Abstract
Background
In a recent study utilizing a saline-lavaged adult rabbit model, we described a significant improvement in systemic oxygenation and pulmonary shunt after the instillation of a novel synthetic peptide-containing surfactant, Synsurf. Respiratory distress syndrome in the preterm lamb more closely resembles that of the human infant, as their blood gas, pH values, and lung mechanics deteriorate dramatically from birth despite ventilator support. Moreover, premature lambs have lungs which are mechanically unstable, with the advantage of being able to measure multiple variables over extended periods. Our objective in this study was to investigate if Synsurf leads to improved systemic oxygenation, lung mechanics, and histology in comparison to the commercially available porcine-derived lung surfactant Curosurf® when administered before first breath in a preterm lamb model.
Materials and methods
A Cesarean section was performed under general anesthesia on 18 time-dated pregnant Dohne Merino ewes at 129–130 days gestation. The premature lambs were delivered and ventilated with an expiratory tidal volume of 6–8 mL/kg for the first 30 minutes and thereafter at 8–10 mL/kg. In a randomized controlled trial, the two surfactants tested were Synsurf and Curosurf®, both at a dose of 100 mg/kg phospholipids (1,2-dipalmitoyl-L-α-phosphatidylcholine; 90% in Synsurf, 40% in Curosurf®). A control group of animals was treated with normal saline. Measurements of physiological variables, blood gases, and lung mechanics were made before and after surfactant and saline replacement and at 15, 30, 45, 60, 90, 120, 180, 240 and 300 minutes after treatment. The study continued for 5 hours.
Results
Surfactant treatment led to a significant improvement in oxygenation within 30 minutes, with the Synsurf group and the Curosurf® group having significantly higher ratios between arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2; P = 0.021) compared to that of the control (saline-treated) animals. Dynamic compliance improved in the three groups over time, with no intergroup differences. All of the surfactant-treated animals survived, and one in the saline group died before the study ended. Histology between groups was not different, showing mild–moderate injury patterns.
Discussion: Treatment with surfactants before first breath clearly resulted in improved systemic oxygenation within 30 minutes of instillation. Both Synsurf- and Curosurf®-treated animals experienced similar and more sustained improvement in oxygenation and decreased calculated shunt compared to saline-treated animals.
Introduction
The essential role of hydrophobic surfactant proteins SP-B and SP-C in natural surfactant function has been well described.Citation1–Citation3 Increasingly, the recombinant production of these hydrophobic proteins has been the focus for the development of synthetic surfactants, especially after the protein-free synthetic surfactants were shown to perform somewhat inferiorly to mammalian-derived surfactants in the acute management of new-born infants with respiratory distress syndrome (RDS).Citation4 Some in vitro studies have shown that mixtures of phospholipids with SP-B yield surfactants that are more efficacious than SP-C-based surfactants, with SP-B-based preparations displaying markedly lower susceptibility to fibrinogen inhibition than SP-C preparations.Citation5,Citation6 However, head-to-head trials comparing various animal-derived surfactants that provide varying amounts of SP-B or phospholipids have shown minor differences in outcomes related to the management of RDS or none at all. Post hoc analysis of data from one study showed better survival using a high initial dose of Curosurf® (Chiesi Farmaceutici SpA, Parma, Italy) when compared with Survanta® (Abbott Laboratories, Abbott Park, North Chicago, IL, USA).Citation7 New surfactant preparations that include peptides or whole proteins that mimic endogenous surfactant protein have recently been developed and tested.Citation8–Citation10 A new-generation synthetic surfactant that contains a peptide mimicking the action of SP-B, Surfaxin (Discovery Laboratories, Warrington, PA, USA), performed better than a protein-free synthetic surfactant (Exosurf; GlaxoKlineSmith, Brentford, UK), and similarly to animal-derived surfactants (Survanta® and Curosurf®).Citation11 A Cochrane review identified two studies that compared protein- containing synthetic surfactants to animal-derived surfactant preparations. In a meta-analysis of these two studies, infants who received protein-containing synthetic surfactant compared to animal-derived surfactant extract did not demonstrate significant differences in primary outcomes, ie, death and chronic lung disease.Citation12
In a recent study utilizing a saline-lavaged adult rabbit model, we described a significant improvement in systemic oxygenation and pulmonary shunt after the instillation of a novel synthetic peptide-containing surfactant, Synsurf.Citation8 Our objective in the present study was to investigate if Synsurf leads to improved systemic oxygenation, lung mechanics, and histology in comparison to the commercially available porcine-derived lung surfactant Curosurf® when administered before first breath in a preterm lamb model.
Materials and methods
Surfactant preparations
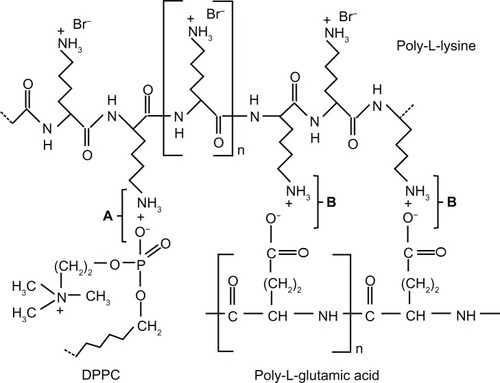
Synsurf was prepared as described previously.Citation8 Briefly, dipalmitoylphosphatidylcholine (DPPC), hexadecanol, and phosphatidylglycerol were mixed in a 10:1.1:1 ratio (w/w) in chloroform. The organic solvent was then removed by rotary evaporation, and the mixture was dried under a continuous stream of nitrogen at room temperature. Poly-L-lysine (~100–120 residues) was mixed with poly-L-glutamate (~80 residues) and incubated at 37°C in 0.1 M NaCl to give a complex which is 50% neutralized. The dried phospholipid film was then hydrated with the polymer mixture (3% by weight of the phospholipid concentration) and gently mixed in the presence of glass beads. The mixture was ultrasonicated on ice under a stream of nitrogen (20 watts for 7 × 13 seconds at 60-second intervals). Thereafter, 24 mg of tyloxapol was added to the preparation, and the tube was sealed under nitrogen before use. The molecular structure of the two-stranded polymer and possible interactions with DPPC is shown in . Both Synsurf and Curosurf® (Chiesi Farmaceutici SpA) were used at a dose of 100 mg/kg phospholipids.
Figure 1 Molecular structures of poly-L-lysine, poly-L-glutamic acid, and dipalmitoylphosphatidylcholine (DPPC).
Delivery and ventilation of lambs
Animal care and experimental procedures were performed under approval from the Faculty of Medicine and Health Sciences Research Committee of Stellenbosch University. Eighteen time-dated pregnant Dohne Merino ewes at 129–130 days gestation (normal gestation 148–154 days) were anesthetized with intravenous 2.5% thiopental sodium, intubated, and mechanically ventilated with inhalation of 1%–2% halothane in oxygen. A Cesarean section was performed and the fetal head and neck were exteriorized. To withdraw lung fluid subcutaneous lignocaine (2%) was infiltrated into the midtracheal region with a 24-gauge needle attached to a syringe. Ten to 15 mL of fetal lung fluid was sampled to determine lung maturity (lamellar body count) and to remove excessive lung fluid. A neck incision was then made, followed by a tracheotomy. An uncuffed 4 mm or 4.5 mm endotracheal tube was placed and advanced to 4 cm. The umbilical cord was cut, the fetus was then delivered weighed dried and placed in a supine position on warming pads under a radiant warmer. Within 2 minutes after umbilical cord ligation, before first breath, two of the groups received 100 mg/kg of either Synsurf or Curosurf®. The third group (control) received normal saline. The lambs were anesthetized and sedated with continuous infusion of midazolam (Dormicum® [Roche, Basel, Switzerland], 0.1 mg/kg/hour) and morphine (5–10 μg/kg/hour). Supplemental intermittent ketamine dosages (10 mg/kg) were administered when spontaneous breathing was observed on the flow curves. Animals were paralyzed with intravenous pancuronium bromide (0.1 mg/kg, hourly). A rectal thermistor was placed to continuously monitor body temperature, which was maintained at 38°C–39°C. To minimize lung injury, ventilation during the first 30 minutes after birth was initiated with time-cycled, pressure-limited assist control ventilation (AVEA® ventilator system; CareFusion, San Diego, CA, USA). The expiratory tidal volume was set at 6–8 mL/kg and was then increased to 8–10 mL/kg. Hereafter, the ventilator settings were held constant throughout the study at an inspired O2 fraction (FiO2) of 1.0, a rate of 50 breaths/minute, an inspiratory time of 0.50 seconds, and a positive end-expiratory pressure of 4 cm H2O. Immediately after ventilation was started, a 5 French catheter was passed via the femoral artery into the aorta for monitoring blood pressure and heart rate and for blood gas measurements. Each lamb received a continuous infusion of 5% dextrose water that was begun at the start of the study. Lambs were assigned to one of three groups (six lambs/group). Measurements of physiological variables, blood gases, and lung mechanics (tidal volume, dynamic compliance of the respiratory system [Cdyn]) were made before and after surfactant and saline replacement and at 15, 30, 45, 60, 90, 120, 180, 240 and 300 minutes after treatment. After 5 hours, all live animals were killed by lethal injection of intra-arterial 15% potassium chloride. The chest wall was opened and quasistatic maximal inspiratory capacity of the intact lung at 35 cm H2O peak plateau pressure and positive end-expiratory pressure of zero was determined after exsanguination. In lambs without a pneumothorax, the lung (right lung caudal, middle, and cranial lobes) was then gravitationally filled with formaldehyde (4%) and inflated under a constant pressure of 25 cm H2O. This lung was used for histology and morphometry. Total lung water was determined after the animals were killed by calculating the difference between the wet weight minus the weight of the lung exposed to 80°C until the weight was constant for 48 hours (~4–6 days). It is expressed as the lung wet weight/dry weight ratio.
Statistical analysis
STATISTICA version 10 (StatSoft, Tulsa, OK, USA) and GraphPad Prism 5 (GraphPad Software Inc, La Jolla, CA, USA) were used to determine comparability of the experimental groups before and after surfactant instillation. Changes of variables between the groups were analyzed with one-way analysis of variance (and nonparametric). The Kruskal–Wallis test and Dunn’s multiple comparison test were used as the discriminating post-test. Data are expressed as mean ± standard deviation. Significant differences were accepted at P-values < 0.05.
Mean linear intercept analysis
The mean linear intercept as an indicator of alveolar airspace size was calculated from counting lines of defined length as previously described by Dunnill.Citation13 Briefly, the lines were randomly placed on every lung section, and the number of intersections crossing the lines were counted. The mean linear intercept is calculated from the length of the lines multiplied by the number of lines, divided by the sum of all counted intercepts.
Results
Respiratory outcomes
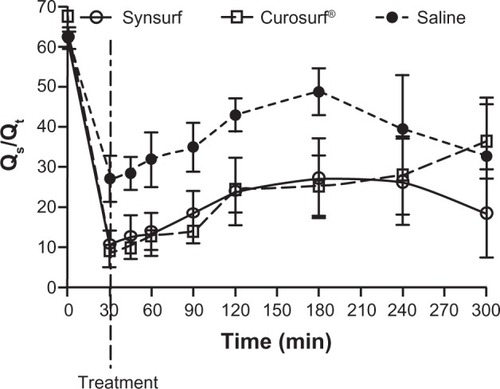
At randomization, arterial blood gases, ventilator indexes, and hemodynamic variables were similar for all three groups. gives an overall summary of the pretreatment parameters of the three groups. All lambs were severely surfactant-deficient as their lamellar body counts were less than 15,000/μL and lecithin/sphingomyelin ratios were less than 2. The surfactant deficiency was further reflected in the overall low mean Cdyn (mean for n = 18, 0.31 ± 0.09 mL/cmH2O kg) and poor oxygenation status as reflected by a low arterial/alveolar ratio (mean for n = 18, 0.03 ± 0.01) and a high oxygenation index (mean for n = 18, 53.69 ± 32.1). There were no significant differences between the three groups. Following instillation of surfactant, the Synsurf group and the Curosurf® group experienced significant improvement in oxygenation (arterial partial pressure of oxygen [PaO2]/FiO2 ratio, P = 0.021) within 30 minutes ( and ), compared to that of the saline-treated animals. Moreover, both surfactant-treated groups showed better oxygenation over the time period of the study. For Synsurf-treated animals, oxygenation was significantly better compared to the saline-treated animals at timepoints 30, 45, 60, 90, 120 and 180 minutes. With exception of the 120-minute timepoint, the finding was similar for the Curosurf® versus saline-treated animals. However, at 300 minutes the Synsurf-treated animals had a significantly better oxygenation status when compared to Curosurf® (P = 0.014863, determined by mixed model repeated measures analysis of variance). A decrease in calculated pulmonary shunt (time period 0–300 minutes; ) in the Synsurf, Curosurf®, and saline-treated group of animals was observed (intergroup differences: Synsurf 18.82 ± 16.19 versus Curosurf® 22.35 ± 18.42, P = 0.09; and Synsurf versus saline 34.41 ± 12.52, P = 0.003; Friedman analysis of variance). At 300 minutes the mean calculated value for Synsurf was 18.44%, versus 36.29% and 32.63% for the Curosurf® group and the saline-treated group, respectively. Significant lower pulmonary shunt values for Synsurf-treated animals occurred at 180 minutes (P < 0.05, two-way analysis of variance, Synsurf versus the saline group) and at 300 minutes (P = 0.0002, t-test, Synsurf versus the saline group and the Curosurf®group). The arterial partial pressure of carbon dioxide (PaCO2) also decreased from about 70 mmHg to about 58 mmHg in the surfactant-treated animals ().
Table 1 Indexes, lung mechanics, cord blood gasses, and calculated shunt
Table 2 Physiological variables, lung mechanics, and postnatal blood gasses
Figure 2 Time profiles for PaO2/FiO2 and PaCO2.
Abbreviations: FiO2, fraction of inspired oxygen; PaCO2, arterial partial pressure of CO2; PaO2, arterial partial pressure of oxygen.
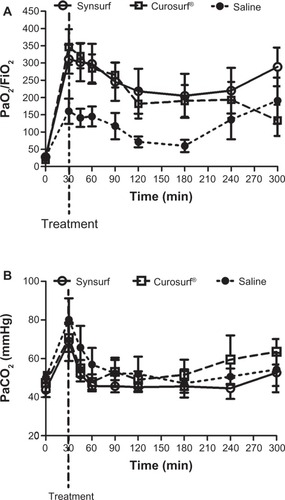
Figure 3 Time profile of a comparison of pulmonary shunt (Qs/Qt) between lamb groups before and after administration of Synsurf or Curosurf® or saline.
Pulmonary mechanics
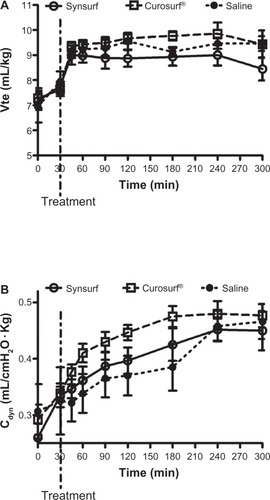
To avoid compromising the effect of surfactant rescue treatment and to minimize lung over-distension, initial ventilation with an expiratory tidal volume of 6–8 mL/kg and thereafter 8–10 mL/kg was used. As a consequence, we found no significant differences between the Synsurf and Curosurf® groups for expiratory tidal volumes () as well as for the arterial PaCO2 values () between the start of the study and at 300 minutes. Total Cdyn () steadily increased in all of the study groups over time between timepoint 0 minutes (start of experiment) and 300 minutes (P = 0.0012, P = 0.00026, and P = 0.0268 for Synsurf, Curosurf®, and saline, respectively). There were no significant intergroup differences at 300 minutes (Synsurf Cdyn 0.45 ± 0.09, Curosurf® Cdyn 0.48 ± 0.05, and saline Cdyn 0.47 ± 0.07). All of the surfactant-treated animals survived and one in the saline group died before the study ended. However, two lambs developed a pneumothorax during the study, one each in the saline group and Synsurf-treated group. Representative lungs in the three groups were used for quasistatic lung volume measurements, as determined in the open-chest state for maximal inspiratory capacity. Values were 36.22 ± 10.21 mL/kg for a saline-treated lamb, 33.95 ± 8.03 mL/kg for a Curosurf®-treated lamb, and 32.96 ± 1.98 mL/kg for a Synsurf-treated lamb. There were no statistical intergroup differences.
Figure 4 Time profiles of expiratory tidal volume (Vte) and dynamic respiratory compliance (Cdyn) of lambs treated with Synsurf or Curosurf ® or saline before first breath.
Indicators of inflammation
Systemic inflammation
The cord blood had the typical lymphocytosis of fetal blood for all study groups (). After 5 hours of ventilation the numbers of white blood cells changed significantly only in the Curosurf®-treatment group. In this group, the mean peripheral white blood cell count rose from 1.2 to 2.5 (P = 0.0236), with significant intragroup increase in the neutrophil count. In all of the groups, the predominant shift from a lymphocytosis in cord blood to a neutrophilic predominance occurred after 5 hours of ventilation. There were, however, no significant differences between the groups.
Table 3 Cord and peripheral white blood cell (WBC) and differential count
Lung inflammation and appearance and morphology
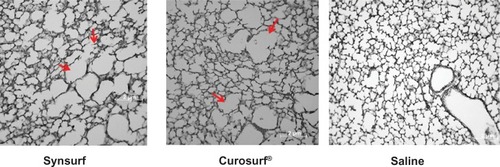
The macroscopic appearance of lungs of the majority of lambs was categorized as mild, moderate, or severe (widespread) atelectasis as reflected by the extent of visible dark areas (). There were no clear visible group differences with regard to the distribution of the injury. In almost all of the groups, except for one lamb in the Curosurf® group, the posterior regions of the lungs, especially the lower and inferior regions of the lobes, showed atelectasis (dark areas) with or without small hemorrhages. Of the Synsurf-treated lambs, two had mild () and four had moderate atelectasis (). Of the Curosurf®-treated lambs, one had a normal, uniform, expanded lung appearance (), three had mild (), and two had moderate () atelectasis. The lungs of only four saline-treated animals were available; one showed mild atelectasis (), two had moderate (), and one had severe () atelectasis. Representing the average size of the alveoli, measurements of the mean linear intercepts () of the Curosurf® group and the Synsurf group were higher than that of the saline group. However, this was not statistically significant (intergroup differences: Synsurf 48.68 ± 5.16 versus Curosurf® 49.82 ± 8.78, P = 0.804; Synsurf versus saline 43.10 ± 3.79, P = 0.115; and Curosurf® versus saline, P = 0.192), and therefore excluded alveolar damage as a result of differences in applied volume/pressure during ventilation. Enlargement of histomorphology microscopic images () in the different groups showed no accumulation of alveolar macrophages or white blood cells in the alveoli of the animals that received Curosurf® or Synsurf.
Figure 5 Representative photographs of the appearance of lungs of Synsurf- (A and B), Curosurf®- (C–E), and saline-treated lambs (F–H).
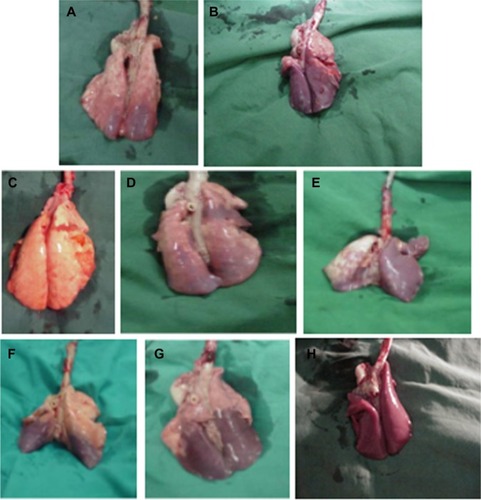
Figure 6 Mean linear intercepts of airspaces for Synsurf-, Curosurf®-, and saline-treated animals.
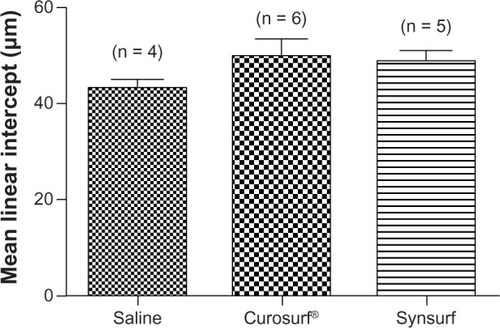
Figure 7 Lung histology findings in representative animals of each treatment group.
Calculations for postmortem lung water content, expressed as the lung wet weight/dry weight ratio (Synsurf 6.23 ± 0.65, Curosurf® 5.67 ± 1.12, and saline 6.49 ± 1.57), showed no significant difference between any of the groups.
Discussion
We have demonstrated that a novel synthetic peptide-containing surfactant (Synsurf), resulted in a more sustained, improved oxygenation response compared to that of Curosurf®- or saline-treated premature lambs when treated before first breath.
Commercially available surfactants for replacement therapy are still mainly mammalian-derived, and their superiority over nonprotein-containing surfactants in the treatment of neonatal RDS has repeatedly been shown.Citation12,Citation14,Citation15 More recently, synthetic formulations containing an SP-C and/or SP-B analog have been developed and tested against mammalian-derived surfactants. Davis et al evaluated the efficacy of recombinant SP-C surfactant against natural sheep surfactant in vitro and in vivo using ventilated preterm lambs and rabbits.Citation16 They found that both surfactants were sensitive to inhibition by plasma and had similar lung mechanics, lung volume measurement, and indicators of lung injury. Interestingly, a synthetic formulation (CHF5633) developed by Chiesi Farmaceutici SpA was recently compared with Survanta®, but not with Curosurf®, in extremely premature lambs (day 124).Citation10 The authors found that the SP-B/SP-C analog-containing synthetic surfactant (200 mg/kg CHF5633) improved lung functions (tidal volumes at 2 minutes and dynamic lung compliance at 300 minutes) over that of Survanta® (100 mg/kg). Both surfactant-treated groups showed large variations in alveolar size, with patchy atelectasis in the peripheral lung and similar degrees of lung inflammation at 5 hours.
Of all the protein components in pulmonary surfactant, SP-B and SP-C have an essential function in the spreading, adsorption, and stability of surfactant lipids. Fourier transform infrared spectroscopy as well as infrared studies of porcine SP-B demonstrate that it possesses approximately 45% α-helical content, four amphipathic helices, and a β-sheet secondary structure (22%) interacting with areas of the phospholipid bilayers.Citation17,Citation18 Various research groups have chemically prepared peptides with sequences based on SP-B.Citation19,Citation20 Recently, the successful treatment of preterm infants with RDS was demonstrated with a peptide/phospholipid mixture KL-surfactant prepared by Cochrane et al.Citation9 This peptide, that possibly mimics the pattern of hydrophobic and hydrophilic residues in SP-B, is 21 amino acids long and contains five basic lysine (hydrophilic) residues, which interact with the negative charges of phospholipid polar head groups, and 16 leucine (hydrophobic) residues that interact with the acyl side chains of the phospholipids.Citation21–Citation23 Moreover, it was designed with the intent to stabilize the phospholipid bilayer through its interaction with lipid head groups and portions of the fatty acid acyl chains. An initial study on the mechanism of interaction of KL4 peptide with phospholipid multilayers showed that it predominantly has α-helical content with a transmembrane orientation in lipid multilayers.Citation21 However, in follow up studies it was shown that with DPPC films, the peptide adopted an antiparallel β-sheet structure.Citation9
In our experiments, we reasoned that the polypeptide poly-L-lysine, which is widely used in membrane research due to its interaction with acidic lipids, was worth studying as a KL4 alternative. To test this idea, poly-L-lysine (~100–120 residues) was mixed with poly-L-glutamic acid (~80 residues) to give a complex (~50% neutralisation) which is held together by strong electrostatic interaction (). The rationale behind using polymers of poly-L-lysine complexed with poly-L-glutamic acid was to have a two-stranded polymer containing a percentage of basic lysyl side chains participating in the formation of protein-lipid bonds, as well as some degree of hydrophobicity in the neutralized double-stranded portion of the molecule. It should be noted that reports indicate that electrostatic binding is coupled with hydrophobic effects in protein-lipid bonding and that electrostatic interaction precedes hydrophobic interaction.Citation24,Citation25 Moreover, poly-L-lysine also becomes increasingly helical with charge cancellation above 50%.Citation26 At present, in preliminary experiments using Fourier transform infrared spectroscopy (van Zyl, unpublished data, 2012), we have found results which suggest the existence of molecular zipper-like properties of poly-L-lysine and poly-L-glutamic acid, which was first described by Dzwolac and Marszalek.Citation27 In these preliminary experiments, at neutral pD (experiments were carried out in D2O), we also found that the two form sequenceless poly-L-lysine-poly-L-glutamic acid polymers in the random coil conformation (amide I′ band) self-assemble to form an antiparallel β-sheet with a characteristic splitting of the amide I′ band into a major and minor spectral component. However, a persisting amide I′ band still reflected residual loose chains (random coil characteristics). At present, we suggest that these chains of the polymer mixture will favor the exposure of the basic-charged surface groups on the lysine side chains, whereby the construct could interact flexibly with other molecules such as phosphatidyl glycerol to perform a functional role in phospholipid monolayers. Furthermore, in agreement with other reports, we also assume that part of the beneficial effect of poly-L-lysine could relate to the interaction of the charged lysine amino groups with the anionic lipid headgroups and the ability to dehydrate the lipid bilayers.Citation28,Citation29 Furthermore, from the finding that positive charges are important for maintaining the structure and function of SP-C,Citation30,Citation31 the argument can be made that the overall positive character of poly-L-lysine residues in Synsurf could contribute to the mimicking of SP-C structural and/or functional properties. This could also facilitate the action of Synsurf in our preterm lamb study, as it was shown that in the presence of phosphatidylglycerol, SP-C decreased the energy barrier limiting the adsorption of phospholipids to the air/liquid interface, possibly by introducing electrostatic interactions. As to the exact mechanism of action of the poly-L-lysine–poly-L-glutamic acid construct, further studies are in progress.
The recently developed Synsurf was also tested earlier, in vivo in an adult rabbit saline-lavaged model.Citation8 In that study, we showed significant improvement in oxygenation and pulmonary shunt in the group of animals treated with Synsurf. The premature lamb model is an alternative test system, with a clinical and pathological course similar to that described for preterm newborns with RDS. We therefore conducted the present randomized trial to compare systemic oxygenation and lung mechanics in surfactant-deficient preterm lambs after treatment with either Synsurf (synthetic) or porcine-derived Curosurf® (natural) and saline as control before first breath.
We removed 10–20 mL/kg lung fluid before instilling vehicle and initiating ventilation in order to avoid ventilation on top of the fluid volume and thus injuring the lung. Furthermore, vehicle at equivalent dosages was administered as bolus before first breath in order to facilitate more uniform spreading, followed by ventilation with lower tidal volume ventilation for the first 30 minutes, again to minimize injurious ventilation.Citation32 Despite attempts to improve distribution of surfactant in a uniform manner, inhomogeneity probably still occurred during ventilation, as between gestation days 120–130 the preterm lamb has lungs that are differentially matured, ie, the upper portions of the lungs are functionally more mature than the lower portions, and possibly receive better tidal volume ventilation.Citation33 This was demonstrated by the distribution of visible dark areas of the excised lungs in the present study. Lambs were ventilated in the supine position, and it was evident that the dark areas which reflect atelectasis were mostly found in the posterior and posterior-inferior regions of the lungs, with no significant differences in distribution between the groups. Nevertheless, before receiving surfactant, all of the lambs had significant surfactant deficiency. Lambs that received surfactant responded with a marked increase in oxygenation and decrease in calculated shunt within 30 minutes of birth. Over the study period, replacement with Synsurf or Curosurf® resulted in a sustained, improved oxygenation response compared to saline-treated animals. The improved oxygenation was accompanied by a significantly improved calculated shunt over time in all three groups. However, at 300 minutes, Synsurf-treated lambs had a significantly higher PaO2/FiO2 ratio accompanied by a lower calculated shunt (Synsurf versus saline at 180 minutes and Synsurf versus saline- and Curosurf®-treated lambs at 300 minutes; ). Notably, the Synsurf-treated lambs not only had a more sustained improvement in oxygenation, they also had lower tidal volumes and associated lower PaCO2 levels (), similar to what was described by Wolfson et al for lambs treated with lucinactant in their study.Citation34
Furthermore, although Cdyn increased significantly only in the surfactant-treated groups, their time profiles did not differ significantly from that of saline-treated animals. In the present study, this trend ( versus ) is similar to the previous study that we conducted in adult rabbits, where we observed an increase in Cdyn in relation to a concurrent decrease in oxygenation over time.Citation8
Our findings of modestly improved Cdyn values over the 5-hour study period in all three study groups is of interest because the changes in Cdyn were not accompanied by similar improvement in oxygenation. In fact, the Curosurf®-treated lambs experienced the steadiest improvement in Cdyn, yet their timepoint-corresponding PaO2/FiO2 ratios were decreasing. Only in the Synsurf-treated group at timepoint 300 minutes did the improved PaO2/FiO2 ratio correlate significantly with their corresponding improved Cdyn values (R2 = 0.7047; P = 0.0366). A novel peptide-containing synthetic surfactant, Surfaxin®, a SP-B mimetic, was recently approved by the US Food and Drug Administration. This surfactant was evaluated against porcine- and bovine-derived surfactants in the 125–128-day preterm lamb model in regard to its potential to modulate lung inflammation following initiation of mechanical ventilation.Citation34 Surfactant treatment after commencing ventilation improved lung compliance to a similar extent compared to no surfactant treatment. In agreement with our findings, the researchers also described “uncoupling” between sustained improved oxygenation and lung mechanics in lucinactant-treated animals. They suggested that the uncoupling is due to the presence of SP-B or SP-B-mimicking peptide, KL4, in the surfactants, which support alveolar capillary shape by reducing surface tension, thereby improving pulmonary blood flow and shunt.Citation35 Our study showed uncoupling between Cdyn and oxygenation and calculated intrapulmonary shunt, favoring the Synsurf-treated animals over that of Curosurf®- and saline-treated lambs.
Discrepancy between the time course of PaO2 and that of Cdyn was also described by Häfner et al in premature lambs at 124–127 days gestation after treatment with bovine lung surfactant at 50 mg/kg body weight.Citation36 Cummings et al reported contradictory findings in preterm lambs treated with a mammalian-derived product (calf lung surfactant extract) before first breath.Citation37 They found significantly improved calculated compliance values, accompanied by improved oxygenation status in preterm lambs (127 days gestation) treated before commencing with artificial ventilation. Interestingly, in that study, the superior oxygenation status and improved calculated compliance observed between lambs treated before first breath and those treated after 5 minutes of ventilation could not be explained by differences in histopathology. The authors therefore speculated that uneven surfactant distribution in an air-filled lung as compared to a liquid-filled lung could have produced the differences.
Does exogenous surfactant instilled before initiating ventilation (prophylactic treatment) protect the immature lung against ventilation-induced lung injury? Contradictory and at times confusing evidence exists, with some research groups showing no significant injury, while others find an incomplete protective effect of natural surfactants that is linked to specific time frames after birth. For instance, Björklund et al studied immature lambs at 127 days gestation.Citation38 They found that three of four lambs treated with high dosage Curosurf® before delivery (235 mg/kg and 265 mg/kg) or before first breath (200 mg/kg) showed no evidence of lung injury after 4 hours of ventilation. In another study, the same research group administered Curosurf® (200 mg/kg) to lambs at various time intervals after birth at 127 days gestation and then subjected the lambs to various inflation treatment groups.Citation39 Although the group did not publish clear quantitative histological data, they found that although surfactant treatment before first breath did not prevent lung injury, it did protect against severe lung injury. In contrast to these findings, Ikegami et al showed that extreme styles of ventilation had minimal effects on lung function, surfactant function, or metabolism in their study group of 131 ± 1 days preterm lambs treated with sheep surfactant before initiation of ventilation.Citation40 Somewhat inexplicably, in that study, lambs ventilated with low-rate (15 breaths/min) and high tidal volume ventilation (15 mL/kg) had higher PaO2/FiO2 ratios for lower mean airway pressures at 24 hours of age compared to a high frequency ventilation and high rate positive pressure ventilation group. The same research group studied 126- or 127-day gestation lambs and assessed tidal volume effects on surfactant treatment responses with the initiation of ventilation.Citation41 In one of their study arms, lambs were randomized to surfactant (Survanta® 100 mg/kg) treatment before initiation of ventilation and then ventilated with 6 mL/kg, 12 mL/kg, or 20 mL/kg tidal volumes for 30 minutes and thereafter with 10 mL/kg tidal volumes to 6 hours of age. In contrast to their previous study findings, lambs initially ventilated with 6 mL/kg had lower dry-to-wet ratios and significantly less recovery of protein in alveolar washes compared to the 12 and 20 mL/kg tidal volume groups. The study showed that the combination of surfactant treatment at birth, followed by ventilation with tidal volumes <12 mL/kg decreases the leak of albumin into the preterm lung and is therefore less injurious.
Lung inflammation and systemic activation of inflammation follows tidal volume ventilation-induced stretch of immature lungs within 5–15 minutes after birth.Citation41–Citation43 However, when low tidal volumes (5–9 mL/kg) are targeted and maintained throughout a 5-hour protocol, minimal lung inflammation, expressed as bronchoalveolar lavage fluid inflammatory cells, or expression of proinflammatory cytokines are found.Citation10
Jaarsma et al demonstrated a drop in polymorphonuclear leukocytes (PMNs) within 5–10 minutes of birth in ventilated 132-day premature lambs as a marker of inflammation.Citation43 Carlton et al described that circulating PMNs transiently disappeared from blood within 2 hours after birth due to sequestration in the lungs of preterm lambs (127 ± 1 days gestation) with RDS.Citation44 They also found that the circulating PMN cell numbers returned to prenatal values by 6–8 hours after birth.Citation44 The peptide-containing synthetic surfactant lucinactant, has recently been shown to be associated with less systemic (plasma IL [interleukin]-6 and IL-8) and lung inflammation (proinflammatory markers), better lung expansion, and lower ventilator requirements than no surfactant treatment or treatment with porcine-and bovine-derived surfactants.Citation34 We found that a systemic inflammatory response as reflected by changes in PMNs was only detectable in the Curosurf® group, but although the total white blood cell count and neutrophil count in this group increased significantly between birth and 5 hours of life, it was no different from the values found in the other two groups at 5 hours. In both the saline- and Synsurf-treated groups, no significant changes in neutrophil counts or total white blood cell count occurred. Our findings are therefore in keeping with those described by Carlton et al, showing comparative PMNs and total white blood cell count numbers between cord blood (prenatal) and 5 hours of life.Citation44 Furthermore, we found no relationship between changes in neutrophil counts and PaO2/FiO2 or PaCO2 respectively, in order to demonstrate an influence of systemic inflammation in the lung on gas exchange and a relationship with respiratory compromise. Moreover, there was no accumulation of alveolar macrophages or white blood cells in the alveoli of the animals that received Curosurf® or Synsurf (histology of saline-instilled lungs were similar to that of surfactant-treated lungs). Taken together with the findings of a relative lack of lung inflammation, we speculate that surfactant administration before first breath did not exert a clear anti-inflammatory protective effect, but possibly the initiation of ventilation utilizing a low tidal volume may have played a role.
Why did the Synsurf-treated animals have a more sustained oxygenation response and significantly better PaO2/FiO2 ratio at 300 minutes? Explanations may include earlier studies that have shown that clinical responses after surfactant treatment in experimental animals is unpredictable when based on in vitro (bench) properties, and surfactants behave differently with respect to loss from the lungs and sensitivity to soluble airway proteins.Citation45,Citation46 In other words, the surfactant with the best in vitro characteristics may not necessarily perform better than others in the preterm lamb model.
Conclusion
To conclude, treatment with surfactants before first breath clearly resulted in improved systemic oxygenation, with the response of Synsurf-treated animals being more sustained at 300 minutes. The functional importance of the synthetic peptide complex in Synsurf was therefore apparent from the results obtained with a control group and the group of lambs treated with an equivalent dosage of Curosurf®.
Acknowledgments
The authors gratefully acknowledge Professor G Maritz of the University of the Western Cape for histomorphology analysis of lung tissue and valuable comments made in preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work. The surfactant has been patented by InnovUS (Stellenbosch University).
References
- Pérez-Gil J Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions Biochim Biophys Acta 2008 1778 7–8 1676 1695 18515069
- Whitsett JA Weaver TE Hydrophobic surfactant proteins in lung function and disease N Engl J Med 2002 347 26 2141 2148 12501227
- Hawgood S Surfactant protein B: structure and function Biol Neonate 2004 85 4 285 289 15218284
- Moya FR Gadzinowski J Bancalari E International Surfaxin Collaborative Study Group A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants Pediatrics 2005 115 4 1018 1029 15805380
- Wang Z Gurel O Baatz JE Notter RH Differential activity and lack of synergy of lung surfactant proteins SP-B and SP-C in interactions with phospholipids J Lipid Res 1996 37 8 1749 1760 8864959
- Seeger W Günther A Thede C Differential sensitivity to fibrinogen inhibition of SP-C- vs SP-B-based surfactants Am J Physiol 1992 262 3 Pt 1 L286 L291 1550251
- Ramanathan R Rasmussen MR Gerstmann DR Finer N Sekar K North American Study Group A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants Am J Perinatol 2004 21 3 109 119 15085492
- van Zyl JM Smith J Hawtrey A The effect of a peptide-containing synthetic lung surfactant on gas exchange and lung mechanics in a rabbit model of surfactant depletion Drug Des Devel Ther 2013 7 139 148
- Cochrane CG Revak SD Merritt TA The efficacy and safety of KL4-surfactant in preterm infants with respiratory distress syndrome Am J Respir Crit Care Med 1996 153 1 404 410 8542150
- Sato A Ikegami M SP-B and SP-C containing new synthetic surfactant for treatment of extremely immature lamb lung PLoS ONE 2012 7 7 e39392 22808033
- Moya F Maturana A Animal-derived surfactants versus past and current synthetic surfactants: current status Clin Perinatol 2007 34 1 145 177 viii 17394936
- Pfister RH Soll RF Wiswell T Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome Cochrane Database Syst Rev 2007 4 CD006069
- Dunnill MS Quantitative methods in the study of pulmonary pathology Thorax 1962 17 4 320 328
- Halliday HL Surfactants: past, present and future J Perinatol 2008 28 Suppl 1 S47 S56 18446178
- Suresh GK Soll RF Lung surfactants for neonatal respiratory distress syndrome: animal-derived or synthetic agents? Pediatric Drugs 2002 4 8 485 492 12126452
- Davis AJ Jobe AH Häfner D Ikegami M Lung function in premature lambs and rabbits treated with a recombinant SP-C surfactant Am J Respir Crit Care Med 1998 157 2 553 559 9476872
- Vandenbussche G Clercx A Clercx M Secondary structure and orientation of the surfactant protein SP-B in a lipid environment. A Fourier transform infrared spectroscopy study Biochemistry 1992 31 38 9169 9176 1390703
- Andersson M Curstedt T Jörnvall H Johansson J An amphipathic helical motif common to tumourolytic polypeptide NK-lysin and pulmonary surfactant polypeptide SP-B FEBS Lett 1995 362 3 328 332 7729523
- Waring A Taeusch W Bruni R Synthetic amphipathic sequences of surfactant protein-B mimic several physicochemical and in vivo properties of native pulmonary surfactant proteins Pept Res 1989 2 5 308 313 2562485
- Bruni R Taeusch HW Waring AJ Surfactant protein B: lipid interactions of synthetic peptides representing the amino-terminal amphipathic domain Proc Natl Acad Sci U S A 1991 88 16 7451 7455 1871144
- Gustafsson M Vandenbussche G Curstedt T Ruysschaert JM Johansson J The 21-residue surfactant peptide (LysLeu4)4 Lys(KL4) is a transmembrane alpha-helix with a mixed nonpolar/polar surface FEBS Lett 1996 384 2 185 188 8612820
- Cochrane CG Revak SD Pulmonary surfactant protein B (SP-B): structure-function relationships Science 1991 254 5031 566 568 1948032
- Cochrane CG Revak SD Protein-phospholipid interactions in pulmonary surfactant. The Parker B. Francis Lectureship Chest 1994 105 Suppl 3 57S 62S 8131614
- Cai P Flach CR Mendelsohn R An infrared reflection-absorption spectroscopy study of the secondary structure in (KL4)4 K, a therapeutic agent for respiratory distress syndrome, in aqueous monolayers with phospholipids Biochemistry 2003 42 31 9446 9452 12899632
- Hammes GG Schullery SE Structure of macromolecular aggregates. II. Construction of model membranes from phospholipids and polypeptides Biochemistry 1970 9 13 2555 2563 5456729
- Kimelberg HK Papahadjopoulos D Interactions of basic proteins with phospholipid membranes. Binding and changes in the sodium permeability of phosphatidylserine vesicles J Biol Chem 1971 246 4 1142 1148 5543681
- Dzwolak W Marszalek PE Zipper-like properties of [poly(L-lysine) + poly(L-glutamic acid)] β-pleated molecular self-assembly Chem Commun (Camb) 2005 28 44 5557 5559 16358062
- Ciferri A Puett D Rajagh L Hermans J Potentiometric titrations and the helix-coil transition of poly(L-glutamic acid) and poly-L-lysine in aqueous salt solutions Biopolymers 1968 6 8 119 136 5663413
- Carrier D Dufourcq J Faucon J-F Pézolet M A fluorescence investigation of the effects of polylysine on dipalmitoylphosphatidylglycerol bilayers Biochim Biophys Acta 1985 820 1 131 139
- Carrier D Pézolet M Investigation of polylysine-dipalmitoylphosphatidylglycerol interactions in model membranes Biochemistry 1986 25 14 4167 4174 3741849
- Creuwels LA Boer EH Demel RA van Golde LM Haagsman HP Neutralization of the positive charges of surfactant protein C. Effects on structure and function J Biol Chem 1995 270 27 16225 16229 7608188
- Ueda T Ikegami M Rider ED Jobe AH Distribution of surfactant and ventilation in surfactant-treated preterm lambs J Appl Physiol 1994 76 1 45 55 8175544
- Brumley GW Chernick V Hodson WA Normand C Fenner A Avery ME Correlations of mechanical stability, morphology, pulmonary surfactant, and phospholipid content in the developing lamb lung J Clin Invest 1967 46 5 863 873 6025487
- Wolfson MR Wu J Hubert TL Gregory TJ Mazela J Shaffer TH Lucinactant attenuates pulmonary inflammatory response, preserves lung structure, and improves physiologic outcomes in a preterm lamb model of RDS Pediatr Res 2012 72 4 375 383 22821059
- Ikegami M Weaver TE Grant SN Whitsett JA Pulmonary surfactant surface tension influences alveolar capillary shape and oxygenation Am J Respir Cell Mol Biol 2009 41 4 433 439 19202005
- Häfner D Hanauer G Kilian U Beume R Dillmann G Randomized placebo-controlled study on the characteristics and duration of action of surfactant treatment in premature lambs Pulm Pharmacol 1993 6 4 255 262 8148579
- Cummings JJ Holm BA Nickerson PA Ferguson WH Egan EA Pre- versus post-ventilatory surfactant treatment in surfactant-deficient preterm lambs Reprod Fertil Dev 1995 7 5 1333 1338 8848608
- Björklund LJ Ingimarsson J Curstedt T Larsson A Robertson B Werner O Lung recruitment at birth does not improve lung function in immature lambs receiving surfactant Acta Anaesthesiol Scand 2001 45 8 986 993 11576050
- Ingimarsson J Björklund LJ Curstedt T Incomplete protection by prophylactic surfactant against the adverse effects of large lung inflations at birth in immature lambs Intensive Care Med 2004 30 7 1446 1453 15045168
- Ikegami M Wada K Emerson GA Rebello CM Hernandez RE Jobe AH Effects of ventilation style on surfactant metabolism and treatment response in preterm lambs Am J Respir Crit Care Med 1998 157 2 638 644 9476883
- Wada K Jobe AH Ikegami M Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs J Appl Physiol 1997 83 4 1054 1061 9338410
- Jaarsma AS Braaksma MA Geven WB Van Oeveren W Oetomo SB Early activation of inflammation and clotting in the preterm lamb with neonatal RDS: comparison of conventional ventilation and high frequency oscillatory ventilation Pediatr Res 2001 50 5 650 657 11641462
- Jaarsma AS Braaksma MA Geven WB van Oeveren W Bambang Oetomo S Activation of the inflammatory reaction within minutes after birth in ventilated preterm lambs with neonatal respiratory distress syndrome Biol Neonate 2004 86 1 1 5 14739550
- Carlton DP Albertine KH Cho SC Lont M Bland RD Role of neutrophils in lung vascular injury and edema after premature birth in lambs J Appl Physiol 1997 83 4 1307 1317 9338441
- Bernhard W Mottaghian J Gebert A Rau GA von Der HARDT H Poets CF Commercial versus native surfactants. Surface activity, molecular components, and the effect of calcium Am J Respir Crit Care Med 2000 162 4 Pt 1 1524 1533 11029372
- Ikegami M Agata Y Elkady T Hallman M Berry D Jobe A Comparison of four surfactants: in vitro surface properties and responses of preterm lambs to treatment at birth Pediatrics 1987 79 1 38 46 3642431