Item Number: 514010; Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer’s specifications. Data were collected checked and revised. Quantitative variables from normal distribution were expressed as means ± standard error of the mean. The significant difference between groups was tested using one-way analysis of variance followed by Tukey’s honestly significant difference test at P < 0.05.
Acute toxicity
The acute toxicity of the most promising compounds (1e and 1h) was determined using standard methods.Citation32 Male TO mice weighing 22–25 g were divided into three groups of six mice each. The test compounds, which were dissolved in DDDT (1.5%), were injected intraperitoneally at a dose of 60 mg/kg in the first group, 120 mg/kg in the second group, and 150 mg/kg in the third group. Toxic signs and the mortality rate in each group were recorded 48 hours after drug administration.
Results
Chemistry
In the present study, new 2-((4-ethylphenoxy)methyl)-N-(substituted-phenylcarbamothioyl) benzamides (1a-h) were synthesized by allowing 2-((4-ethylphenoxy)methyl)benzoyl isothiocyanate (2), as the key intermediate, to react with variously substituted primary aromatic amines in a 1:1 molar ratio. The new compounds were synthesized by a series of reactions as shown in .
Pharmacology
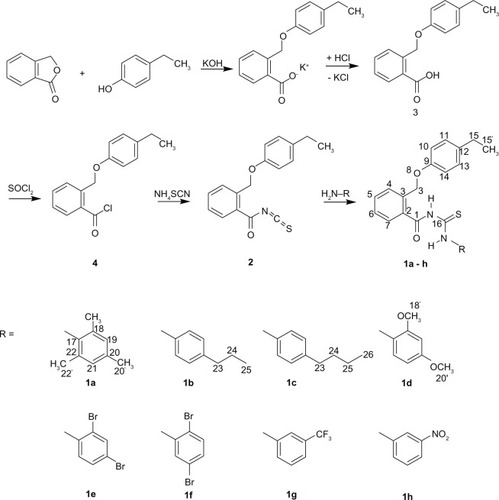
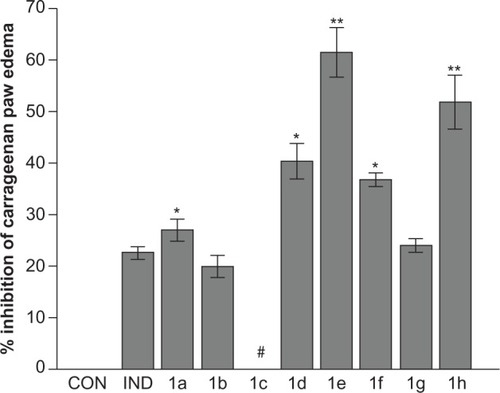
The results obtained from the CPE test indicate that 1a and 1d-h exhibited significantly higher anti-inflammatory activity (23.85%–61.45%) compared with the reference drug indomethacin (22.43%) (P = 0.049 for 1a, 0.0015 for 1d, and 0.033 for 1f; P = 0.00067 for 1e and 0.00081 for 1h). Notably, 1e and 1h showed the most potent anti-inflammatory activity (61.45% and 51.76%, respectively). The data observed for ulcerogenic properties clearly demonstrate that ulcerogenic potentials (ulcer incidence of 20% for 1e and 1h) were lower than those of the standard drug indomethacin (ulcer incidence of 80%). In particular, compound 1e, with an ulcer index of 0.2, showed significantly reduced ulcerogenicity when compared with indomethacin (ulcer index 0.6, P = 0.014). Moreover, PGE2 inhibition was found to be highest with 1e and 1h, which were found to have PGE2 levels of 68.32 pg/mL and 54.15 pg/mL, respectively. Notably, the PGE2 inhibition of 1h was significantly different from that of the placebo control group (P < 0.001) and the indomethacin group, which decreased PGE2 synthesis to a level of 96.13 pg/mL (P < 0.05). The significant inhibition of PGE2 observed with 1a, 1e, and 1h (106.31 pg/mL, 68.32 pg/mL, and 54.15 pg/mL, respectively) agree with the observed ulcer incidences of 80%, 20%, and 20%, respectively.
In addition, the acute toxicity of the most promising anti-inflammatory derivatives (1e and 1h) was observed closely, and there was no morbidity or mortality in mice at a dose of up to 150 mg/kg (body weight); that is, up to tenfold of the used effective dose. Further, during the 48-hour observation period, neither physical energy levels nor food and water intake were influenced, suggesting a good safety profile of the developed compounds.
Discussion
Chemistry
The new N-phenylcarbamothioylbenzamides 1a–h were white or light yellow crystalline solids. Although soluble at room temperature in DDDT or organic solvents like acetone and chloroform, they are soluble only when heated in lower alcohols, benzene, toluene, and xylene, and absolutely insoluble in water. The chemical structures of the newly synthesized compounds 1a–h were revealed by 1H-NMR, 13C-NMR, IR, mass spectrometry, and elemental analysis spectral studies, which confirmed the proposed structures. The IR bands were given as: “w,” weak; “m,” medium; “s,” strong; “vs,” very strong. The IR spectra provide valuable information regarding the nature of functional groups. The titled compounds were characterized mainly using the N–H of amide and the thioamide group, C–H of the methyl group, C–H of the methylene group, alkyl-aryl-ether, and the C=O and C=S bands. The IR spectra showed the expected frequencies of the νN-H of the amide group (3,357–3,349 cm−1) and the νN-H of the thioamide group (3,178–3,117 cm−1). The absorption bands for the antisymmetric stretching vibrations, the νC–H of the methyl and methylene groups, appeared at 2,971–2,958 cm−1 and 2,918–2,934 cm−1, respectively. These bands are typical for aromatic compounds containing some saturated carbon. The medium-strong νC=O band appeared at 1,693–1,669 cm−1, which is lower than that of ordinary carbonyl absorption (1,730 cm−1); this may be attributed to the formation of hydrogen bonds. The IR spectra of the target compounds showed very strong characteristic absorption of the νN–H amide group in the range of 1,503–1,516 cm−1. These compounds also showed typical alkyl-aryl ether at 1,248–1,223 cm−1 for the antisymmetric vibration and 1,044–1,026 cm−1 for the symmetric one. The spectra showed absorption bands at 1,159–1,176 cm−1, which can be attributed to the νC=S stretching frequency.
The chemical structures of the developed compounds were also supported by NMR spectra. The new N-phenyl-carbamothioylbenzamides were dissolved in DDDT-d6 and the chemical shift values, expressed in ppm, were referenced downfield to tetramethylsilane for 1H-NMR and 13C-NMR and upfield to trichlorofluoromethane for 19F-NMR and the constants (J) values in Hz. The chemical shifts for hydrogen and carbon atoms were also established by gradient correlation spectroscopy, gradient heteronuclear multiple bond coherence (GHMBC), and gradient heteronuclear single quantum coherence (GHSQC) experiments. As explained in the “Chemistry” subsection of “Methods and materials,” the 1H-NMR data are reported in the following order: chemical shifts, multiplicity, the coupling constants, number of protons and signal/atom attribution. The apparent resonance multiplicity is described as: s (singlet), d (doublet), t (triplet), q (quartet), qv (quartet), sxt (sextet), m (multiplet), dd (double doublet), td (triple doublet), and br (broad) signal. For the 13C-NMR data the order is: chemical shifts, the coupling constants in some cases, and signal/atom attribution (Cq). In the 1H-NMR spectra, the titled compounds exhibited broad signal in the ranges 12.56–11.81 ppm and 12.12–11.67 ppm, which were assigned to the NH protons. Generally, the NMR signal of NH protons for amides is observed in the range of 9–10 ppm. The low-field shift of the signal for the proton can be attributed to the de-shielding effect of the electron-withdrawing carbonyl and thiocarbonyl groups and hydrogen bonds. The ethyl group protons appear as a triplet (–CH3) at 2.54–2.50 ppm and as a quartet (–CH2–) at 1.13–1.11 ppm. 13C-NMR spectra showed the peaks at about δ 180.38–178.28 ppm for C=S. The carbon atom of the carbonyl group appeared at δ 169.99–170.78 ppm, due to the existence of the intramolecular hydrogen bond related to the carbonyl oxygen atom. The mass spectra of all the analyzed compounds showed the molecular ion peaks. The peak at m/z 239.1 is derived from the 2-(4-ethylphenoxymethyl)benzoyl cation.
Pharmacology
The results obtained from the CPE test model indicate that, of the derivatives developed, 1e and 1h demonstrated the most potent anti-inflammatory activity (61.45% and 51.76%, respectively) when compared with reference the drug indomethacin (22.43%) (P < 0.005) (). Since N-phenylcarbamothioylbenzamide derivatives with (N-2,4-dibromophenyl)-(1e) and N-(3-nitrophenyl)-substituent (1h) showed the most potent anti-inflammatory activity of the current series, it was surmised that modulation of the C20 position might greatly affect the anti-inflammatory activity of the compounds investigated. This was demonstrated by the anti-inflammatory results obtained, which were significantly decreased through prolongation of the C20-positioned alkyl chain to n-propyl or n-butyl in compounds 1a (26.81%), 1b (19.79%), and 1c (no anti-inflammatory activity). Importantly, our results show that anti-inflammatory activity was achieved by small-sized substitution as present in 1a (methyl moiety) with an anti-inflammatory activity of 26.81%, an activity comparable to that of the reference drug indomethacin (22.43%) (P > 0.05). The same observation of decrease in anti-inflammatory activity was obtained by shifting one bromo substituent from the C20 to C21 position, as achieved by comparing compound 1e and 1f with 61.45% and 36.68%, respectively (P < 0.005). An explanation for the observed activities of 1a–c and 1e–f might be the alteration in pharmacokinetic profile in accordance with the changes in the substitution pattern of the respective N-phenylcarbamothioylbenzamide derivative.
Figure 2 Percent inhibition of carrageenan paw edema for N-phenylcarbamothioylbenzamides 1a–h in mice.a
aDoses administered: indomethacin and test compounds 1a–h (0.028 mmol). Percentage of inhibition of edema expressed as means of five replicates ± standard error of the mean. *Significantly statistically different from IND group (P = 0.049 for 1a, 0.0015 for 1d, and 0.033 for 1f); **significantly statistically different from IND group and groups treated with 1a (P = 0.0014 for 1e and 0.0022 for 1h), 1b, 1c, and 1d (P = 0.031 for 1e), 1f (P = 0.026 for 1e), and 1g (P = 0.0011 for 1h); #no activity observed.
Abbreviations: CON, placebo control; IND, positive control.
The ulcerogenic properties for the most active derivatives, 1e and 1h, clearly indicate that ulcerogenic potentials – with ulcer incidence of 20% for 1e and 1h, as well as the ulcer indices of 0.20 ± 0.02 and 0.40 ± 0.12, respectively – were significantly reduced when compared with the reference drug indomethacin, which had an ulcer incidence of 80% and ulcer index of 0.6 (P < 0.05) (). Interestingly, the results observed for 1a, with an ulcer incidence of 80% and ulcer index of 0.50 ± 0.16, were comparable to those for the reference drug indomethacin and in agreement with the obtained moderate anti-inflammatory effect in the CPE test model (26.81%, and ).
Table 1 Effect of indomethacin and compounds 1a, 1e, and 1h on ulcerogenic properties
PGE2 inhibitory properties were determined for compounds 1a, 1e, and 1h, in comparison to the reference drug indomethacin. The results were in agreement with the anti-inflammatory observations. The inhibition of PGE2 synthesis was found to be high with 1e and 1h, which had PGE2 levels of 68.32 pg/mL and 54.15 pg/mL, respectively, and was significantly different from the other derivatives and indomethacin, which had a PGE2 level of 96.13 pg/mL (P < 0.05) ().
Figure 3 Inhibition of prostaglandin E2 (PGE2) by test compounds 1a, 1e, and 1h.
Abbreviations: CON, placebo control; IND, positive control.
The comparison of the chemical structures of 1e and 1h with that of known COX-2 inhibitors (celecoxib, valdecoxib, and lumiracoxib) was of interest because of their reduced ulcerogenic potential; however, there were no structural similarities to which to attribute the reduction in the ulcerogenic effects.
Conclusion
The developed N-phenylcarbamothioylbenzamides 1a–h showed moderate to high anti-inflammatory activity in the CPE test, potent inhibition of PGE2, and low ulcerogenic properties. In particular, the compounds with N-(2,4-dibromophenyl)- and N-(2-nitrophenylcarbamothioyl) benzamide substitution, 1e and 1h, respectively, significantly improved in vivo anti-inflammatory potency, potently inhibited PGE2 synthesis, and were endowed with low ulcerogenicity, proving that the newly developed class form potential candidates for further pharmacological investigation as well as assays on appropriate cell lines to validate their intrinsic cytotoxicity.
Supplementary materials
Key to data
The new N-phenylcarbamothioylbenzamides were dissolved in DDDT-d6 and the chemical shift values, expressed in ppm, were referenced downfield to tetramethylsilane for 1H-NMR and 13C-NMR and upfield to trichlorofluoromethane for 19F-NMR and the constants (J) values in Hz. In the next paragraphs, the 1H-NMR data are reported in the following order: chemical shifts, multiplicity, the coupling constants, number of protons and signal/atom attribution. The apparent resonance multiplicity is described as: s (singlet), d (doublet), t (triplet), q (quartet), qv (quartet), sxt (sextet), m (multiplet), dd (double doublet), td (triple doublet), and br (broad) signal. For the 13C-NMR data, the order is: chemical shifts, the coupling constants in some cases, and signal/atom attribution (quaternary carbon [Cq]). The explanation of the notes is as follows: J, coupling constant (Hz); IR, infrared; mp, melting point; ATR, attenuated total reflectance; MS, mass spectrometry; Anal calcd elemental analysis calculated; MH+, pseudomolecular ion; S, sulfur; C, carbon; H, hydrogen; N, nitrogen; m/z, mass/charge ratio; GHMBC, gradient heteronuclear multiple bond coherence; GHSQC, gradient heteronuclear single quantum coherence; w, weak; m, medium; s, strong; vs, very strong.
2-((4-Ethylphenoxy)methyl)-N-(2,4,6-trimethylphenylcarbamothioyl)benzamide (1a)
Yield 77%; mp 139°C–141°C; 1H-NMR (hexadeuteriodimethyl sulfoxide [DDDT-d6]): 11.81 (br s, 1H, NH), 11.67 (br s, 1H, NH), 7.62 (dd, J = 1.4 Hz, J = 7.4 Hz, 1H, H-7), 7.59 (dd, J = 1.6 Hz, J = 7.4 Hz, 1H, H-4), 7.55 (td, J = 1.4 Hz, J = 7.4 Hz, 1H, H-5), 7.47 (td, J = 1.6 Hz, J = 7.5 Hz, 1H, H-6), 7.06 (d, J = 8.6 Hz, 2H, H-11, H-13), 6.90 (d, J = 8.6 Hz, 2H, H-10, H-14), 6.89 (s, 2H, H-19, H-21), 5.25 (s, 2H, H-8), 2.50 (q, J = 7.6 Hz, 2H, H-15), 2.24 (s, 3H, H-20′), 2.07 (s, 6H, H-18′, H-22′), 1.11 (t, J = 7.6 Hz, 3H, H-15′); 13C-NMR (DDDT-d6): 180.38 (C-16), 170.06 (C-1), 156.28 (C-9), 136.49 (Cq), 136.23 (Cq), 135.61 (Cq), 134.69 (C-18, C-22), 133.69 (Cq), 133.50 (Cq), 130.92 (C-5), 128.81 (C-4), 128.62 (C-11, C-13), 128.51 (C-19, C-21), 128.45 (C-7), 127.89 (C-6), 114.60 (C-10, C-14), 67.61 (C-8), 27.31 (C-15), 20.53 (C-20′), 17.68 (C-18′, C-22′), 15.87 (C-15); FT-IR (solid in ATR, ν cm−1): 3,162 m, 3,001 w, 2,965 m, 2,924 m, 2,862 w, 1,679 m, 1,606 w, 1,506 vs, 1,381 m, 1,332 w, 1,301 w, 1,232 s, 1,168 s, 1,073 w, 1,032 m, 1,026 m, 875 w, 830 w, 748 m, 653 w, 617 w; MS: m/z (%) 433.19 (MH+ 100), 239.1 (5). Anal calcd for C26H28N2O2S (432.56): C, 72.19; H, 6.52; N, 6.48; S, 7.41%; Found: C, 72.48; H, 6.58; N, 6.34; S 7.48%.
2-((4-Ethylphenoxy)methyl)-N-(4-n-propylphenylcarbamothioyl)benzamide (1b)
Yield 82%; mp 139°C–140°C; 1H-NMR (DDDT-d6): 12.38 (br s, 1H, NH), 11.77 (br s, 1H, NH), 7.61 (br d, J = 7.4 Hz, 1H, H-7), 7.58 (m, 1H, H-4), 7.55 (td, J = 1.4 Hz, 7.4 Hz, 1H, H-5), 7.49 (d, J = 8.4 Hz, 2H, H-18, H-22), 7.46 (td, J = 1.4 Hz, 7.5 Hz, 1H, H-6), 7.21 (d, J = 8.4 Hz, 2H, H-19, H-21), 7.08 (d, J = 8.6 HZ, 2H, H-11, H-13), 6.89 (d, J = 8.6 Hz, 2H, H-10, H-14), 5.27 (s, 2H, H-8), 2.54 (q, J = 7.5 Hz, 2H, H-15), 2.49 (t, J = 7.3 Hz, 2H, H-23), 1.60 (sxt, J = 7.3 Hz, 2H, H-24), 1.12 (t, J = 7.5 Hz, 3H, H-15′), 0.90 (t, J = 7.33 Hz, H, H-25); 13C-NMR (DDDT-d6): 178.78 (C-16), 170.16 (C-1), 156.32 (C-9), 140.29 (Cq), 136.22 (Cq), 135.81 (Cq), 135.53 (Cq), 133.36 (Cq), 130.98 (C-5), 128.61 (C-19, C-21), 128.46 (C-7), 128.42 (C-11, C-13), 128.37 (C-4), 127.74 (C-6), 124.04 (C-18, C-22), 114.60 (C-10, C-14), 67.56 (C-8), 36.75 (C-23), 27.27 (C-15), 23.96 (C-24), 15.76 (C-15′), 13.57 (C-25); FT-IR (solid in ATR, ν cm−1): 3,357 w, 3,117 w, 3,038 w, 2,961 m, 2,926 m, 2,869 w, 1,669 m, 1,594 w, 1,506 vs, 1,339 m, 1,226 s, 1,175 w, 1,143 s, 1,028 m, 836 m, 734 m, 664 m, 601 w. Anal calcd for C26H28N2O2S (432.56): C, 72.19; H, 6.52; N, 6.48; S, 7.41%. Found: C, 72.37; H, 6.61; N, 6.42; S, 7.37%.
2-((4-Ethylphenoxy)methyl)-N-(4-n-butylphenylcarbamothioyl)benzamide (1c)
Yield 81%; mp 126°C–127°C; 1H-NMR (DDDT-d6): 12.38 (br s, 1H, NH), 11.77 (br s, 1H, NH), 7.61 (br d, J = 7.4 Hz, 1H, H-7), 7.59 (m, 1H, H-4), 7.56 (td, J = 1.4 Hz, 7.4 Hz, 1H, H-5), 7.49 (d, J = 8.4 Hz, 2H, H-18, H-22), 7.46 (td, J = 1.4 Hz, 7.5 Hz, 1H, H-6), 7.21 (d, J = 8.4 Hz, 2H, H-19, H-21), 7.08 (d, J = 8.6 Hz, 2H, H-11, H-13), 6.89 (d, J = 8.6 Hz, 2H, H-10, H-14), 5.27 (s, 2H, H-8), 2.58 (t, J = 7.5 HZ, 2H, H-23), 2.51 (q, J = 7.5 Hz, 2H, H-15), 1.56 (qv, J = 7.5 Hz, 2H, H-24), 1.31 (sxt, J = 7.5 Hz, 2H, H-25), 1.12 (t, J = 7.5 Hz, 3H, H-15′), 0.91 (t, J = 7.5 Hz, 3H, H-25); 13C-NMR (DDDT-d6): 178.76 (C-16), 170.14 (C-1), 156.27 (C-9), 140.43 (Cq), 136.15 (Cq), 135.77 (Cq), 135.45 (Cq), 133.32 (Cq), 130.91 (C-5), 128.55 (C-19, C-21), 128.42 (C-7), 128.30 (C-11, C-13), 128.29 (C-4), 127.66 (C-6), 124.01 (C-18, C-22), 114.56 (C-10, C-14), 67.50 (C-8), 34.28 (C-23), 32.98 (C-24), 27.22 (C-15), 21.66 (C-25), 15.73 (C-15′), 13.70 (C-25); FT-IR (solid in ATR, ν cm−1): 3,349 w, 3,233 w, 3,154 w, 3,116 w, 3,039 w, 2,958 m, 2,927 m, 2,864 w, 1,673 m, 1,593 m, 1,546 vs, 1,378 w, 1,339 s, 1,223 s, 1,176 w, 1,142 vs, 1,028 m, 955 w, 832 m, 789 w, 735 m, 668 m, 600 w. Anal calcd for C27H30N2O2S (446.60): C, 72.61; H, 6.77; N, 6.27; S, 7.18%. Found: C, 72.39; H, 6.71; N, 6.22; S, 7.18%.
2-((4-Ethylphenoxy)methyl)-N-(2,4-dimethoxyphenylcarbamothioyl) benzamide (1d)
Yield 71%; mp 114°C–115°C; 1H-NMR (DDDT-d6): 12.56 (s, 1H, NH), 11.71 (s, 1H, NH), 8.28 (d, J = 8.8 Hz, H-22), 7.61 (br d, J = 7.4 Hz, 1H, H-7), 7.58 (dd, J = 1.6 Hz, 7.4 Hz, 1H, H-4), 7.55 (td, J = 1.4 Hz, 7.4 Hz, 1H, H-5), 7.46 (td, J = 1.4 Hz, 7.8 Hz, 1H, H-6), 7.07 (d, J = 8.6 Hz, 2H, H-11–13), 6.88 (d, J = 8.6 Hz, 2H, H-10-14), 6.67 (d, J = 2.5, 1H, H-19), 6.56 (dd, J = 2.5 Hz, 8.9 Hz, 1H, H-21), 5.26 (s, 2H, H-8), 3.80 (s, 3H, H-18′ or H-20′), 3.79 (s, 3H, H-18′ or H-20′), 2.50 (q, J = 7.6 Hz, 2H, H-15), 1.11 (t, J = 7.6 Hz, 3H, H-15); 13C-NMR (DDDT-d6): 178.28 (C-16), 170.78 (C-1), 158.85 (C-18 or C-20), 156.91 (C-9), 152.75 (C-18 or C-20), 136.89 (Cq), 136.34 (Cq), 134.14 (Cq), 131.61 (C-5), 129.23 (C-11, C-13), 129.20 (C-4), 128.49 (C-6, C-7), 125.26 (C-22), 120.72 (C-17), 115.36 (C-10, C-14), 104.57 (C-21), 99.35 (C-19), 68.33 (C-8), 56.70 (C-18′ or C-20′), 56.09 (C-18′ or C-20′), 27.95 (C-15), 16.43 (C-15′); MS: m/z (%) 451.1 (MH+ 100), 239.1 (5); FT-IR (solid in ATR, ν cm−1): 3,214 m, 3,125 m, 3,031 m, 2,959 m, 1,670 m, 1,608 m, 1,548 vs, 1,503 vs, 1,465 s, 1,369 m, 1,326 s, 1,285 m, 1,229 vs, 1,160 vs, 1,120 s, 1,069 w, 913 w, 856 w, 827 m, 788 m, 721 m, 668 w, 629 w. Anal calcd for C25H26N2O4S (450.55): C, 66.65; H, 5.82; N, 6.22; S, 7.12%. Found: C, 66.34; H, 5.71; N, 6.24; S, 7.19%.
2-((4-Ethylphenoxy)methyl)-N-(2,4-dibromophenylcarbamothioyl)benzamide (1e)
Yield 76%; mp 153°C–154°C; 1H-NMR (DDDT-d6): 12.36 (br s, 1H, NH), 12.09 (br s, 1H, NH), 7.95 (d, J = 2.4 Hz, 1H, H-19), 7.90 (d, J = 8.6 Hz, 1H, H-22), 7.63 (br d, J = 7.4 Hz, 1H, H-7), 7.62 (dd, J = 2.4 Hz, 8.6 Hz, 1H, H-21), 7.60 (m, 1H, H-4), 7.58 (td, J = 1.4 Hz, 7.4 Hz, 1H, H-5), 7.47 (td, J = 1.4 Hz, 7.5 Hz, 1H, H-6), 7.08 (d, J = 8.6 Hz, 2H, H-11, H-13), 6.89 (d, J = 8.6 Hz, 2H, H-10, H-14), 5.27 (s, 2H, H-8), 2.50 (q, J = 7.5 Hz, 2H, H-15), 1.13 (t, J = 7.5 Hz, 3H, H-15′); 13C-NMR (DDDT-d6): 180.16 (C-16), 170.24 (C-1), 156.22 (C-9), 136.45 (Cq), 136.16 (Cq), 135.79 (Cq), 134.46 (C-19), 133.17 (Cq), 131.08 (C-5), 130.67 (C-21), 129.89 (C-22), 128.55 (C-1 1, C-13, C-4, C-7), 127.78 (C-6), 120.32 (C-20), 119.70 (C-18), 114.55 (C-10, C-14), 67.49 (C-8), 27.25 (C-15), 15.79 (C-15′); FT-IR (solid in ATR, ν cm−1): 3,260 w, 3,082 w, 2,961 w, 2,918 w, 2,867 w, 1,677 m, 1,615 w, 1,559 m, 1,512 vs, 1,377 m, 1,311 m, 1,238 s, 1,161 s, 1,076 w, 1,041 m, 940 w, 860 w, 765 w, 735 w, 675 m, 605 w; MS: m/z (%) 548.9 (MH+ 100), 467 (26), 270.2 (25), 239.1 (5). Anal calcd for C23H20Br2N2O2S (548.29): C, 50.38; H, 3.68; N, 5.11; S, 5.85%. Found: C, 50.15; H, 3.61; N, 5.02; S, 5.89%.
2-((4-Ethylphenoxy)methyl)-N-(2,5-dibromophenylcarbamothioyl)benzamide (1f)
Yield 89%; mp 128°C–129°C; 1H-NMR (DDDT-d6): 12.42 (br s, 1H, NH), 12.12 (br s, 1H, NH), 8.06 (d, J = 2.4 Hz,1H, H-22), 7.66 (d, J = 8.6 Hz, 1H, H-19), 7.63 (br d, J = 7.4 Hz, 1H, H-7), 7.60 (m, 1H, H-4), 7.58 (td, J = 1.4 Hz, 7.4 Hz, 1H, H-5), 7.47 (td, J = 1.4 Hz, 7.5 Hz, 1H, H-6), 7.43 (dd, J = 2.4 Hz, 8.6 Hz, 1H, H-20), 7.08 (d, J = 8.6 Hz, 2H, H-11, H-13), 6.89 (d, J = 8.6 Hz, 2H, H-10, H-14), 5.26 (s, 2H, H-8), 2.50 (q, J = 7.52 Hz, H, 2H-15), 1.13 (t, J = 7.5 Hz, 3H, H-15′); 13C-NMR (DDDT-d6): 180.14 (C-16), 170.28 (C-1), 156.22 (C-9), 138.26 (Cq), 136.14 (Cq), 135.71 (Cq), 134.13 (C-19), 133.25 (Cq), 131.03 (C-5), 131.00 (C-20), 130.67 (C-22), 128.55 (C-7), 128.51 (C-11, C-13), 128.46 (C-4), 127.61 (C-6), 119.64 (C-21), 118.34 (C-18), 114.49 (C-10, C-14), 67.53 (C-8), 27.24 (C-15), 15.74 (C-15′); FT-IR (solid in ATR, ν cm−1): 3,240 m, 2,964 w, 1,674 m, 1,610 w, 1,569 m, 1,516 vs, 1,397 w, 1,313 m, 1,243 s, 1,159 s, 1,077 w, 1,044 m, 875 w, 823 w, 795 w, 755 w, 706 m, 611 w; MS: m/z (%) 548.9 (MH+ 100), 467 (14), 239.1 (5). Anal calcd for C23H20Br2N2O2S (548.29): C, 50.38; H, 3.68; N, 5.11; S, 5.85%. Found: C, 50.21; H, 3.59; N, 5.12; S, 5.81%.
2-((4-Ethylphenoxy)methyl)-N-(3-trifluor omethylphenylcarbamothioyl)benzamide (1g)
Yield 73%; mp 119°C–120°C; 1H-NMR (DDDT-d6): 12.48 (br s, 1H, NH), 11.96 (br s, 1H, NH), 8.07 (bs, 1H, H-18), 7.80 (dq, J = 7.1 Hz, 1H, H-22, 4 J (H18, 20-H22) = 1.6 Hz), 7.67-7.60 (m, 4H, H-4, H-7, H-20, H-21), 7.57 (td, J = 1.4 Hz, 7.4 Hz, 1H, H-5), 7.48 (td, J = 1.4 Hz, 7.5 Hz, 1H, H-6), 7.09 (d, J = 8.6 Hz, 2H, H-11, H-13), 6.90 (d, J = 8.6 Hz, 2H, H-10, H-14), 5.27 (s, 2H, H-8), 2.51 (q, J = 7.5 Hz, 2H, H-15), 1.11 (t, J = 7.5 Hz, 3H, H-15′); 13C-NMR (DDDT-d6): 179.58 (C-16), 170.03 (C-1), 156.27 (C-9), 138.68 (Cq), 136.16 (Cq), 135.78 (Cq), 133.26 (Cq), 130.96 (C-5), 129.73 (C-22), 129.12 (q, C-19, 2J (3F-C19) = 31.9 Hz), 128.60 (C-21), 128.49 (C-11, C-13), 128.37 (C-7), 128.30 (C-4), 127.69 (C-6), 123.82 (q, CF3, J (3F-C) = 271.0 Hz), 122.70 (q, C-20, 3J (3F-C20) = 3.3 Hz), 121.00 (q, C-18, 3J (3F-C18) = 4.0 Hz), 114.51 (C-10, C-14), 67.51 (C-8), 27.17 (C-15), 15.63 (C-15′); 19F-NMR (DDDT-d6): −56.92 (3F); FT-IR (solid in ATR ν cm−1): 3,178 s, 3,030 m, 2,971 s, 2,934 m, 2,877 m, 1,693 s, 1,600 m, 1,555 vs, 1,531 vs, 1,513 vs, 1,451 s, 1,397 m, 1,331 s, 1,309 s, 1,282 m, 1,248 vs, 1,230 s, 1,164 vs, 1,112 vs, 1,075 s, 1,033 m, 966 w, 951 w, 889 w, 859 w, 827 m, 795 m, 761 m, 737 s, 692 m, 680 m, 660 w, 646 m, 623 w, 572 w; MS: m/z (%) 459.1 (MH+ 100), 425.1 (7), 239.1 (7). Anal calcd for C24H21F3N2O2S (458.49): C, 62.87; H, 4.62; N, 6.11; S, 6.99%. Found: C, 62.65; H, 4.37; N, 6.12; S, 6.93%.
2-((4-Ethylphenoxy)methyl)-N-(3-nitrophenylcarbamothioyl)benzamide (1h)
Yield 72%; mp 148°C–149°C; 1H-NMR (DDDT-d6): 12.54 (s, 1H, NH), 12.01 (br s, 1H, NH), 8.65 (t, J = 2.3 Hz, 1H, H-18), 8.12 (ddd, J = 1.0 Hz, 2.3 Hz, 8.2 Hz, 1H, H-20), 7.93 (ddd, J = 1.0 Hz, 2.3 Hz, 8.2 Hz, 1H, H-22), 7.69 (t, J = 8.2 Hz, 1H, H-21), 7.61 (br d, J = 7.4 Hz, 1H, H-7), 7.58 (dd, J = 1.6 Hz, 7.4 Hz, 1H, H-4), 7.55 (td, J = 1.4 Hz, 7.4 Hz, 1H, H-5), 7.46 (td, J = 1.4 Hz, 7.8 Hz, 1H, H-6), 7.06 (d, J = 8.6 Hz, 2H, H-11, H-13), 6.88 (d, J = 8.6 Hz, 2H, H-10-14), 5.28 (s, 2H, H-8), 2.51 (q, J = 7.6 Hz, 2H, H-15), 1.12 (t, J = 7.6, 3H, H-15); 13C-NMR (DDDT-d6): 179.64 (C-16), 169.99 (C-1), 156.22 (C-9), 147.43 (C-19), 139.03 (Cq), 136.19 (Cq), 135.79 (Cq), 133.19 (Cq), 131.05 (C-21), 130.01 (C-5), 129.87 (C-22), 128.51 (C-11, C-13), 128.37 (C-4), 128.32 (C-7), 127.71 (C-6), 120.78 (C-20), 119.00 (C-18), 114.49 (C-10, C-14), 67.48 (C-8), 27.16 (C-15), 15.64 (C-15′); FT-IR (solid in ATR, ν cm−1): 3,156 m, 2,966 w, 2,923 w, 2,859 w, 1,678 m, 1,606 w, 1,509 vs, 1,384 w, 1,344 m, 1,313 m, 1,232 m, 1,171 m, 1,070 w, 1,033 m, 898 w, 822 w, 766 w, 737 m, 695 m, 601 w, 538 w; MS: m/z (%) 436.1 (MH+ 100), 239.1 (9). Anal calcd for C23H21N3O4S (435.51): C, 63.43; H, 4.86; N, 9.65; S, 7.36%. Found: C, 63.61; H, 4.77; N, 9.58; S, 7.34%.
Acknowledgments
The authors of this manuscript highly appreciate the financial support for this project provided by the Department of Pharmaceutical Sciences, College of Pharmacy, Al Ain University of Science and Technology, and by the College of Medicine and Health Sciences, United Arab Emirates University.
The authors would also like to acknowledge Dr Sayed Mohammed Nurulain for his technical assistance.
Disclosure
The authors declare no conflicts of interest in this work other than the financial support received for this study, as outlined in the “Acknowledgments.”
References
- Gülkok Y Biçer T Kaynak Onurdag F Özgen S Şahin MF Doğruer DS Synthesis of some new urea and thiourea derivatives and evaluation of their antimicrobial activities Turkish Journal of Chemistry 2012 36 279 291
- Struga M Rosolowski S Kossakowski J Stefanska J Synthesis and microbiological activity of thiourea derivatives of 4-azatricyclo[5.2.2.0(2,6)]undec-8-ene-3,5-dione Arch Pharm Res 2010 33 1 47 54 20191342
- Liav A Angala SK Brennan PJ Jackson M N-D-aldopentofuranosyl-N′-[p-(isoamyloxy)phenyl]-thiourea derivatives: potential anti-TB therapeutic agents Bioorg Med Chem Lett 2008 18 8 2649 2651 18362068
- Walchshofer N Delabre-Defayolle I Paris J Petavy AF In vivo morphological damage induced by a new benzimidazole prodrug in Echinococcus multilocularis metacestodes J Pharm Sci 1990 79 7 606 608 2118956
- Mishra A Srivastava K Tripathi R Puri SK Batra S Search for new pharmacophores for antimalarial activity. Part III: synthesis and bioevaluation of new 6-thioureido-4-anilinoquinazolines Eur J Med Chem 2009 44 11 4404 4412 19586687
- Çıkla P Küçükgüzel ŞG Küçükgüzel I Synthesis and evaluation of antiviral, antitubercular and anticancer activities of some novel thioureas derived from 4-aminobenzohydrazide hydrazones Marmara Eczacılık Dergisi [Marmara Pharmaceutical Journal] 2010 14 1 13 20
- Patel RB Chikhalia KH Pannecouque C de Clercq E Synthesis of novel PETT analogues: 3,4-dimethoxy phenyl ethyl 1,3,5-triazinyl thiourea derivatives and their antibacterial and anti-HIV studies J Braz Chem Soc 2007 18 2 312 321
- Kayser H Eilinger P Metabolism of diafenthiuron by microsomal oxidation: procide activation and inactivation as mechanisms contributing to selectivity Pest Manag Sci 2001 57 10 975 980 11695192
- Ramadas K Suresh G Janarthanan N Masilamani S Antifungal activity of 1,3-disubstituted symmetrical and unsymmetrical thioureas Pest Manag Sci 1998 52 2 145 151
- Ke SY Xue SJ Synthesis and herbicidal activity of N-(o-fluoro-phenoxy-acetyl) thioureas derivatives and related fused heterocyclic compounds Arkivoc 2001 10 63 68
- Kumar S Awasthi V Kanwar JK Influence of growth regulators and nitrogenous compounds on in vitro bulblet formation and growth in oriental lily Horticultural Science (Prague) 2007 34 2 77 83
- Lee J Kim J Kim SY N-(3-Acyloxy-2-benzylpropyl)-N′-(4-hydroxy-3-methoxybenzyl) thiourea derivatives as potent vanilloid receptor agonists and analgesics Bioorg Med Chem 2001 9 1 19 32 11197340
- Liu W Zhou J Zhang T Design and synthesis of thiourea derivatives containing a benzo[5,6]cyclohepta[1,2-b]pyridine moiety as potential antitumor and anti-inflammatory agents Bioorg Med Chem Lett 2012 22 8 2701 2704 22450132
- Cherala S Lingabathula H Ganta R Ampati S Manda S Synthesis and anti-inflammatory activity of a novel series of diphenyl-1,2,4-triazoles and related derivatives J Chem 2012 9 4 2510 2515
- Amr AE Sabry NM Abdulla MM Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon Monatshefte für Chemie – Chemical Monthly 2007 138 7 699 707
- Wang JY Yamasaki S Takeuchi K Okabe S Delayed healing of acetic acid-induced gastric ulcers in rats by indomethacin Gastroenterology 1989 96 2 Pt 1 393 402 2910759
- Konturek PK Brzozowski T Konturek SJ Dembiński A Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions Gastroenterology 1990 99 6 1607 1615 2227276
- Takeuchi K Pathogenesis of NSAID-induced gastric damage: importance of cyclooxygenase inhibition and gastric hypermotility World J Gastroenterol 2012 18 18 2147 2160 22611307
- Limban C Missir AV Chirita IC Neagu AF Draghici C Chifiriuc MC Synthesis and antimicrobial evaluation of some new 2-(4-fluoro-phenoxymethyl) benzoic acid thioureides Revista de Chimie (Bucharest) 2011 62 168 173
- Limban C Marutescu L Chifiriuc MC Synthesis, spectroscopic properties and antipathogenic activity of new thiourea derivatives Molecules 2011 16 9 7593 7607 21900862
- Bahi A Sadek B Schwed SJ Walter M Stark H Influence of the novel histamine H3 receptor antagonist ST1283 on voluntary alcohol consumption and ethanol-induced place preference in mice Psychopharmacology (Berl) 2013 228 1 85 95 23474889
- Koo HJ Lim KH Jung HJ Park EH Anti-inflammatory evaluation of gardenia extract, geniposide and genipin J Ethnopharmacol 2006 103 3 496 500 16169698
- Komeshima N Osowa T Nishitoba T Jinno Y Kiriu T Synthesis and anti-inflammatory activity of antioxidants, 4-alkylthio-o-anisidine derivatives Chem Pharm Bull (Tokyo) 1992 40 2 351 356 1535026
- Winter CA Risley EA Nuss GW Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs Proc Soc Exp Biol Med 1962 111 544 547 14001233
- Ackrell J Antonio Y Franco F Synthesis and antiinflammatory activity of 6,11-dihydro-11-oxodibenzo[b,e]thiepinalkanoic acids and related compounds J Med Chem 1978 21 10 1035 1044 309946
- Dunn JP Muchowski JM Nelson PH Antiinflammatory 5,6-dihydro-11-oxodibenz[b,e]azepine-3-acetic acids J Med Chem 1981 24 9 1097 1099 6974784
- Erol DD Sunal R Duru S Synthesis and screening of analgesic and anti-inflammatory activity of 6-acyl-3-(4-substituted benzoylmethyl)-2(3H)-benzoxazolones Arzneimittelforschung 1990 40 4 478 480 2357250
- Barsoum FF Hosni HM Girgis AS Novel bis(1-acyl-2-pyrazolines) of potential anti-inflammatory and molluscicidal properties Bioorg Med Chem 2006 14 11 3929 3937 16460945
- Khayyal MT El-Ghazaly MA Abdallah DM Okpanyi SN Kelber O Weiser D Mechanisms involved in the anti-inflammatory effect of a standardized willow bark extract Arzneimittelforschung 2005 55 11 677 687 16366042
- Edwards JC Sedgwick AD Willoughby DA The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system J Pathol 1981 134 2 147 156 7019400
- Rasheed A Kumar CK Mishra A Synthesis, hydrolysis studies and phamacodynamic profiles of amide prodrugs of dexibuprofen with amino acids J Enzyme Inhib Med Chem 2011 26 5 688 695 21250819
- Bekhit AA Fahmy HT Design and synthesis of some substituted 1H-pyrazolyl-oxazolidines or 1H-pyrazolyl-thiazolidines as anti-inflammatory-antimicrobial agents Arch Pharm (Weinheim) 2003 336 2 111 118 12761764