Abstract
Objective
Catalpol, as a natural medicine small-molecule drug, has been proven to have anti-inflammatory and antioxidant pharmacological effects.
Methods
The effect of catalpol on oxidative damage of mouse epidermal fibroblast L929 model and its mechanism were investigated by using hydrogen peroxide model, CCK8 method, flow cytometry, and Western blot.
Results
The effect of catalpol on Nrf2/HO-1 signaling pathway was further studied to improve oxidative stress in cell models. The results showed that catalpol had no cytotoxicity to L929 cells, and inhibited the apoptosis of L929 cells after oxidative damage in a concentration-dependent manner, thus playing a role in cell protection. The oxidative damage of cells was inhibited by up-regulating the expression of the signature protein of Nrf2/HO-1 signaling pathway and inhibiting the interstitial formation of cells.
Conclusion
This study is a preliminary study on the protective function of catalpol against oxidation and apoptosis in dermal fibroblasts, which can provide a theoretical basis and drug guidance for promoting skin wound healing in the later stage.
Introduction
Dermal fibroblasts are the key cells in the wound healing process. When the skin is injured, the corium initiates an inflammatory response to clear tissue and pathogens from the damaged site. Dermal fibroblasts participate in the inflammatory process by releasing cytokines. These cytokines help attract more immune cells to the wound site and stimulate them to release more inflammatory substances in response to injury and infection.Citation1–4
A close relationship exists between the inflammatory response of skin wounds and oxidative stress (OS). During the inflammatory process caused by trauma, cells release reactive oxygen species (ROS), increasing oxidative stress. Under normal circumstances, the ROS production and clearance system in the body is in a state of dynamic balance.Citation3,Citation5 When the body is in the OS state, the ROS content in tissues and cells in the body is relatively increased, exceeding the body’s scavenging ability, which can lead to the increase in lipid peroxidation level in tissues, resulting in oxidative damage of DNA and abnormal expression of protein, causing damage to the body.Citation6,Citation7 Therefore, ROS levels are often used to assess the occurrence of OS.
Catalpol is a traditional Chinese medicine component of iridoid, widely existing in many Chinese medicine components, and can play a therapeutic role in many organs in the body.Citation8 Recent studies have proven that it has multiple pharmacological effects such as antioxidant, anti-inflammatory, anti-tumor, neuroprotective, hypoglycemic, etc.Citation9,Citation10 In recent years, many studies have focused on the mechanism of catalpol’s hypoglycemic and anti-tumor actions in vivo and in vitro.Citation9,Citation11,Citation12 However, the intervention’s effect on dermal fibroblasts’ function is still unclear. Through these experiments, we hope to know whether catalpol can also regulate the oxidative damage of dermal fibroblasts. This could expand the number of cell types catalpol regulates and explore its mechanisms of action in different cells.
Nrf2/HO-1 signaling pathway plays a key role in regulating oxidative stress response, and it has been confirmed that this pathway plays a significant role in oxidative stress.Citation13 As an antioxidant enzyme, Heme Oxygenase-1 (HO-1) has anti-inflammatory, antioxidant, and anti-proliferative effects when stimulating oxidative stress in the body, and is involved in the body’s compensatory mechanism for oxidative stress, hypertension, and vascular reconstruction.Citation14 Nuclear Factor E2-associated Factor 2 (Nrf2) is inhibited in the cytoplasm and is rapidly degraded by the ubiquitin proteasome pathway.Citation15 When stimulated by oxidative stress signals, Nrf2 rapidly decouples with allosteric Kelch-like ECH Associated Protein 1 (Keap1), translocation into the nucleus to form a heterodimer in a stable state. Then, it binds to the gene’s antioxidant response element (ARE). The expression of target genes mediated by ARE is increased to regulate the transcription and translation of antioxidant genes.Citation16,Citation17
This experiment first studied whether catalpol affected the oxidation-damaged mouse epidermal fibroblast L929 model and its mechanism of action.Citation18,Citation19 In addition, we further investigated whether catalpol is involved in the Nrf2/HO-1 signaling pathway to affect oxidative stress in cell models.Citation20 Our study provides new insights into the mechanism of catalpol in oxidation-damaged dermal fibroblasts and valuable data and experience for our team’s subsequent in vivo experiments.Citation21,Citation22 shows the preliminary thinking and experimental prediction of this study.
Materials and Methods
Cell Culture
Mouse dermal fibroblasts L929 (Starfish Biology, TCM-C749) were taken from L929 cells at the logarithmic growth stage and supplemented with 1% penicillin and streptomycin mixture in DMEM/F12 (Corning, 10-092-CVRC) medium containing 10% fetal bovine serum (Soleboll, P1400). Penicillin (10kU/mL) and streptomycin (10mg/mL) in the stock solution were cultured at 37°C and 5% CO2 in a constant temperature incubator.
Materials and Reagents
Catalpol (CAT) (Aladdin, C110215, China), Hydrogen peroxide (H2O2) (Tianjin Damao Chemical Reagent Factory, analytical pure, China), N-acetylcysteine (NAC) (Aladdin, N170064, China), CCK8 (MCE, HY-K0301, NJ, USA), Medium (Corning, 10–092-CVRC, NY, USA), Fetal bovine serum (ExCell Bio, FSP500, China), 0.25% pancreatic enzyme (Gibco, 25200-072, CA, USA), Annexin V-FITC apoptosis detection kit (Beyotime Biotechnology, C1062L, China), Reactive oxygen species detection kit (Beyotime Biotechnology, S0033M, China), RIPA Lysis Solution (Beyotime Biotechnology, P0013, China), 5×loading buffer (Kang Weishi, CW0027S, China), PAGE Gel rapid preparation kit (Yi Sheng 10%: 20325ES, Yi Sheng 15%: 20327ES, China), Nrf2 antibody (Proteintech, lot No. 16396-1-AP, China), HO-1 antibody (Proteintech, Lot No. 16396-1-AP, Batch No. 10701-1-AP, China), Fibroblast-specific protein 1 (S100A4) antibody (Proteintech, Batch No. 16105-1-AP, China), fibroblast marker protein: Anti-smooth muscle antibody (alpha-SMA) antibody (Proteintech, batch No. 14395-1-AP, China).
Instruments
Enzymoscope, VICTOR NivoTM, PerkinElmer Corporation, USA. Flow cytometry, BD FACS CantoII, USA.
The Effect of Catalpol on Cells Was Detected
L929 cells at logarithmic growth stage were washed with PBS and collected after trypsinization at 37°C for 2 min. Then, L929 cells were centrifuged at 200 g for 5 min and resuspend with fresh medium. Thereafter, the cells were inoculated into 96-well plates at 5×103/ well and cultured overnight at 37°C and 5% CO2. The experimental groups treated with different concentrations of catalpol were added (catalpol concentration was 2, 5, 10, 50, 100 μM, respectively) and continued to culture and treat at 5% CO2 and 37°C for 24 hours. After 24 hours, CCK8 was added to detect cell viability in each group. Each group is provided with six multiple holes. Cell relative survival rate = (experimental group OD value − blank group OD value)/(regular control group OD value − empty group OD value). Each experiment was repeated three times.
Establishment of Oxidative Damage Model of Fibroblasts
L929 cells in the logarithmic growth phase were seeded at a density of 5 × 103 cells per well in 96-well plates and incubated overnight at 37°C with 5% CO2. At the second day, the cells were treated with varying concentrations of H2O2 (50, 100, 200, 400, 600, 800, and 1000 μM) for 2 hours. The complete culture medium was used as the control group. After 2 hours, the H2O2-containing medium was replaced with fresh complete medium and incubated for an additional 24 hours. After 24 hours, CCK8 was performed to assess cell activity. The % relative survival rates of cells were plotted against concentrations of H2O2 and IC50 was calculated from the graph. Finally, according to the IC50, the optimal induced concentration of the oxidative damage model was determined. Each group is provided with six multiple holes. Each experiment was repeated three times.
Detect the Effect of Catalpol on Cell Viability
L929 cells were seeded into 96-well plate at a density of 5 × 103 cells/200 μL/well and cultured overnight. L929 cells were treated with different concentrations of catapol (2 μM, 5 μM, 10 μM, 50 μM, and 100 μM), and the positive control NAC (500 μM). After 24 hours incubated, 400 μM H2O2 was added to each well for a 2 hours co-incubation at 37°C. Thereafter, the supernatant was discarded and replaced with a fresh complete culture medium cultured for 24 hours, 48 hours, and 72 hours, respectively. At different time points, the cells were incubated with 10% CCK8 solution for cell viability assay. The OD of each well at 450 nm was detected by a microplate reader after the 2 hours reaction. Each group was set up with three multiple holes, and the experiment was repeated three times. Each experiment was repeated three times.
Detect the Effect of Catalpol on Cell Apoptosis
Grouping and treatment were the same as the Detect the Effect of Catalpol on Cell Viability. The adherent cells were washed with PBS, collected after trypsinization, and washed twice with PBS. Then, resuspend cells in 1× Binding Buffer at a concentration of 1 × 106 cells/mL and transfer 100 µL of the solution to the tube. Add 5µL Annexin V-FITC, mix gently, then add 10 µL propyl iodide stain, and mix gently. Incubate at room temperature (20–25°C) without light for 10–20 minutes and then place in an ice bath and test on the machine within 1 hour. Each experiment was repeated three times.
Detect the ROS Content of the Cells
Grouping and treatment were the same as the Detect the Effect of Catalpol on Cell Viability. The adherent cells were washed with PBS and collected after trypsinization and washed twice with PBS. Then, the cells were resuspended in serum-free medium, and 100 μL DCFH-DA (1:1000) was added and incubated at 37°C without light for 30 min. The mean DCFH-DA was measured by flow cytometric analysis. Each experiment was repeated three times.
Determination of SOD, MDA, and GSH Activity
Group and drug intervention followed the same procedures outlined in the section Establishment of Oxidative Damage Model of Fibroblasts. After 24 hours, except for the blank control, each group was treated with H2O2 for 2 hours, and the H2O2 culture medium was removed and replaced with a complete medium for continuous culture for 24 hours. After 24 hours, L929 cells were broken up by ultrasound treatment and subjected to centrifugation by 8000g for 10 min. The SOD kit (Solarbio, #BC0025), MDA kit (solarbio, #BC0175), and GSH kit (Beytime, #S0053) were used, respectively, for the detections. Different OD values (560 nm for SOD, 532 nm for MDA, and 412 nm for GSH) were measured by a microplate reader. The activity of SOD, MDA, and GSH in each group was calculated according to the kit instructions.
Determination of the Expression Levels of Fibroblast Marker Proteins and Nrf2/HO-1 Signaling Pathway Marker Proteins
Grouping and drug intervention followed the same procedures outlined in the section Detect the Effect of Catalpol on Cell Viability. The total protein was extracted by discarding the supernatant medium and adding protein extraction reagents containing protease and phosphatase inhibitors. A BCA protein kit was used to detect total protein concentration. After 20 μg protein denaturation, the sample was transferred to 0.2 μm PVDF membrane by 10% SDS-PAGE gel electrophoresis, 260 mA, 1 h wet transfer, and sealed with 1×TBST containing skim milk powder /BSA according to the instructions of the primary antibody. The primary antibody (Nrf2, HO-1, S100A4, and α-SMA diluted at 1:1000 and β-actin diluted at 1:500) was added. The film was incubated overnight on a shaking bed at 4°C, washed four times with 1×TBST for 10 min each, and then incubated with a secondary antibody diluted at a ratio of 1:3000 for 1 hour. Following this, the film underwent three washes with 1×TBST for 10 min each, and the image was developed by chemiluminescence gel imager. Each group was set up with three multiple holes, and the experiment was repeated three times. β-actin was the internal reference protein, and Image J was used for gray analysis of the bands. Each experiment was repeated three times.
Statistical Analysis
FlowJo 7.6.1 software for flow analysis. GraphPad Prism 8.0 software plotted each experimental data, and all data were expressed as mean ± standard deviation (). IBM SPSS 23 software was used for statistical analysis of experimental data and one-way AVONA compared differences between groups. The Kolmogorov–Smirnov (K-S) test was used to test the normality of the data, and the F-test for the homogeneity of the variance. Fisher's LSD test was used for planned post-hoc pairwise comparisons. p < 0.05 indicates a statistically significant difference.
Results and Discussion
Toxicity of Catalpol to L929 Cells
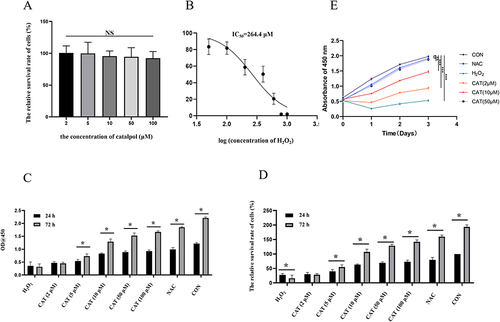
Compared with the regular group, different concentrations of catalpol treated normal L929 cells for 24 hours, and the decreasing trend of cell viability was not noticeable. Cell viability was slightly decreased in the 100 μmol·L−1 catalpol group (p < 0.05), and the lowest value in the six repeated experiments was also above 80%, as shown in .
Figure 2 H2O2-induced oxidative damage cell model and effect of catalpol on viability of cell model with oxidative damage. (A) CCK8 assay for evaluating the effect of catalpol on L929 cell proliferation. (B) Assessment of oxidative damage on L929 cells with H2O2 treated. (C) CCK8 assay evaluating protective effects of catalpol on H2O2 treated L929 cells. (D) IC50 values of H2O2 in L929 cells. Data are presented as the average of repeated samples, with error bars representing standard deviations. *p <0.05. (E) The cell proliferation ability of L929 cells which were pretreatment with NAC and different concentrations of catalpol in H2O2 induced oxidative stress model for 24 h, 48 h, 72 h.
The Cell Model of H2O2-Induced Oxidative Damage Was Optimized
With the increase in H2O2 concentration and time, the activity of L929 cells decreased gradually. After 2-hour treatment with different concentrations of H2O2, the difference in H2O2 concentrations (50, 100, 200, 400, 600, 800, and 1000 μM) was statistically significant, and the IC50 value was 264.4 μM. In order to prevent excessive oxidative damage to L929 cells, 400 μM was selected as the appropriate concentration for the oxidative damage cell model, as shown in .
Effect of Catalpol on Viability of Cell Model with Oxidative Damage
Compared with the negative control group, OD value and relative cell survival rate induced by H2O2 in the catalpol intervention group increased in a concentration-dependent way after incubation for 24 hours and 72 hours, and viability was significantly increased, as shown in and .
Compared to the negative control group, the cells underwent ROS and apoptosis, so after 24 hours and 72 hours, the cell survival rate was lower than that of the standard control group. The relative survival rate of cells in different concentration groups of catalpol increased significantly (p < 0.05), suggesting that catalpol effectively protected L929 cells.
Meanwhile, we evaluated the cell proliferation ability using CCK8 assay, As shown in , H2O2 inhibited the growth of L929 cells. The cell proliferation ability was significantly increased with NAC and different concentrations of catalpol.
Effect of Catalpol on Apoptosis of Model Cells Damaged by Oxidation
Compared with the negative control group, the H2O2-induced apoptosis rate in the catalpol intervention group decreased in a concentration-dependent manner after incubation for 24 hours, and apoptosis was significantly inhibited. Twenty-four-hour flow cytometry showed that the apoptosis rates of negative control group, catalpol intervention group (2, 5, 10, 50, 100 μM) and positive control group were, respectively, 92.31%±1.12%; 86.39%±3.22%; 66.86%±1.81%; 26.01%±2.32%; 5.68%±1.14%; 3.62%±0.79%; 2.91%±0.78%; 1.26%±0.12%. By .
Figure 3 Effect of catalpol on apoptosis of model cells damaged by oxidation. Flow cytometry for evaluating the effect of catalpol on apoptosis in H2O2 treated L929 cells.
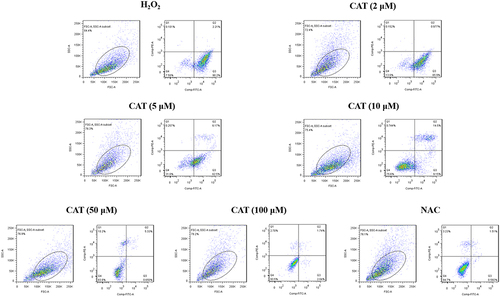
Compared with the negative control group, the apoptosis rate of catalpol in different concentration groups was significantly decreased (p < 0.05), suggesting that catalpol effectively protected L929 cells and prevented apoptosis induced by oxidative damage to a certain extent. Through the data analysis of 3.1–3.4, the appropriate concentration of catalpol in the next experiment was set as follows: 2, 10, and 50 μM.
Effects of Catalpol on ROS in L929 Cells Damaged by Oxidation
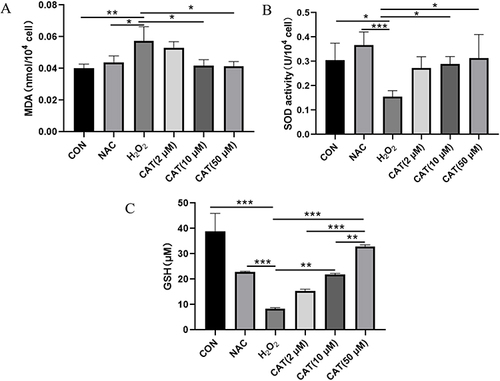
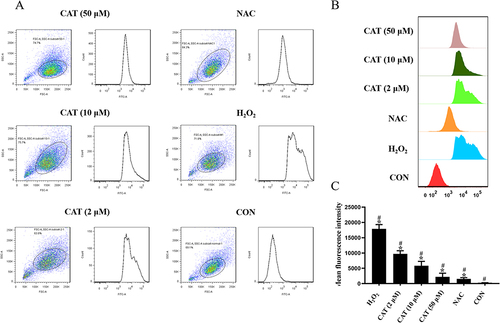
Compared with the blank control group, ROS content in the negative control group was increased (p < 0.05). Compared with the negative control group, ROS content in the catalpol group (2, 10, 50 μM) was dose-dependent decreased (p < 0.05), as shown in . When the concentration of catalpol reached 50 μM, its ROS content was similar to that of the NAC group, thereby reducing cellular oxidative damage, as shown in . shows the average fluorescence intensity of different experimental groups. The moderate fluorescence intensity is proportional to the ROS level, which can also reflect the changes in ROS level in other experimental groups.
Figure 4 Effects of catalpol on ROS in L929 cells damaged by oxidation. (A and B) Flow cytometric analysis of the ROS production in L929 cells with different treatments. (C) Quantitative analysis of DCFH-DA accumulation by FACS. Data are presented as the average of repeated samples, with error bars representing standard deviations. *p <0.05, #p <0.05.
Effects of Catalpol on H2O2-Induced Oxidative Injury in L929 Cells
To examine the effects of catalpol on oxidative stress, we measured the level of SOD, GSH, and MDA. As shown in and , the levels of SOD and GSH were significantly reduced in H2O2-treated L929 cells, but catalpol significantly restored the levels of SOD and GSH dose-dependently. H2O2 leads to the production of ROS and subsequently causes lipid peroxidation of the biomembrane, which leads to the production of a large amount of MDA. Therefore, we measured the levels of MDA in H2O2-treated L929 cells. As shown in , the levels of MDA were significantly elevated with H2O2 treated, whereas decreased in L929 cells pretreated with catalpol. Therefore, catalpol could enhance antioxidant capacity.
Effects of Catalpol on Oxidative Damage of L929 Cell Marker Protein and Nrf2/HO-1 Pathway Protein
Nrf2 and HO-1 are antioxidant stress-related proteins that play a protective role in cells. We assessed the effects of catalpol on the Nrf2/HO-1 pathway. As shown in and , the level of Nrf2 and HO-1 was decreased in H2O2-treated L929 cells. Pretreatment with N-acetyl cysteine (NAC), a free radical scavenger, reversed the H2O2-induced Nrf2/HO-1 down-regulation. Meanwhile, catalpol increased their levels in a dose–effect manner. Thus, Nrf2/HO-1 pathway may be activated by catalpol in L929 cells.
Figure 6 Effects of catalpol on oxidative damage of L929 cell marker protein and Nrf2/HO-1 pathway protein. (A) Western blot analyzed Nrf2, HO-1, α-SMA and S100A4 expression in H2O2-treated L929 cells which were pretreatment with NAC and different concentrations of catalpol for 24 hours. (B) Statistical analysis of Nrf2, HO-1, α-SMA and S100A4 expression in H2O2-treated L929 cells which were pretreatment with NAC and different concentrations of catalpol for 24 hours. Data are presented as the average of repeated samples, with error bars representing standard deviations. *p <0.05, **p <0.01, ***p <0.001.
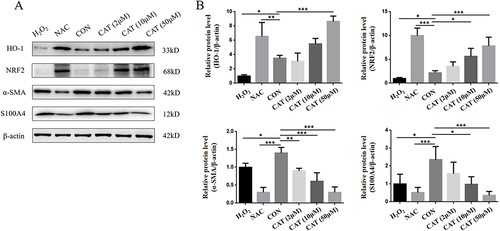
α-SMA and S100A4 are important markers for fibroblasts. In this study, we investigated the effect of Catalpol on α-SMA and S100A4. As shown in , H2O2 decreased fibroblast marker expression with oxidative damage. Oxidative stress is an important factor in activating EMT. Both α-SMA and S100A4 are also epithelial and mesenchymal markers. And considering the antitumor properties of catalpol, L929 cells altered their mesenchymal phenotypes with catalpol treatment. As shown in and , the expressions of α-SMA and S100A4 were reversed in L929 cells pretreated with catalpol. Our result indicated that catalpol altered mesenchymal phenotypes of L929 cells induced by H2O2.
Discussion
The results showed that pretreatment with catalpol significantly increased cell viability and reduced apoptosis in H2O2-treated L929 cells. Catalpol also effectively reduced the production of ROS and free radicals in the cells, indicating its antioxidant properties. Furthermore, catalpol was found to upregulate the expression of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), which play a crucial role in protecting cells from oxidative damage. Overall, these findings suggest that catalpol exerts protective effects on L929 cells against H2O2-induced oxidative stress and apoptosis by enhancing antioxidant defense mechanisms. Therefore, catalpol may have potential therapeutic applications in conditions involving oxidative stress and cell damage. Further research is needed to elucidate the underlying molecular mechanisms of catalpol’s protective effects and explore its potential clinical applications.Citation23,Citation24
The pharmacodynamic experiment of catalpol showed that different concentrations of catalpol (2–100 μM) had no inhibitory effect on the viability of normal L929 cells. According to the analysis of CCK8 and flow cytometry results, catalpol 2, 10, 50 μM were selected as the appropriate dosage concentrations for follow-up experiments. The results of this study showed that catalpol alleviated the inhibition of Nrf2 and up-regulated HO-1 expression, as shown in . briefly describes the process of oxidative stress. depicts the Nrf2/HO-1 signaling pathway. The conclusion is that catalpol can reduce the damage caused by oxidative stress by up-regulating the protein expression of Nrf2/HO-1 signaling pathway. Catalpol and NAC can clear ROS and reduce cell damage caused by ROS.Citation25–27 Catalpol and NAC can protect cells from apoptosis but cannot promote cell viability after treatment. In the previous CCK8 experiment, it was only found that catalpol or NAC-treated cells had no effect on cell viability, while hydrogen-peroxide-treated cells generated ROS and apoptosis, so the cell survival rate was lower than that of the standard control group after 24 hours and 72 hours.
Figure 7 (A) Mechanism diagram of catalpol obtained by experiment; (B) Relationship between OS and H2O2. (C) Schematic diagram of Nrf2/HO-1 signaling pathway.
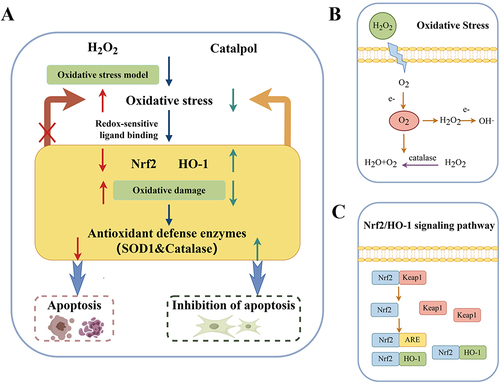
So far, the published studies on catalpol are about its mechanism of action on tumor cells, islet cells, and so on. There is no cell research on the skin yet. Therefore, these experiments are to fill the gap in this aspect.
Conclusion
Catalpol, a new terpenoid hypoglycemic active monomer type, was approved for clinical trials of a new class of traditional Chinese medicine in China in 2017.Citation28 Because it is one of the newly discovered small-molecule drugs, there are still few studies related to catalpol, which can bring us not too much enlightenment. Fortunately, more and more researchers have begun to pay attention to monomer drugs and are keen to use them in the clinic. This is an increasingly clear development for Chinese medicine in the field of medical research around the world. Although the bioavailability of catalpol is low, part of its activity and mechanism of action has been determined, and the optimization of the structure or the use of nano preparation technology is expected to develop drugs to promote skin wound healing in the future. Therefore, it is essential to strengthen the basic research on the antioxidant mechanism of catalpol on fibroblasts.Citation29–34 Before the experiment began, the research group had predicted the correlation between catalpol and the functional targets of L929 cells by molecular docking simulation and speculated that catalpol could reduce the apoptosis induced by excessive oxidative stress and improve the function of L929 cells. This study is a preliminary study on the protective function of catalpol against oxidation and apoptosis in dermal fibroblasts, which can provide a theoretical basis and drug guidance for promoting skin wound healing at a later stage.
Ethical Approval
No ethical approval is required for this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships construed as a potential conflict of interest.
Data Sharing Statement
All the data in the article are obtained by the authors themselves, and the pictures are also drawn by themselves. So no reference to any data set, all original. Six samples were prepared for each data, and the repeated experiment of each sample was carried out as a separate and independent experiment.
Additional information
Funding
References
- Abbasi S, Sinha S, Labit E, et al. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell. 2020;27(3):396–412 e6. doi:10.1016/j.stem.2020.07.008
- Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21(1):18–24. doi:10.1038/s41556-018-0237-6
- Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm Sin B. 2022;12(3):1163–1185. doi:10.1016/j.apsb.2021.08.020
- Wu F, Yang J, Liu J, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6(1):218. doi:10.1038/s41392-021-00641-0
- Komi DEA, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2020;58(3):298–312. doi:10.1007/s12016-019-08729-w
- Lin Y, Jiang M, Chen W, Zhao T, Wei Y. Cancer and ER stress: mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother. 2019;118:109249. doi:10.1016/j.biopha.2019.109249
- Sahoo DK, Heilmann RM, Paital B, et al. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front Endocrinol. 2023;14:1217165. doi:10.3389/fendo.2023.1217165
- Hu H, Wang C, Jin Y, et al. Catalpol inhibits homocysteine-induced oxidation and inflammation via inhibiting Nox4/NF-kappaB and GRP78/PERK pathways in human aorta endothelial cells. Inflammation. 2019;42(1):64–80. doi:10.1007/s10753-018-0873-9
- Bhattamisra SK, Koh HM, Lim SY, Choudhury H, Pandey M. Molecular and biochemical pathways of catalpol in alleviating diabetes mellitus and its complications. Biomolecules. 2021;11(2). doi:10.3390/biom11020323
- Chen H, Deng C, Meng Z, Meng S. Effects of catalpol on Alzheimer’s disease and its mechanisms. Evid Based Complement Alternat Med. 2022;2022:2794243. doi:10.1155/2022/2794243
- Bai Y, Zhu R, Tian Y, et al. Catalpol in diabetes and its complications: a review of pharmacology, pharmacokinetics, and safety. Molecules. 2019;24(18). doi:10.3390/molecules24183302
- Qiao PF, Yao L, Zeng ZL. Catalpol-mediated microRNA-34a suppresses autophagy and malignancy by regulating SIRT1 in colorectal cancer. Oncol Rep. 2020;43(4):1053–1066. doi:10.3892/or.2020.7494
- Li B, Nasser MI, Masood M, et al. Efficiency of Traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed Pharmacother. 2020;126:110074. doi:10.1016/j.biopha.2020.110074
- Ryter SW. Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation. Cells. 2021;10(3). doi:10.3390/cells10030515
- He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21(13). doi:10.3390/ijms21134777
- Gao X, Hu W, Qian D, et al. The mechanisms of ferroptosis under hypoxia. Cell Mol Neurobiol. 2023;43(7):3329–3341. doi:10.1007/s10571-023-01388-8
- Khoshandam A, Razavi BM, Hosseinzadeh H. Interaction of saffron and its constituents with Nrf2 signaling pathway: a review. Iran J Basic Med Sci. 2022;25(7):789–798. doi:10.22038/IJBMS.2022.61986.13719
- Patil S, Licari FW, Bhandi S, et al. The cytotoxic effect of thermoplastic denture base resins: a systematic review. J Funct Biomater. 2023;14(8). doi:10.3390/jfb14080411
- Peranidze K, Safronova TV, Kildeeva NR. Electrospun nanomaterials based on cellulose and its derivatives for cell cultures: recent developments and challenges. Polymers. 2023;15(5). doi:10.3390/polym15051174
- Zhang Z, Dai Y, Xiao Y, Liu Q. Protective effects of catalpol on cardio-cerebrovascular diseases: a comprehensive review. J Pharm Anal. 2023;13(10):1089–1101. doi:10.1016/j.jpha.2023.06.010
- Bhattamisra SK, Yap KH, Rao V, Choudhury H. Multiple biological effects of an iridoid glucoside, catalpol and its underlying molecular mechanisms. Biomolecules. 2019;10(1). doi:10.3390/biom10010032
- Fu Z, Su X, Zhou Q, Feng H, Ding R, Ye H. Protective effects and possible mechanisms of catalpol against diabetic nephropathy in animal models: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1192694. doi:10.3389/fphar.2023.1192694
- He L, Zhao R, Wang Y, Liu H, Wang X. Research progress on catalpol as treatment for atherosclerosis. Front Pharmacol. 2021;12:716125. doi:10.3389/fphar.2021.716125
- Zheng XW, Yang WT, Chen S, et al. Neuroprotection of catalpol for experimental acute focal ischemic stroke: preclinical evidence and possible mechanisms of antioxidation, anti-inflammation, and antiapoptosis. Oxid Med Cell Longev. 2017;2017:5058609. doi:10.1155/2017/5058609
- Jia J, Chen J, Wang G, Li M, Zheng Q, Li D. Progress of research into the pharmacological effect and clinical application of the traditional Chinese medicine Rehmanniae Radix. Biomed Pharmacother. 2023;168:115809. doi:10.1016/j.biopha.2023.115809
- Teschke R. Treatment of Drug-Induced Liver Injury. Biomedicines. 2022;11(1). doi:10.3390/biomedicines11010015
- Yuan H, Yang M, Han X, Ni X. The therapeutic effect of the Chinese herbal medicine, rehmanniae radix preparata, in attention deficit hyperactivity disorder via reversal of structural abnormalities in the cortex. Evid Based Complement Alternat Med. 2018;2018:3052058. doi:10.1155/2018/3052058
- Dinda B, Dinda M, Kulsi G, Chakraborty A, Dinda S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: a review. Eur J Med Chem. 2019;169:185–199. doi:10.1016/j.ejmech.2019.03.009
- Bai R, Jie X, Yao C, Xie Y. Discovery of small-molecule candidates against inflammatory bowel disease. Eur J Med Chem. 2020;185:111805. doi:10.1016/j.ejmech.2019.111805
- Kalkreuter E, Pan G, Cepeda AJ, Shen B. Targeting bacterial genomes for natural product discovery. Trends Pharmacol Sci. 2020;41(1):13–26. doi:10.1016/j.tips.2019.11.002
- Liu Y, Yang S, Wang K, et al. Cellular senescence and cancer: focusing on traditional Chinese medicine and natural products. Cell Prolif. 2020;53(10):e12894. doi:10.1111/cpr.12894
- Nasim N, Sandeep IS, Mohanty S. Plant-derived natural products for drug discovery: current approaches and prospects. Nucleus. 2022;65(3):399–411. doi:10.1007/s13237-022-00405-3
- Yin B, Fang DM, Zhou XL, Gao F. Natural products as important tyrosine kinase inhibitors. Eur J Med Chem. 2019;182:111664. doi:10.1016/j.ejmech.2019.111664
- Zheng W, Wu Y, Gao H, Ouyang D. Traditional Chinese medicine injections: where we are after 80-year development. Chin Med. 2022;17(1):127. doi:10.1186/s13020-022-00681-w