Abstract
B-type natriuretic peptide (BNP) is a member of the natriuretic peptide family, a group of widely distributed, but evolutionarily conserved, polypeptide mediators that exert myriad cardiovascular effects. BNP is a potent vasodilator with mitogenic, hypertrophic and pro-inflammatory properties that is upregulated in pulmonary hypertensive diseases. Circulating levels of BNP correlate with mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance (PVR) in patients with pulmonary arterial hypertension (PAH). Elevated plasma BNP levels are associated with increased mortality in patients with PAH and a fall in BNP levels after therapy is associated with improved survival. These findings have important clinical implications in that a noninvasive blood test may be used to identify PAH patients at high-risk of decompensation and to guide pulmonary vasodilator therapy. BNP also has several biologic effects that could be beneficial to patients with PAH. However, lack of a convenient method for achieving sustained increases in circulating BNP levels has impeded the development of BNP as a therapy for treating pulmonary hypertension. New technologies that allow transdermal or oral administration of the natriuretic peptides have the potential to greatly accelerate research into therapeutic use of BNP for cor pulmonale and pulmonary vascular diseases. This review will examine the basic science and clinical research that has led to our understanding of the role of BNP in cardiovascular physiology, its use as a biomarker of right ventricular function and its therapeutic potential for managing patients with pulmonary vascular disease.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive vasculopathy characterized by extensive remodeling of the pulmonary circulation that results in narrowing of the arterial lumen and impaired flow-mediated vasodilation. The marked increase in pulmonary vascular resistance limits the rate at which the right ventricle can pump blood through the lungs, causing shortness of breath and reduced functional capacity. The disease can occur at any age, but frequently affects young adults in the third or fourth decade of life who are otherwise healthy. Until recently, there were no specific therapies available to treat this disease and most patients progressed to right ventricular failure and death within 2 to 5 years of diagnosis.Citation1 In 1995, intravenous epoprostenol became the first drug approved for the treatment of PAH in the United States. Since that time 6 more medications have been approved and several more await consideration by the FDA. These medications have improved survival and exercise capacity, but none have been able to achieve a cure and the majority of patients continue to progress to right ventricular failure, albeit at a slower pace. The lack of a cure and the severity with which PAH strikes has generated considerable interest in furthering the understanding of the pathogenesis of this disease and the development of new diagnostic and therapeutic modalities to manage it.
Figure 1 Structure of the gene and the biosynthetic pathway of human brain natriuretic peptide (BNP). The major storage form of BNP in the heart is the cleaved mature peptide, although in atrial tissue also prohormones may be stored. Reproduced with permission from Barr CS, Rhodes P, Struthers AD. C-type natriuretic peptide. Peptides. 1996;17(7):1243–1251.Citation197 Copyright © 1996 Elsevier.
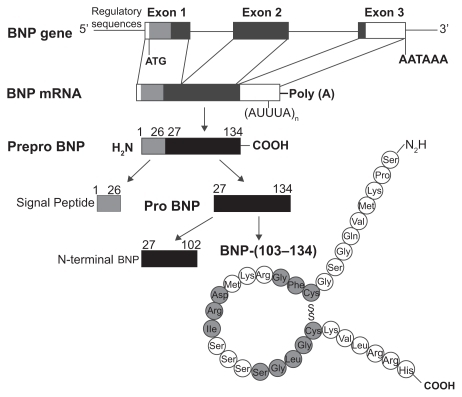
Brain natriuretic peptide (BNP) is one of several members of the natriuretic peptide family that are widely distributed, but evolutionarily conserved throughout the animal kingdom. The natriuretic peptides play important roles in cardiovascular homeostasis including regulation of vascular tone, blood volume, endothelial permeability, and cardiac hypertrophy. Cardiac BNP expression is markedly upregulated in response to ventricular hypertrophy or overload and circulating BNP levels can increase by 1 to 2 orders of magnitude. There is increasing evidence that BNP may be a useful marker for right ventricular dysfunction and predict outcome in patients with PAH. As such, plasma BNP may help guide clinical decision making in patients with newly diagnosed PAH and help gauge response to treatment. BNP is also a pulmonary vasodilator and anti-hypertrophic agent and thus, may have therapeutic potential to inhibit pulmonary vascular remodeling and maladaptive increases in right ventricular mass.
This paper examines the role of BNP in assessing the severity of pulmonary vascular disease and its potential as a therapeutic agent for the treatment of PAH. The synthesis, secretion and biologic properties of BNP, relevant to its role in mediating pulmonary vascular tone, is discussed first followed by a review of its inhibitory effects on pulmonary vascular remodeling, cardiac hypertrophy and endothelial cell apoptosis. Finally, potential therapeutic applications for the treatment of PAH and right heart failure are presented.
Pulmonary arterial hypertension
Pulmonary hypertension (PH) refers to a variety of conditions in which pulmonary arterial pressure (PAP) is elevated above normal. Mean pulmonary arterial pressure (mPAP) in a healthy adult at rest averages about 14 to 17 mmHg. Pulmonary hypertension is defined as a resting mPAP > 25 mmHg or a mPAP > 30 mmHg during exercise and a pulmonary capillary wedge pressure (PCWP) < 15 mmHg. Providing that cardiac output is not elevated, this results in a pulmonary vascular resistance (PVR) > 3 Wood units. Most patients with PAH have a mPAP that is considerably higher, however. In fact, the mean mPAP of over 600 PAH patients in the French pulmonary hypertension registry was 55 ± 15 mmHg, more than double the cut off level of 25 mmHg.Citation2 The most common causes of elevated PAP are chronic lung and left sided heart disease. Elevated PAP in these conditions occurs as the result of hypoxia, loss of the pulmonary vascular bed or elevated pulmonary venous pressure due to impaired left sided heart function. Rarely, PH occurs as the result of abnormal constriction and remodeling of the pulmonary arteries in the absence of any other underlying cardiopulmonary disease. This type of PH represents a true pulmonary artery vasculopathy, described by Dresdale et al in 1951 as primary pulmonary hypertension.Citation2 Since then, the term secondary PH has been used to describe PH associated with heart and lung disease or other identifiable causes of increased pulmonary vascular resistance. Major advances in our understanding of pulmonary vascular disease led to new terminology proposed during the 4th World Symposium on PH held in 2008 at Dana Point, California that organizes pulmonary hypertension into 5 groups to reflect the major underlying etiologies and sites of injury ().Citation3
Table 1 Updated clinical classification of pulmonary hypertension (Dana Point 2008)Citation3
PAH is a disease that is characterized by hypertrophy and proliferation of pulmonary vascular smooth muscle and subsequent thickening of the medial wall of mid sized pulmonary arteries and the muscularization of more peripheral, normally non-muscularized vessels.Citation4 Marked proliferation of endothelial cells in distal pulmonary arteries is also evident and in some areas causes near obliteration of the vascular lumen with evidence of recanulized channels (plexiform lesions). These changes in both the smooth muscle and endothelial layers substantially reduce the cross sectional area of the pulmonary vascular tree.Citation5 At the same time, pulmonary vascular tone may be increased and the ability of the pulmonary circulation to dilate or recruit underutilized vessels in response to increased flow is reduced.Citation6 As remodeling of the pulmonary vascular bed progresses, PVR rises, increasing right ventricular afterload and leading to decreased functional capacity and eventually right ventricular failure.Citation7
The diagnosis and treatment of PAH is hampered by difficulty in obtaining accurate measurements of PAP. Unlike the systemic circulation where intravascular pressure is easily measured by syphgmometry, there is no accurate, readily available noninvasive test for measuring PAP. Right heart catheterization (RHC) provides the direct measurement of PAP and is vital in the appropriate diagnosis and evaluation of unexplained PH, but it is invasive and expensive and not amenable to repeated measurements that are necessary to monitor disease progression. As a result, the clinician must rely on a combination of indirect assessments of PAP and cardiac output such as transthoracic echocardiography, 6-minute walking distance, and WHO functional class. A simple noninvasive, but objective measure of right ventricular afterload would contribute significantly to the management of PAH patients. Biomarkers that correlate with PAP and/or right sided filling pressures would allow the practitioner insight into how a patient’s disease is progressing and allow the physician to evaluate the effect of pulmonary vasodilator therapy. Plasma BNP levels rise in response to right as well as left atrial or ventricular overload and may be an important tool for assessing right ventricular performance in PAH.
The treatment of PAH has grown considerably in the last decade. Presently, 7 different medications belonging to 3 distinct pharmaceutical classes are available for the management of PAH. However, in the great majority of patients, none of these treatments are capable of restoring normal pulmonary hemodynamics and exercise capacity and have limited ability to improve right heart function. Furthermore, all 3 classes focus primarily on vasodilation or remodeling of the pulmonary circulation. Additional therapies are needed that aid the right ventricle in maintaining adequate systolic function and inhibit maladaptive hypertrophic changes in response to persistent elevation in afterload.
Brain natriuretic peptide
BNP release
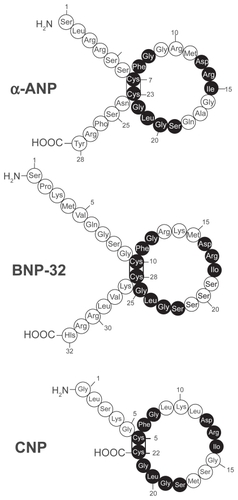
Human BNP is a 32-amino acid polypeptide secreted predominantly by the cardiac atria and ventricles. BNP is synthesized as a preprohormone of 134 residues that is cleaved to yield a 108-amino-acid prohormone (proBNP1 – 108). The prohormone is subsequently cleaved by an unknown protease to an inactive 76-residue amino-terminal (NT) fragment known as NT-BNP and the 32 aminoacid, carboxyl-terminal 32 fragment that is the biologically active peptide.Citation8–Citation12 The size of the final, fully processed BNP fragment varies between species. Human, pig, and dog BNP contain 32 amino acids,Citation13,Citation14 whereas rat and mouse BNP is a 45 amino acid peptide.Citation8,Citation10,Citation11,Citation15–Citation17 Compared to the sequence homology of atrial natriuretic peptide (ANP) and C-type natriuretic peptide (CNP) that are tightly conserved, the sequence homology of BNP differs across species both in size and amino acid sequenceCitation17,Citation18 (). This sequence variability may explain, in part, the variations of BNP biological activity in different species.
Figure 2 Amino acid structure of the 3 natriuretic peptides, atrial, brain and c-type natriuretic peptide (ANP, BNP, CNP). All 3 peptides share a similar 17 amino acid loop. Shaded circles represent amino acid sequence that is identical in each peptide. Reproduced with permission from Barr CS, Rhodes P, Struthers AD. C-type natriuretic peptide. Peptides. 1996;17(7):1243–1251.Citation197 Copyright © 1996 Elsevier.
Although originally discovered in porcine brain, human BNP is expressed primarily in the cardiac atria and ventricles. The tissue concentration of BNP in the ventricle constitutes only about 1% of the atrium. However, the total BNP mRNA content is almost 3-fold higher in the ventricle.Citation19 The majority of circulating BNP (>60%) is released from ventricular tissues.Citation19–Citation22 This is in distinction from ANP, the great majority of which is synthesized and released by the cardiac atria.Citation19 These findings have prompted some investigators to suggest that ANP and BNP make up a dual cardiac peptide system with ANP the primary atrial peptide and BNP the primary ventricular peptide. In fact, some investigators have suggested that BNP be renamed to ventricular natriuretic peptide to denote its greater importance in the cardiovascular system and to distinguish it from ANP.
Both atrial and ventricular BNP expression is induced within 1 hr of myocardial stretch from increases in venous volume or arterial pressure.Citation23,Citation24 Other stimuli that induce cardiac BNP secretion include hypoxia, ischemia, and states of nephrosis and cirrhosis that are associated with an increase in central blood volume.Citation25–Citation27 Several neuroendocrine mechanisms also mediate cardiac BNP release.Citation28,Citation29 For example, α-adrenergic stimulation with phenylephrine results in increases in both BNP gene expression and BNP secretion, whereas myocardial stretch and endothelin-1 (ET-1) induce a marked increase in BNP gene expression alone.Citation29,Citation30
Atrial myocytes store pre-synthesized ANP and BNP in secretory granules and release them via a regulated pathway.Citation31 Unlike ANP, however, most studies in normal subjects show no significant change in BNP levels in response to exercise.Citation32–Citation35 In patients with cardiac disease such as ischemia, hypertension or heart transplant recipients, exercise does induce a significant increase in BNP secretion.Citation32–Citation37 These findings suggest that only under pathologic conditions is atrial BNP secreted during exercise-induced heart stress via a regulatory pathway in quantities sufficient to increase circulating levels. Ventricular myocytes also synthesize and secrete both peptides, but through a constitutive pathway.Citation20 Hence, it is possible that BNP is secreted by ventricular tissue during exercise. Most of the immediate release of BNP likely derives from atrial stores followed later by gene transcription and new protein synthesis from atrial and ventricular sources. BNP mRNA has a faster turnover rate than ANP due to the presence of mRNA-destabilizing AUUUA repeats in the 3′-untranslated region. This allows the synthesis and secretion of BNP in the ventricles to be closely controlled by mRNA levels according to changing amounts of load on the myocardium. The 5′-untranslated region of the gene possesses shear stress response elements and activation of MAPK and nuclear factor κB (NF-κB) is thought to be important in upregulating expression.Citation38 The nuclear transcription factor, GATA 4, plays a dominant role in regulating this process.Citation39–Citation41 The ability of BNP to be secreted from the heart in response to neurohormonal as well as hemodynamic stimuli and the rapid induction of BNP gene expression in response to ventricular strain make it a robust biomarker of cardiac stress and facilitates its use as a clinical tool.
BNP receptors
The diverse biological effects of BNP are mediated primarily by a membrane-bound particulate guanylyl cyclase receptor called natriuretic peptide receptor-A (NPR-A). NPR-A is widely expressed in the cardiovascular system including the cardiac atria and the ventricles as well as the aorta and peripheral vasculature, lungs, kidney, skin, platelets, and presynaptic sympathetic fibers.Citation42–Citation47 NPR-A has much higher affinity for ANP and BNP, than for CNP, but BNP is approximately 10-fold less potent than ANP in generating particulate cGMP.Citation48–Citation50 Another membrane bound guanylyl-cyclase linked receptor, NPR-B, demonstrates high affinity for CNP and much lower affinity for ANP and BNP.Citation49,Citation51 Both NPR-A and NPR-B function as particulate guanylyl cyclasesCitation43,Citation52 distinct from the cytoplastic soluble gaunylyl cyclase stimulated by nitric oxide (NO).Citation53
Ligand binding to NPR-A or NPR-B activates the intracellular guanalyl cyclase domain of the receptor and catalyzes the synthesis of cGMP. There are three known proteins that bind cGMP with high affinity and act as downstream targets: cGMP-dependent protein kinase (PKG), cGMP binding phosphodiesterases (PDEs), and cyclic nucleotide-gated ion channels. PKG appears to be the principal intracellular mediator of cGMP signals.Citation54–Citation58 Natriuretic peptide-induced elevation of intracellular cGMP causes a binding-dependent activation of PKG leading to the catalytic transfer of phosphate from ATP to a serine or threonine residue of target proteins. The phosphorylated proteins then determine the translation of the extracellular stimulus into a specific biological function.Citation59
Cyclic nucleotides such as cGMP and cAMP are metabolized by a family of phosphodiesterases (PDE).Citation60–Citation63 PDE-1, -2, -3, -10, and -11 degrade both cGMP and cAMP; PDE-4, -7, and -8 specifically hydrolyze cAMP; whereas PDE-5, -6, and -9 are highly selective for cGMP.Citation64 In vascular tissue, PDE-3 and -4 are responsible for most of the degradation of cAMP and PDE-5 is the major metabolic enzyme for cGMP. Despite the relative selectivity of PDE isoforms, both cAMP and cGMP can act as substrate for the other’s primary PDE and thereby impede its metabolism. There is clear evidence that this interaction between cyclic nucleotides and PDEs allows for crosstalk and integration between different pathways.Citation60 PDE-5 is expressed in most vascular smooth muscle, is found in high concentration in the lung, and is increased in the lungs in experimental models of pulmonary hypertension.Citation65,Citation66 PDE-I in heart, lungs, brain, and smooth muscle is activated by binding of Ca2+-calmodulin to cause a decrease in cyclic nucleotide (cGMP or cAMP) concentration. Some PDEs are regulated allosterically by cGMP. For instance, in addition to its catalytic domain, PDE-5 has additional binding sites for both cGMP and PKG that serve to stabilize the enzyme and enhance cGMP metabolism resulting in a feed-forward mechanism
BNP metabolism and clearance
Circulating natriuretic peptides are removed or metabolized via two major routes. One involves binding of the natriuretic peptide to natriuretic peptide receptor-C (NPR-C). NPR-C has an extracellular domain similar to that of NPR-A and NPR-B, but no intracellular guanylyl cyclase domain. Binding to the receptor is followed by endocytosis and lysosomal degradation,Citation67 NPR-C has high affinity for all natriuretic peptides with a rank order of ANP ≥ CNP > BNP in both humans and ratsCitation51,Citation68 and has been proposed to function primarily as a clearance receptor,Citation46,Citation69 although other biologic effects have been described.Citation42
The second mode of metabolism is peptide cleavage and inactivation by neutral endopeptidase 24.11 (NEP).Citation70 This membrane bound zinc metalloproteinase is found on the luminal surface of endothelial cells and is particularly concentrated at the brush border membranes in the proximal tubule of the kidney. NEP inactivates and degrades both ANP and BNP,Citation70,Citation71 by opening their ring structures.Citation72 The relative importance of the two clearance mechanisms is controversial, but it has been shown that in chronic heart failure, the clearance receptor NPR-C may play a lesser role than NEP in natriuretic peptide metabolism.Citation73 Renal excretion of the peptides has also been described,Citation74 but probably plays a minor role in regulating circulating concentrations of natriuretic peptides.
The circulating BNP level measured at any given time point is the result of the balance between BNP secretion and degradation. BNP has a plasma half-life of about 20 minutes, which is substantially longer than the 4 minute half-life reported for ANP.Citation75 The longer half-life may be the result of more resistance to degradation by NEPs and less avid binding to NPR-C than ANP.Citation76 Healthy individuals have plasma BNP concentrations around 1 fmol/ml (3.5 pg/ml) or approximately one-tenth that of ANP.Citation77 However, plasma BNP levels in congestive heart failure patients can be 200- to 300-fold higher. The enormous range of plasma BNP concentrations between healthy subjects and patients with heart failure and the rapid induction of BNP expression in response to acute overload or ischemia make it well suited to serve as an indicator of elevated myocardial loading and cardiac stress.Citation78,Citation79 The amino terminal fragment of proBNP (NT-proBNP) is less susceptible to degradation by NEP and is not cleared by NPR-C. As such, it may represent a more reliable indicator of cardiac BNP expression and secretion than circulating BNP levels. In fact, some studies suggest that plasma NT-proBNP levels may be a more sensitive and specific marker of cardiovascular disease than BNP.Citation80,Citation81 In patients, with left ventricular (LV) dysfunction NT-proBNP levels are up to 10 times higher than BNP.Citation82 Unfortunately, NT-proBNP assays are not readily available in most clinical laboratories. A number of factors have been associated with higher BNP levels in addition to ventricular wall stress. Both BNP and NT-proBNP increase with age independent of the increased incidence of diastolic dysfunction.Citation83 BNP and NT-proBNP levels are also higher in women than men at any age.Citation84 The association between BNP and renal function is complex. Patients with chronic renal disease tend to have higher atrial pressure, systemic pressure, and ventricular mass, all of which would be expected to increase BNP synthesis and secretion. As mentioned previously, renal excretion of BNP has been described, but alterations in circulating levels in patients with renal disease are more likely related to decreased clearance by NPR-C and endopeptidases. NT-proBNP, in contrast, is predominantly excreted by the kidney, although plasma NT-proBNP, appears to maintain it’s clinical utility even in the presence of renal dysfunction.Citation85,Citation86
Use of BNP as a biomarker in PH
Pulmonary arterial pressure and RV strain
As in systemic hypertension, PAH is a hemodynamic diagnosis. The clinical manifestations of PAH and RV failure are non-specific, and are usually not evident on exam or general diagnostic tests such as chest roentgenogram and electrocardiogram until the disease is fairly advanced. Circulating levels of ANP and BNP correlate with mPAP,Citation87 but elevations in BNP levels are usually not seen until PAP is high enough to cause right ventricular strain. Therefore, BNP has limited diagnostic value as a screening tool for excluding PAH in populations at low risk for the disease. In select patient groups however, where the risk of PAH is substantially increased, such as those with connective tissue disease, portal hypertension, congenital systemic to pulmonary shunts, or a family history of IPAH, elevated BNP may help identify patients in whom further testing is warranted. For example, BNP may prove useful as a screening test for PAH in systemic sclerosis, where the prevalence of PH has been reported to be 12% to 35% and where PAH treatment has been shown to be beneficial.Citation88–Citation92 In 2003, Allanore et al obtained plasma NT-proBNP levels and echocardiographic estimates of peak PAP in 40 consecutive patients hospitalized for follow-up care of scleroderma (limited or diffuse disease).Citation93 All patients were without symptoms of heart failure, had normal LV ejection fraction, and a creatinine clearance >58 mL/min. At baseline, 10 patients (25%) were found to have PAH as defined by a peak right ventricular systolic pressure (RVSP) > 40 mmHg. Thirteen patients had high NT-proBNP values (adjusted for age), including all 10 patients classified as PAH by TTE. There was a moderate correlation between peak RVSP and NT-proBNP level (r = 0.44, P = 0.006). A high NT-proBNP concentration (cutoff values supplied by the manufacturer and defined as above the 97th percentile) identified PH with a sensitivity of 90%, specificity of 90%, positive predictive value of 69%, and negative predictive value of 96%. Although the sensitivity and specificity of transthoracic echo for detecting PH is only about 90% and 75% respectively, subsequent studies using right heart catheterization to define PAH have obtained similar results.Citation94 Mukerjee et al identified found PAH by RHC in 23 of 49 patients with SSc.Citation95 The mean value of NT-proBNP in patients with PAH was 3365 ± 1095) pg/mL compared to 347 ± 174 pg/mL for patients without PAH. There was a statistically significant correlation (P < 0.05) between NT-proBNP values and (i) mPAP (r = 0.53), (ii) right ventricular end diastolic pressure (r = 0.59) and (iii) PVR (r = 0.49). Receiver operator characteristic curve analysis showed that a cut-off value of 395.34 pg/mL had a sensitivity of 0.69 and specificity of 1.0. The same group performed a larger study to assess prospectively the specificity of 395 pg/mL in a larger population and to evaluate the prognostic value of NT-proBNP in a homogenous group of patients with scleroderma associated PAH (SSc-PAH).Citation94 The study population included 68 individuals with PAH mPAP > 25 mmHg and PCWP < 15 mmHg and 41 individuals without PAH. The patients without PAH had a lower mean NT-proBNP level than those with SSc-PAH baseline NT-proBNP levels were correlated positively with mPAP (r = 0.62; P < 0.0001), PVR (r = 0.81; P < 0.0001), and inversely with 6 minute walking distance (6MWD) (r = −0.46; P < 0.0001). At an NT-proBNP level of 395 pg/mL, the sensitivity and specificity for predicting the presence of SSc-PAH were 56% and 95% respectively. Thus NT-proBNP estimation in systemic sclerosis-related PAH is a potentially useful diagnostic tool. Similarly, subjects with lung fibrosis and elevated BNP levels (n = 20) had significantly higher PAP than those with normal BNP levels (mean pulmonary arterial pressure (40.85 ± 3.2 mmHg vs 23.42 ± 1.44 mmHg, (P < 0.001).Citation96
Although TTE is the most commonly used test to diagnose or exclude PH, its specificity is low in early disease.Citation95 For example, a RVSP > 40 mmHg commonly used cut off level to separate normal from elevated PAP has been found in 6% of individuals older than 50 years of age and in 5% of patients with a body mass index >30 kg/m2 in the absence of pulmonary hypertension.Citation97 In patients such as these, an elevated plasma BNP level may improve the diagnostic accuracy of TTE in screening for PAH. Two recent studies have demonstrated the potential utility of exercise echocardiography in identifying individuals at risk for future development of PH in at risk populations.Citation98,Citation99 It is interesting to speculate whether increased levels of BNP during exercise would improve the predictive accuracy of this emerging technology.
In addition to PAP, plasma BNP levels are a reliable marker of right heart strain, which is a significant predictor of morbidity and mortality in PAH and other forms of pulmonary disease causing pulmonary hypertension.Citation100,Citation101 Elevated BNP is not specific for right or left sided heart disease, but in the absence of left sided heart disease, may prove to be useful adjunct to echocardiography in evaluating right heart stress. In patients with right ventricular pressure overload associated with idiopathic PAH or CTEPH, BNP levels were significantly higher than in controls (294 ± 72 pg/mL versus 48 ± 14 pg/mL (P < 0.05).Citation100 In this study, BNP levels correlated with, right ventricular end diastolic pressure (r = 0.76) as well as mPAP (r = 0.73) and total pulmonary resistance (TPR) (r = 0.79). Long-term treatment with either prostacyclin or prostaglandin E reduced TPR (from 23 to 15 Wood units) and cut plasma BNP by more than half (315 ± 120 to 144 ± 54 pg/mL). BNP levels have also been shown to be higher in acute pulmonary embolism and to fall following thromboendarterectomy in patients with CTEPH.Citation100,Citation102 Conversely, right ventricular dilation is exceedingly uncommon in acute PE when plasma troponin and BNP levels are normal.Citation103 Souza et al demonstrated a close correlation between PVR and NT-proBNP (r = 0.80, P < 0.001) in 42 patients with IPAH.Citation104 BNP levels also correlate with functional capacity in PAH. In one study, BNP levels were inversely correlated with 6MWD (r = −0.70; P < 0.001) and peak VO2 (r = −0.61; P < 0.01), and positive correlation with WHO functional class (r = 0.79; P < 0.001) in 42 patients with IPAH.Citation96
Survival and response to therapy
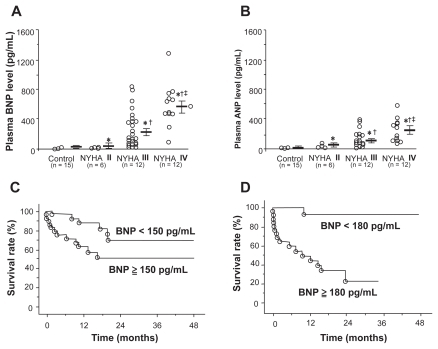
Plasma BNP levels have been shown to have significant prognostic capabilities in patients with PAH.Citation105 One study measured plasma BNP levels in 60 patients with IPAH at initial right heart catheterization and compared the prognostic significance of baseline and follow-up BNP levels with clinical, echocardiographic, and hemodynamic variables. Patients with renal insufficiency were excluded. Measurements were repeated in 53 patients at 3 months. Plasma BNP was increased at baseline and correlated positively with New York Heart Association (NYHA) functional class (), mPAP, mean RAP, and TPR, and correlated negatively with cardiac output. Mean follow-up averaged 24 months, during which time 18 patients died of cardiopulmonary causes. Among the noninvasive baseline parameters studied, only plasma BNP was an independent predictor of mortality by multivariate analysis. Kaplan-Meier analysis demonstrated that follow-up BNP provided a more distinct separation of survival curves than did baseline BNP measurement (). Survival was markedly improved in those patients with a follow-up BNP level below 180 pg/mL. In fact, receiver operating characteristic analysis suggested that baseline BNP was at least equal to mPAP and superior to CO in predicting mortality. These findings were substantiated by Fijalkowska et al who demonstrated in their 36 IPAH patients that a serum NT-proBNP level of ≥1400 pg/mL identified patient with a poor long term prognosis estimated by Kaplan-Meier cumulative survival curves.Citation106
Figure 3 A) and B) Correlation between plasma brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) and New York Heart Association (NYHA) functional class in patients with pulmonary arterial hypertension. C) Effect of plasma BNP levels at time of diagnosis on survival in patients with pulmonary arterial hypertension. D) Effect of plasma BNP levels after treatment on survival in the same patients. Reproduced with permission from Nagaya N, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102(8): 865–870.Citation105 Copyright © 2002 Elsevier.
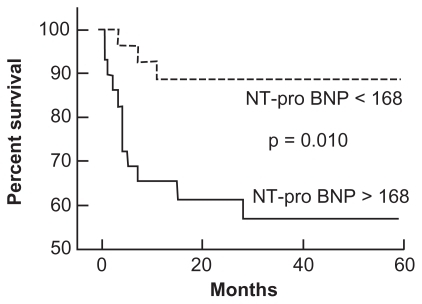
Plasma NT-proBNP levels have also been shown to predict survival in patients with PH (). The use of NT-proBNP to predict survival was assessed in 68 patients with SSc-PAHCitation94 over a mean duration of 10 months (range 1 to 18 months). The mean NT-proBNP level in patients without PAH was significantly lower than in those with SSc-PAH (P = 0.0002). For every order of magnitude increase in NT-proBNP level in patients with PAH, the risk of death increased 4-fold (P = 0.002 for baseline level and P = 0.006 for follow-up level). Baseline NT-proBNP levels correlated directly with mPAP (r = 0.62; P < 0.0001), PVR (r = 0.81; P < 0.0001), and inversely with 6MWD (r = −0.46; P < 0.0001). Plasma NT-proBNP levels also identified differences in functional class among patients with SSc-PAH. The 13 patients (19%) in WHO functional class II had mean N-TproBNP levels of 325 ± 388 pg/mL, whereas the 53 patients (78%) in WHO classes III and IV had mean NT-proBNP levels that were 5-fold higher (1677 ± 2835 pg/mL, P = 0.02).
Figure 4 Effect of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) levels on survival in patients with pulmonary arterial hypertension. Reproduced with permission from Andreassen AK, Wergeland R, Simonsen S, Geiran O, Guevara C, Ueland T. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol. 2006;98:528–529.Citation198 Copyright © 2002 Elsevier.
The correlation of circulating BNP levels with pulmonary hemodynamics, right heart strain and survival has generated interest in using it as a noninvasive assessment of response to therapy. In an early study of nine patients with IPAH who underwent prostaglandin therapy,Citation87 plasma BNP levels fell in conjunction with TPR after a mean follow-up of 35 days (TPR 23 ± 4 to 15 ± 3 Wood units, P < 0.05; plasma BNP 315 ± 120 to 144 ± 54 pg/mL P < 0.05). In a larger follow-up study by Nagaya and colleagues, BNP measurements were repeated after 3 months of vasodilator therapy in 49 patients with idiopathic PAH.Citation105 Changes in plasma BNP were associated with changes in RV end-diastolic pressure and TPR but not mPAP or right ventricular dimension. During prostacyclin therapy, plasma BNP significantly decreased in survivors but increased in non-survivors. Park and colleagues studied 20 patients with PAH on epoprostenol and similarly found that BNP may help predict those who are refractory to treatment.Citation107 Leuchte et al found that BNP levels parallel changes in pulmonary hemodynamics and functional parameters, including 6MWD, in 30 patients with PAH.Citation108 Progression of the disease was accompanied by an increase in BNP levels, whereas improvement of pulmonary hemodynamics and functional status was accompanied by a significant decline in plasma BNP concentrations. Findings from studies such as these suggest that serial plasma BNP could be useful in monitoring the efficacy of therapy in PAH patients and this approach is reflected in recent guidelines from the American College of Cardiology that suggest the use of plasma BNP levels in assessing the risk of deterioration in patients with PAH.Citation109 Although one-time measures of circulating BNP may be difficult to interpret, serial BNP testing may provide a non-invasive measure to help identify patients who are failing their current therapeutic regime and predict RV decompensation before it becomes clinically apparent. This is supported by the finding that higher BNP levels at presentation with pulmonary embolism is associated with increased mortality in two recent studies with large cohorts of patients.Citation110,Citation111 These findings suggest that in pulmonary embolism at least, BNP can help identify patients with increased right ventricular strain at higher risk for acute decompensation.
Effect of BNP on right ventricle and pulmonary circulation
In addition to being a biomarker of elevated PAP and right heart failure, BNP has many biologic properties that could be beneficial in combating progressive pulmonary vascular disease. These properties have piqued the interest of investigators and clinicians in BNP as a potential therapeutic target in the management of PAH. Before discussing therapeutic approaches, a review of the current understanding of BNP effects on the heart and pulmonary circulation is presented.
Effect on PA pressure
BNP relaxes preconstricted isolated pulmonary arteries and blunts hypoxic pressor responses in isolated perfused rat lungs with a potency similar to that of ANP ().Citation112 Although circulating BNP levels are an order of magnitude lower than ANP, the percent increases in plasma BNP levels and right heart BNP expression during exposure to chronic hypoxia are similar for both peptides.Citation112 These data suggest that BNP, in concert with ANP, may play a role in modulating pulmonary hypertensive responses and protecting the right heart from the development of hypoxic pulmonary hypertension. In one study, BNP was more effective than ANP at blunting pulmonary hypertension in rats exposed to 3 weeks of hypoxia.Citation113 Furthermore, mice deficient in NPR-A, the receptor that mediates the pulmonary vasodilator effects of both ANP and BNP develop greater pulmonary hypertension and right ventricular hypertrophy than wild type mice after exposure to chronic hypoxia.Citation114,Citation115 These studies suggest that ANP and BNP signaling through NPR-A play important roles in regulating pulmonary vascular tone and in the pathogenesis of hypoxia-induced pulmonary hypertension. Interestingly, chronic hypoxia causes a significant downregulation of NPR-C expression in pulmonary vascular smooth muscle in vitro and a marked decrease in pulmonary clearance of ANP in intact rats.Citation116,Citation117 This may represent a compensatory response of the lungs to increase the circulating levels of natriuretic peptides in the pulmonary vasculature and attenuate the effect of hypoxic pulmonary hypertension.
Figure 5 Vasodilator effect of atrial and brain natriuretic peptide (ANP, BNP) on pulmonary arterial rings isolated from normoxic A) and hypoxia-adapted rats B) and preconstricted with phenylephrine. C) Vasodilator effect of ANP and BNP on acute hypoxic pulmonary vasoconstriction in isolated rat lungs. D) Distribution of change in pulmonary vascular resistance following administration of ANP and BNP in isolated rat lungs exposed to acute hypoxia. Pressure in the pulmonary arteries, veins, and capillaries were assessed by occluding the pulmonary artery catheter (PAO), pulmonary venous catheter (PVO) and both catheters (double occlusion, PDO), respectively.Citation112
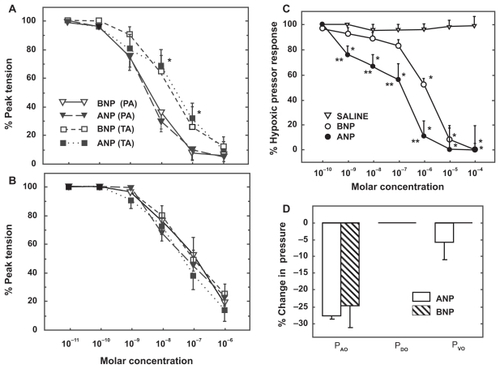
Effect on pulmonary vascular smooth muscle proliferation
In addition to its vasorelaxant effects, BNP has antiproliferative effects on vascular smooth muscle cells in culture and thus may play a role in inhibiting pulmonary vascular remodeling.Citation118 ANP has been shown to inhibit the effect of several growth factors on the proliferation of smooth muscle cells isolated from the systemic vascular bed, however few studies have examined the effect of the natriuretic peptides on inhibition of pulmonary vascular smooth muscle cell proliferation. Arjona et al found that BNP inhibited proliferation of serum stimulated pulmonary vascular smooth muscle cells and increased generation of intracellular cGMP.Citation119 In preliminary studies we have shown that ANP inhibits PDGF-induced proliferation of pulmonary vascular smooth muscle cells as well and that this effect is absent in cells isolated from NPR-A knock out mice.Citation120 Together, these studies suggest that ANP and BNP are capable of modulating proliferation of pulmonary vascular smooth muscle via signaling through NPR-A and elevation of cGMP. These findings are consistent with reports showing that pulmonary vascular remodeling is reduced in rats given ANP or BNP infusion during exposure to chronic hypoxiaCitation113, Citation121 and that mice with gene-targeted disruption of ANP or NPR-A have greater pulmonary vascular remodeling under normoxic conditions and after hypoxic exposure than wild-type littermates.Citation114,Citation115,Citation122
Effect on right ventricular hypertrophy
The natriuretic peptide system also plays an important role in inhibiting maladaptive hypertrophic responses in cardiac myocytes. Numerous studies have demonstrated that cardiac hypertrophy in response to a sustained increased in ventricular afterload is associated with a marked increase in ventricular ANP and BNP mRNA expression. This response is seen in both the right and left ventricle in response to a variety of hypertrophic stimuli such as chronic hypoxia, monocrotaline-induced pulmonary hypertension or aortic banding.Citation122–Citation124 Initially, this finding was attributed to the activation of a program of fetal genes during hypertrophy of cardiac myocytes.Citation125 As a result, increased ventricular expression of ANP and BNP was considered a marker of maladaptive cardiac hypertrophy. However, other studies have shown that the degree of hypertrophy is inversely related to ANP expression and genetic disruption of ANP, BNP or NPR-A results in increased biventricular cardiac mass under baseline conditions and exaggerated hypertrophic responses to aortic banding or chronic hypoxia.Citation114,Citation122,Citation124,Citation125 In addition, hearts of mice with disrupted ANP/BNP-NPR-A signaling have increased deposition of collagen and decreased contractility suggesting that the natriuretic peptides play important roles in mediating maladaptive cardiac hypertrophic effects.Citation126
Although BNP and ANP appear to signal through the same receptor, mice with targeted deletion of BNP exhibit a different phenotype than ANP-deficient mice. Mice without ANP are hypertensive at sexual maturity and this has not been demonstrated in mice without BNP. Thus, gene deletion experiments suggest that ANP and BNP have distinct physiologic effects and that ANP and BNP may play complementary roles in the regulation of cardiovascular homeostasis through NPR-A.
BNP has been shown to inhibit proliferation of cardiac fibroblasts in culture.Citation127 This observation was validated in vivo when ventricular pressure overload caused by abdominal aortic banding induced an increase of multi-focal fibrotic lesions in the ventricles in mice lacking BNP, but not in wild-type mice, despite a similar increase in ventricular mass between the two genotypes.Citation128 In this study, ventricular fibrosis was associated with a marked increase in expression of angiotensin converting enzyme, transforming growth factor-β3 (TGFβ3), and pro-α1(I) collagen, factors that are implicated in the generation and progression of ventricular fibrosis.Citation128 Finally, BNP has been shown to almost completely abolish TGFβ-induced increases in collagen secretion from cardiac fibroblasts.Citation129 Taken together, these results indicate that BNP exerts antifibrotic as well as antihypertrophic effects on the heart and plays an important role in mitigating maladaptive cardiac hypertrophic responses.
The mechanism involved in BNP regulation of fibroblasts is unclear. Kapoun et al found that BNP treatment inhibited TGFβ-induced expression of genes related to fibrosis, (collagen 1, fibronectin, CTGF, PAI-1, and TIMP3, myofibroblast conversion (alpha-smooth muscle actin 2 and nonmuscle myosin heavy chain), proliferation (PDGFA, IGF1, FGF18, and IGFBP10), and inflammation (COX2, IL6, TNFα-induced protein 6, and TNF superfamily).Citation129 Other studies have found that ANP and BNP modulate the matrix metalloproteinases, enzymes that play important roles in regulating the production of extracellular matrix proteins.Citation129–Citation131
It can be difficult to separate the antihypertrophic effect of BNP from its vasorelaxant properties. Studies of BNP and NPR-A knockout mice typically show increases in systemic arterial and pulmonary arterial pressure as well as cardiac hypertrophy, although the hypertrophic effect of disrupted NPR-A signaling has been more pronounced than its effect on arterial pressure. To differentiate the cardiac from the vascular effects of ANP and BNP, Holtwick et al developed mice with conditional, selective deletion of NPR-A in cardiomyocytes mice.Citation120 The endocrine, blood pressure- and volume-regulating effects of ANP were found to be preserved in these mice but significant cardiac hypertrophy was still observed. These findings suggest that NPR-A signaling indeed functions as counter-regulatory hypertrophic circuit in the heart under both basal and pressure-overload conditions.Citation132 Interestingly, in humans, certain ANP- and BNP-receptor polymorphisms have been associated with left ventricular mass in essential hypertension.Citation133 In addition, a deletion in the 5′-flanking region of the gene encoding NPRA has been associated with hypertension and hypertrophy.Citation134
Effect on pulmonary endothelial proliferation and apoptosis
Preservation of endothelial function is critical in combating pulmonary vascular disease.
Recent studies demonstrate that the NO/sGC/cGMP pathway complements natriuretic peptide/pGC/cGMP such that suppression of one pathway decreases the sensitivity of the other.Citation135,Citation136 The reciprocal regulation of natriuretic peptides and nitric oxide suggest that natriuretic peptides could play a key role in treating diseases like PAH that are associated with deficiencies in NO production. Interactions between natriuretic peptides and ET-1 may also be important. BNP can directly inhibit ET-1 synthesis as well as functionally antagonize ET-1-induced vasoconstriction and cardiomyocyte hypertrophy.Citation137 These molecular interactions may play a significant role in the cardioprotective function of natriuretic peptides in PAH. Furthermore, classic heterologous desensitization of NPR-A, ie, the ability of other cGMP-elevating enzymes to desensitize NPR-A, does not seem to occur.Citation138 This supports important reciprocal regulation of vascular tone by natriuretic peptides and NO, which activate the particulate and soluble isoforms of guanylate cyclase, respectively.Citation135,Citation136
Impaired apopotosis of pulmonary vascular endothelial cells may play an important role in the pathogenesis of pulmonary arterial hypertension. Tuder et al have proposed that impaired apoptotic responses in pulmonary vascular endothelial cells favors the emergence of apoptosis- resistant endothelial cells leading to uncontrolled proliferation in PAH.Citation4,Citation139,Citation140 ANP, BNP and CNP are all capable of inducing apoptosis in cultured rat endothelial cells,Citation141 but the extent to which they modify apoptosis under such circumstances has not been fully defined. During ischemia/reperfusion in rodents and humans, 5% to 30% of cardiac myocytes in the area at risk undergo apoptosis within 16 hours.Citation142 Kato et al reported that ANP significantly inhibits apoptosis in rat cardiac myocytes and that this effect is mediated by the increase in cGMP followed by activation of the Akt/PI3K pathway.Citation143 Conversely, ANP has been shown to induce cell cycle entry in terminally differentiated chick cardiac myocytes, and to induce apoptosis in a model of neonatal rat cardiac myocytes.Citation144 Therefore, it appears that natriuretic peptides can modulate apoptosis in cardiac myocytes as well as endothelial cells, but to what extent this occurs in pulmonary vascular endothelial cells and/or the right ventricle in PAH is uncertain.
Effect of BNP on inflammation and oxidative stress
The pathological lesions in IPAH include areas of marked intima remodeling and near complete obliteration by fibrous tissue. Intravascular and perivascular inflammatory infiltrates have also been described, suggesting that vascular inflammation may contribute to the pulmonary vascular remodeling seen in some forms of PAH. BNP has the ability to modulate the production of inflammatory mediators in activated macrophages such as leukotriene B4, prostaglandin E2 and the cytokines (TNFα, IL-12 and IL-10).Citation145 Interestingly, NPR-A knockout mice exhibit decreased neutrophil infiltration to cardiac tissue after ischemic injury compared with wild-type mice by decreasing activation of the transcription factor NF-κβ.Citation146
Oxidative stress also contributes to the pathophysiology of inflammation in the cardiovascular system.Citation147 Reactive oxygen species are produced in the lung tissue of patients with severe pulmonary hypertension as a consequence of tissue hypoxiaCitation148,Citation149 or ischemiaCitation150 or through the activation of inflammatory cascades and increased production of inflammatory cytokines.Citation151,Citation152 BNP reduces the production of free radicals, such as reactive oxygen- and nitrogen species from activated macrophages thereby reducing oxidative stress during inflammation.Citation145 This may represent another potential mechanism by which BNP could protect against the progression of vascular disease and endothelial dysfunction in PAH.
Potential role for BNP in treatment of PH
As described above, BNP influences a variety of biologic processes in a manner that has the potential to benefit patients with PAH. Unfortunately, the peptide structure of BNP results in rapid degradation in the gastrointestinal tract following ingestion, making oral delivery challenging. Intravenous infusion of BNP is feasible using recombinant human BNP developed for the treatment of acute decompensated heart failure (nesiritide; Natrecor®). Inconvenience, expense and potential safety concerns of continuous intravenous infusion are barriers to conducting even small proof of concept clinical trials. Although it should be acknowledged that long-term intravenous infusion has been used successfully for prostacyclin therapy in PAH. Most of the information available on the use of BNP to treat cardiovascular disease derives from clinical trials in congestive heart failure and acute renal failure and a handful of reports of its acute effects on the pulmonary circulation.Citation153,Citation154 As a result, there are limited data on the efficacy or safety of BNP for the treatment of PAH.
BNP has biological effects that are similar to those of ANP, but its circulating levels are much lower and in the absence of any physiological derangements, small increases in plasma BNP levels are unlikely to have significant cardiovascular effects. This is supported by most human studies in which physiological doses of BNP given to healthy volunteers have little effect on blood pressure, heart rate, angiotensin II, or aldosterone synthesis.Citation155–Citation160 However, at supra-physiological doses, BNP has been shown to have hypotensive and diuretic properties. For example, infusion of pharmacological doses of BNP into normal subjects leads to a rapid and sustained reduction in mean arterial blood pressure.Citation161 The fall in blood pressure is not solely a consequence of vasodilation, but also related to reduced stroke volume and intravascular volume. Pharmacologic doses of BNP have also been shown to have an acute pulmonary vasodilator effect in humans. Intravenous infusion of human BNP (10 pmol kg−1 min−1) blunts the acute pulmonary vasoconstrictor response to hypoxiaCitation162 and reduces PA pressure in patients with cor pulmonale.Citation163 These preliminary studies demonstrate that BNP is capable of reducing elevated PAP in some forms of pulmonary vascular disease.
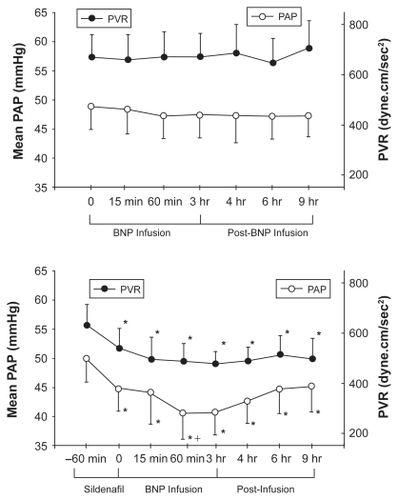
Clinical experience with BNP infusion in patients with PAH is extremely limited. Michaels et al reported the hemodynamic effects of intravenous nesiritide infusion in five patients with precapillary PH.Citation164 At 15 and 30 minutes following infusion, there was a decrease in systemic arterial pressure but no change in right-sided heart pressures or CO. Klinger et al found that a 3-hour BNP infusion alone, although well tolerated in adult patients with PAH, had no effect on mPAP or PVR.Citation165 However, the combination of sildenafil followed by BNP infusion caused a statistically significant reduction in both parameters that persisted for up to 6 h after stopping the BNP infusion (). The reduction in mPAP with sildenafil plus BNP was at least as great as that seen with any of the other pulmonary vasodilators used in this trial and greater than that observed 1 hour after sildenafil alone. Thus, BNP may be able to potentiate the acute vasodilator effects PDE-5 inhibitors.
Figure 6 Upper panel: Mean pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR) in patients with pulmonary arterial hypertension during a 3-hour infusion of human BNP (nesiritide) and 6 hours after infusion was completed. Lower panel: Effect of BNP infusion on PAP and PVR when given 1 hour after sildenafil.Citation165
In addition to its diuretic and acute pulmonary vasodilator effect, the natriuretic peptides generally oppose the actions of angiotensin II, ET-1, and the sympathetic nervous systemCitation137 and thereby have the potential to improve right ventricular function in patients with PAH. Nesiritide administration leads to a rapid vasodilatory effect and decrease in both right and left ventricular filling pressures and pulmonary capillary wedge pressuresCitation166 and has been shown to have therapeutic potential in slowing progression of right ventricular dysfunction.Citation167
There is increasing recognition that left heart failure may have a relative natriuretic deficient or resistant state. Elevated plasma levels of ANP and BNP are found in patients with congestive heart failure and the magnitude of increase coincides with the severity of heart failure.Citation168–Citation170 Studies of early LV dysfunction have demonstrated that natriuretic peptides play an important role in maintaining cardio-renal homeostasis through systemic actions that antagonize volume overload.Citation171 Gene deletion studies confirm that NPR-A signaling protects against heart failure induced by volume overload in mice.Citation172 However, in severe symptomatic HF, there is sodium and water retention associated with increased systemic vascular resistance and high cardiac filling pressures despite extremely high plasma concentration of immunoreactive ANP and BNP. The discrepancy in plasma levels of natriuretic peptides and their apparent lack of effect on cardiorenal physiology is not clearly understood, but may be due to impaired NP synthesis and release, a down regulation of NPRs at the tissue level, or increased metabolism of cGMP. Animal studies have shown an up-regulation of PDE expression in left ventricular failure, causing impaired cGMP activity despite high stimulation of the guanylyl cyclase-linked NPRs.Citation173 Supaporn et al have reported similar increases in PDE-1 and PDE-5 in severe congestive HF in humans.Citation174 Abnormal processing of proBNP into less active forms has also been described in congestive heart failure.Citation175 Resistance to natriuretic peptides may explain the variable response rate and the need for high doses that characterized the early clinical trials of BNP in congestive heart failure.Citation176–Citation179 Recent analyses of clinical trial data of nesiritide have raised concerns about adverse renal effects.Citation180,Citation181 The use of nesiritide at the dosages described in the package insert (0.01 to 0.03 μg kg−1 min−1) was noted to be associated with a dose-dependent increase in serum creatinine. Furthermore, this was exacerbated by systemic hypotension a known adverse effect of nesiritide. This clinical experience needs to be considered in future trials of BNP in pulmonary hypertension.
As discussed previously, BNP has significant antihypertrophic and anti-fibrotic effects on myocardiocytes. It is conceivable that prolonged administration of BNP may mitigate maladaptive hypertrophic responses in the right ventricle. In a small randomized trial of PAH, Wilkins et alCitation182 found that patients treated with sildenafil had a significant reduction in right ventricular mass as measured by MRI that was not found in those who received the endothelin receptor antagonist bosentan, despite a similar degree of improvement in 6MWD. Further augmentation of cGMP with the combination of BNP infusion and PDE inhibition may inhibit RV hypertrophy and fibrosis and help to preserve RV function. This idea is supported by the report Khush et al of 10 patients with precapillary PH that had acute elevations in pulmonary NO and cGMP levels post nesiritide infusion.Citation183
To advance the use of BNP as a therapy for PAH, more practical methods will likely be needed to increase plasma BNP levels. Subcutaneous administration of BNP has been used successfully in animal and in clinical studies to increase plasma BNP and its second messenger 3’,5’-cyclic guanosine monophosphate (cGMP) with subsequent natriuresis and reduction in cardiac filling pressures.Citation184–Citation189 In one canine study,Citation188 tolerance was not observed following chronic administration of BNP. Development of orally available peptides has long been a challenge, given various barriers to protein absorption and penetration.Citation190 Recently, Cataliotti et alCitation191 reported the application of proprietary technology that enabled oral delivery of BNP by covalently attaching short, amphiphilic oligomers to peptides. In normal conscious dogs, this novel oral conjugated human BNP activated cGMP and exerted hypotensive effects.Citation191 Recently, Cataliotti et alCitation192 further demonstrated in a canine model of acute hypertension that a more advanced oral conjugated human BNP significantly increased cGMP and lowered MAP. The availability of an orally active NP such as BNP could lead to a much wider application of these peptides. An alternative approach would be to impede degradation of endogenous BNP through NEP inhibition. Neutral endopeptidase is a major enzymatic pathway for the degradation of BNP and also plays a role in kinin and adrenomedulin breakdown.Citation193 Inhibition of NEP increases plasma levels of BNPCitation193 and has been shown to inhibit hypoxic pulmonary hypertension in rats,Citation194 although the mechanism by which this was achieved is not known.
In summary, although few clinical data currently exist, there is evidence to suggest that enhancement of BNP activity, especially in combination with other agents that increase cGMP signaling has the potential to aid in the treatment of PAH.Citation195,Citation196 Perhaps most intriguing of the pleiotropic effects of BNP is its antiproliferative/antifibrotic properties and the potential for reversing the maladaptive remodeling associated with PAH.
Summary
BNP is an integral part of the natriuretic peptide system that functions as a hormonal regulator of cardiovascular homeostasis. Like ANP, it is capable of relaxing pulmonary vascular smooth muscle, especially under condition in which vascular tone is increased. It also shares with ANP diuretic, antimitogenic, antifibrotic and antihypertrophic effects that have the potential to limit pulmonary vascular remodeling and maladaptive hypertrophic responses in the right ventricle. Right ventricular expression and circulating levels of BNP rise markedly in patients with pulmonary hypertension and correlate with severity of disease and mortality. Changes in BNP expression are more prominent in the ventricle than ANP expression, particularly during times of increased ventricular overload. This ventricular response, along with its slower clearance from the circulation appears to be responsible for BNP acting as a better biomarker of ventricular function than ANP. Monitoring of BNP and its precursor NT-ProBNP provides important objective information regarding the ability of the right ventricular to compensate for increased after load as pulmonary vascular disease progresses. The prognostic significance of plasma BNP on survival in pulmonary hypertension is independent of hemodynamic variables and provides the practitioner another tool with which to assess prognosis and response to therapy. Findings from in vitro and intact animal studies suggest that administration of exogenous BNP is capable of blunting pulmonary vasoconstriction and inhibiting the development of pulmonary hypertension, right ventricular hypertrophy and pulmonary vascular remodeling. However, lack of a convenient method for achieving sustained increases in circulating BNP levels has impeded the development of BNP as a therapy for treating pulmonary hypertension. Further study is needed to determine if BNP can potentiate the pulmonary vasodilator effects of phosphodiesterase inhibitors and blunt or reverse maladaptive hypertrophic responses in the right ventricular. New technologies that allow transdermal or oral administration of the natriuretic peptides have the potential to greatly accelerate research into therapeutic use of BNP for cor pulmonale and pulmonary vascular diseases.
Abbreviations
| ANP | = | atrial natriuretic peptide |
| BNP | = | brain natriuretic peptide |
| CTEPH | = | chronic thromboembolic pulmonary hypertension |
| cGMP | = | dependent protein kinase |
| cGMP | = | cyclic guanosine monophosphate |
| CNP | = | C-type natriuretic peptide |
| CO | = | cardiac output |
| ET-1 | = | endothelin-1 |
| FDA | = | Food and Drug Administration |
| MPAP | = | mean pulmonary arterial pressure |
| NEP | = | neutral endopeptidase |
| NO | = | nitric oxide |
| NPR-A | = | natriuretic peptide receptor-A |
| NPR-B | = | natriuretic peptide receptor-B |
| NPR-C | = | natriuretic peptide receptor-C |
| NYHA | = | New York Heart Association |
| PAH | = | pulmonary arterial hypertension |
| PAP | = | pulmonary arterial pressure |
| PCWP | = | pulmonary capillary wedge pressure |
| PDE | = | phosphodiesterase |
| PH | = | pulmonary hypertension |
| PKG, PVR | = | pulmonary vascular resistance |
| RVSP | = | right ventricular systolic pressure |
| RHC | = | right heart catheterization |
| SSc-PAH | = | scleroderma associated PAH |
| TGF b | = | transforming growth factor-b |
| TPR | = | total pulmonary resistance |
| TTE | = | transthoracic echocardiogram |
| WHO | = | World Health Organization |
Disclosures
The authors declare no conflicts of interest.
References
- D’AlonzoGESurvival in patients with primary pulmonary hypertension. Results from a national prospective registryAnn Intern Med199111553433491863023
- DresdaleDTPrimary pulmonary hypertension. I. Clinical and hemodynamic studyAm J Med195111668670514902839
- SimonneauGUpdated clinical classification of pulmonary hypertensionJ Am Coll Cardiol2009541 SupplS43S5419555858
- TuderRMThe pathobiology of pulmonary hypertensionEndotheliumClin Chest Med2001223405418
- TuderRMPathology of pulmonary hypertensionClin Chest Med20072812342vii17338926
- ChanSYLoscalzoJPathogenic mechanisms of pulmonary arterial hypertensionJ Mol Cell Cardiol2008441143017950310
- GrantonJMoricJPulmonary vasodilators – treating the right ventricleAnesthesiol Clin2008262337353vii18456218
- AburayaMIsolation and identification of rat brain natriuretic peptides in cardiac atriumBiochem Biophys Res Commun198916312262322673236
- AburayaMDistribution and molecular forms of brain natriuretic peptide in porcine heart and bloodBiochem Biophys Res Commun198916528728792532010
- NakaoKRat brain natriuretic peptide. Isolation from rat heart and tissue distributionHypertension1990156 Pt 27747782351430
- OgawaYMolecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide geneJ Clin Invest1994935191119218182124
- SaitoYBrain natriuretic peptide is a novel cardiac hormoneBiochem Biophys Res Commun198915823603682521788
- SudohTA new natriuretic peptide in porcine brainNature1988332615978812964562
- WuCFBishopricNHPrattREAtrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytesJ Biol Chem19972722314860148669169455
- HinoJIsolation and identification of human brain natriuretic peptides in cardiac atriumBiochem Biophys Res Commun199016726937002138890
- KambayashiYIsolation and sequence determination of human brain natriuretic peptide in human atriumFEBS Lett199025923413452136732
- KojimaMCloning and sequence analysis of cDNA encoding a precursor for rat brain natriuretic peptideBiochem Biophys Res Commun19891593142014262522776
- PorterJGCloning of a cDNA encoding porcine brain natriuretic peptideJ Biol Chem1989264126689668922708334
- OgawaYNatriuretic peptides as cardiac hormones in normotensive and spontaneously hypertensive rats. The ventricle is a major site of synthesis and secretion of brain natriuretic peptideCirc Res19916924915001830518
- BlochKDNeonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathwaysCell19864756957022946416
- MinaminoNCharacterization of immunoreactive human C-type natriuretic peptide in brain and heartBiochem Biophys Res Commun199117915355421831979
- NakamuraSAtrial natriuretic peptide and brain natriuretic peptide coexist in the secretory granules of human cardiac myocytesAm J Hypertens19914119099121838692
- HamaNRapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarctionCirculation1995926155815647664440
- NakagawaORapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overloadJ Clin Invest1995963128012877657802
- D’SouzaSPB-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel openingAm J Physiol Heart Circ Physiol20032845H1592160012521930
- TothMHypoxia stimulates release of ANP and BNP from perfused rat ventricular myocardiumAm J Physiol19942664 Pt 2H1572H15808184936
- WongFBlendisLPathophysiology of sodium retention and ascites formation in cirrhosis: role of atrial natriuretic factorSemin Liver Dis199414159708016663
- BruneauBGBNP gene expression is specifically modulated by stretch and ET-1 in a new model of isolated rat atriaAm J Physiol19972736 Pt 2H2678H26869435604
- OgawaTEvidence for load-dependent and load-independent determinants of cardiac natriuretic peptide productionCirculation19969311205920678640983
- BruneauBGAlpha 1-adrenergic stimulation of isolated rat atria results in discoordinate increases in natriuretic peptide secretion and gene expression and enhances Egr-1 and c-Myc expressionEndocrinology199613711371438536605
- PotterLRNatriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functionsEndocr Rev2006271477216291870
- MarumotoKIncreased secretion of atrial and brain natriuretic peptides during acute myocardial ischaemia induced by dynamic exercise in patients with angina pectorisClin Sci (Lond)19958855515567614814
- MorimotoAEffect of exercise on plasma adrenomedullin and natriuretic peptide levels in myocardial infarctionClin Exp Pharmacol Physiol19972453153209143780
- NicholsonSAtrial and brain natriuretic peptide response to exercise in patients with ischaemic heart diseaseClin Exp Pharmacol Physiol199320785355408432042
- NishikimiTDifferent secretion patterns of adrenomedullin, brain natriuretic peptide, and atrial natriuretic peptide during exercise in hypertensive and normotensive subjectsClin Exp Hypertens19971945035189140711
- GenyBEnhanced brain natriuretic peptide response to peak exercise in heart transplant recipientsJ Appl Physiol1998856227022769843552
- MatsumotoAEffects of exercise on plasma level of brain natriuretic peptide in congestive heart failure with and without left ventricular dysfunctionAm Heart J199512911391457817907
- CrabtreeGRGeneric signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-ATCell199996561161410089876
- GrepinCA hormone-encoding gene identifies a pathway for cardiac but not skeletal muscle gene transcriptionMol Cell Biol1994145311531298164667
- LaPointeMCTissue-specific expression of the human brain natriuretic peptide gene in cardiac myocytesHypertension1996273 Pt 27157228613230
- ThueraufDJRegulation of rat brain natriuretic peptide transcription. A potential role for GATA-related transcription factors in myocardial cell gene expressionJ Biol Chem19942692717772177758027030
- Anand-SrivastavaMBThe presence of atrial-natriuretic-factor receptors of ANF-R2 subtype in rat platelets. Coupling to adenylate cyclase/cyclic AMP signal-transduction systemBiochem J1991278Pt 12112171652938
- ChinkersMA membrane form of guanylate cyclase is an atrial natriuretic peptide receptorNature1989338621078832563900
- GutkowskaJNemerMStructure, expression, and function of atrial natriuretic factor in extraatrial tissuesEndocr Rev19891045195362533069
- KoeslingDStudying the structure and regulation of soluble guanylyl cyclaseMethods199919448549310581148
- KollerKJGoeddelDVMolecular biology of the natriuretic peptides and their receptorsCirculation1992864108110881327579
- SchulzSThe primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor familyCell1989586115511622570641
- BestPJDendroaspis natriuretic peptide relaxes isolated human arteries and veinsCardiovasc Res200255237538412123777
- NakaoKMolecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptidesJ Hypertens19921099079121328371
- SchweitzHA new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps)J Biol Chem19922672013928139321352773
- SugaSReceptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptideEndocrinology199213012292391309330
- SchulzSThe primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor familyCell1989586115511622570641
- HobbsAJIgnarroLJNitric oxide-cyclic GMP signal transduction systemMethods Enzymol19962691341488791644
- FeilRCyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified miceCirc Res2003931090791614615494
- LohmannSMFischmeisterRWalterUSignal transduction by cGMP in heartBasic Res Cardiol19918665035141664725
- LucasKAGuanylyl cyclases and signaling by cyclic GMPPharmacol Rev200052337541410977868
- SilberbachMRobertsCTJrNatriuretic peptide signalling: molecular and cellular pathways to growth regulationCell Signal200113422123111306239
- VaandragerABde JongeHRSignalling by cGMP-dependent protein kinasesMol Cell Biochem19961571223308830286
- HametPCyclic GMP as mediator and biological marker of atrial natriuretic factorJ Hypertens Suppl198642S49562873213
- BeavoJACyclic nucleotide phosphodiesterases: functional implications of multiple isoformsPhysiol Rev19957547257487480160
- D’SouzaSPAutocrine and paracrine actions of natriuretic peptides in the heartPharmacol Ther2004101211312914761702
- EssayanDMCyclic nucleotide phosphodiesterasesJ Allergy Clin Immunol2001108567168011692087
- MehatsCCyclic nucleotide phosphodiesterases and their role in endocrine cell signalingTrends Endocrinol Metab2002131293511750860
- MauriceDHCyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular systemMol Pharmacol200364353354612920188
- CorbinJDHigh lung PDE5: a strong basis for treating pulmonary hypertension with PDE-5 inhibitorsBiochem Biophys Res Commun2005334393093816023993
- MacleanMRPhosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertensionJ Pharmacol Exp Ther199728326196249353377
- AlmeidaFAClearance function of type C receptors of atrial natriuretic factor in ratsAm J Physiol19892562 Pt 2R469R4752537040
- BennettBDExtracellular domain-IgG fusion proteins for three human natriuretic peptide receptors. Hormone pharmacology and application to solid phase screening of synthetic peptide antiseraJ Biol Chem19912663423060230671660465
- MaackTFunctional properties of atrial natriuretic factor receptorsSemin Nephrol199313150608434186
- SoleilhacJMA 94-kDa protein, identif ied as neutral endopeptidase-24.11, can inactivate atrial natriuretic peptide in the vascular endotheliumMol Pharmacol19924146096141533267
- RademakerMTClearance receptors and endopeptidase: equal role in natriuretic peptide metabolism in heart failureAm J Physiol19972735 Pt 2H2372H23799374774
- DavidsonNCStruthersADBrain natriuretic peptideJ Hypertens19941243293368064155
- KishimotoIDownregulation of C-receptor by natriuretic peptides via ANP-B receptor in vascular smooth muscle cellsAm J Physiol19932654 Pt 2H1373H13798238425
- ShimaMIntrarenal localization of degradation of atrial natriuretic peptide in isolated glomeruli and cortical nephron segmentsLife Sci19884343573632969444
- EspinerEANatriuretic hormonesEndocrinol Metab Clin North Am19952434815098575406
- SmithMWDelayed metabolism of human brain natriuretic peptide reflects resistance to neutral endopeptidaseJ Endocrinol2000167223924611054637
- PotterLRDomain analysis of human transmembrane guanylyl cyclase receptors: implications for regulationFront Biosci2005101205122015769619
- MukoyamaMAugmented secretion of brain natriuretic peptide in acute myocardial infarctionBiochem Biophys Res Commun199118014314361834057
- TokolaHMechanical load-induced alterations in B-type natriuretic peptide gene expressionCan J Physiol Pharmacol200179864665311558673
- O’DonoghueMThe effects of ejection fraction on N-terminal ProBNP and BNP levels in patients with acute CHF: analysis from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) studyJ Card Fail2005115 SupplS9S1415948094
- SeinoYApplication of NT-proBNP and BNP measurements in cardiac care: a more discerning marker for the detection and evaluation of heart failureEur J Heart Fail20046329530014987579
- BiondaCPlasma BNP and NT-proBNP assays by automated immunoanalyzers: analytical and clinical studyAnn Clin Lab Sci200636329930616951271
- RedfieldMMPlasma brain natriuretic peptide concentration: impact of age and genderJ Am Coll Cardiol200240597698212225726
- WangTJImpact of age and sex on plasma natriuretic peptide levels in healthy adultsAm J Cardiol200290325425812127613
- LeuchteHHN-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertensionChest2007131240240917296640
- DanielsLBMaiselASNatriuretic peptidesJ Am Coll Cardiol200750252357236818154959
- NagayaNPlasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertensionJ Am Coll Cardiol19983112022089426041
- BadeschDBContinuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trialAnn Intern Med2000132642543410733441
- BattleRWPrevalence of pulmonary hypertension in limited and diffuse sclerodermaChest19961106151515198989070
- MacGregorAJPulmonary hypertension in systemic sclerosis: risk factors for progression and consequences for survivalRheumatology (Oxford)200140445345911312386
- MukerjeeDSignificance of plasma N-terminal pro-brain natriuretic peptide in patients with systemic sclerosis-related pulmonary arterial hypertensionRespir Med200397111230123614635979
- OudizRJTreprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue diseaseChest2004126242042715302727
- AllanoreYN-terminal pro-brain natriuretic peptide as a diagnostic marker of early pulmonary artery hypertension in patients with systemic sclerosis and effects of calcium-channel blockersArthritis Rheum200348123503350814674001
- WilliamsMHRole of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertensionEur Heart J200627121485149416682379
- MukerjeeDEchocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosisRheumatology (Oxford)200443446146615024134
- LeuchteHHClinical signif icance of brain natriuretic peptide in primary pulmonary hypertensionJ Am Coll Cardiol200443576477014998614
- McQuillanBMClinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjectsCirculation2001104232797280211733397
- AlkotobMLReduced exercise capacity and stress-induced pulmonary hypertension in patients with sclerodermaChest2006130117618116840399
- GrunigEStress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxiaCirculation2009119131747175719307479
- NagayaNPlasma brain natriuretic peptide as a noninvasive marker for efficacy of pulmonary thromboendarterectomyAnn Thorac Surg2002741180184 discussion 18412118754
- Oswald-MammosserMPrognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressureChest19951075119311987750305
- TulevskiIIIncreased brain natriuretic peptide as a marker for right ventricular dysfunction in acute pulmonary embolismThromb Haemost20018651193119611816706
- BinderLN-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolismCirculation2005112111573157916144990
- SouzaRNT-proBNP as a tool to stratify disease severity in pulmonary arterial hypertensionRespir Med20071011697516781131
- NagayaNPlasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertensionCirculation2000102886587010952954
- FijalkowskaASerum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertensionChest200612951313132116685024
- ParkMHUsefulness of B-type natriuretic peptide as a predictor of treatment outcome in pulmonary arterial hypertensionCongest Heart Fail200410522122515470298
- LeuchteHHCharacterization of brain natriuretic peptide in long-term follow-up of pulmonary arterial hypertensionChest200512842368237416236896
- McLaughlinVVACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension AssociationJ Am Coll Cardiol200953171573161919389575
- KucherNPrognostic role of brain natriuretic peptide in acute pulmonary embolismCirculation2003107202545254712742987
- ten WoldeMBrain natriuretic peptide as a predictor of adverse outcome in patients with pulmonary embolismCirculation2003107162082208412707233
- HillNSBrain natriuretic peptide: possible role in the modulation of hypoxic pulmonary hypertensionAm J Physiol19942663 Pt 1L308L3158166300
- KlingerJRBrain natriuretic peptide inhibits hypoxic pulmonary hypertension in ratsJ Appl Physiol1998845164616529572812
- KlingerJRTargeted disruption of the gene for natriuretic peptide receptor-A worsens hypoxia-induced cardiac hypertrophyAm J Physiol Heart Circ Physiol20022821H586511748047
- ZhaoLNPR-A-Deficient mice show increased susceptibility to hypoxia-induced pulmonary hypertensionCirculation19999956056079950655
- KlingerJRC-receptor ligand blocks pulmonary clearance of atrial natriuretic peptide in isolated rat lungsProc Soc Exp Biol Med199220121541581409730
- SunJZHypoxia reduces atrial natriuretic peptide clearance receptor gene expression in ANP knockout miceAm J Physiol Lung Cell Mol Physiol20002793L51151910956626
- SchirgerJAVascular actions of brain natriuretic peptide: modulation by atherosclerosis and neutral endopeptidase inhibitionJ Am Coll Cardiol200035379680110716485
- ArjonaAAEffects of natriuretic peptides on vascular smooth-muscle cells derived from different vascular bedsGen Pharmacol19972833873929068978
- KlingerJRMurrayJDAtrial natriuretic peptide inhibits murine pulmonary vascular smooth muscle cell proliferation via natriuretic peptide receptor-AProc Am Thor Soc20063A857
- JinHAtrial natriuretic peptide attenuates the development of pulmonary hypertension in rats adapted to chronic hypoxiaJ Clin Invest19908511151202136863
- KlingerJRGenetic disruption of atrial natriuretic peptide causes pulmonary hypertension in normoxic and hypoxic miceAm J Physiol19992765 Pt 1L868L87410330043
- HirataYRole of endogenous ANP in sodium excretion in rats with experimental pulmonary hypertensionAm J Physiol19922626 Pt 2H1684H16891535756
- KnowlesJWPressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient miceJ Clin Invest2001107897598411306601
- CameronVAEllmersLJMinireview: natriuretic peptides during development of the fetal heart and circulationEndocrinology20034462191219412746273
- OliverPMHypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor AProc Natl Acad Sci U S A1997942614730147359405681
- CaoLGardnerDGNatriuretic peptides inhibit DNA synthesis in cardiac fibroblastsHypertension19952522272347843772
- TamuraNCardiac fibrosis in mice lacking brain natriuretic peptideProc Natl Acad Sci U S A20009784239424410737768
- KapounAMB-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammationCirc Res200494445346114726474
- TsurudaTBrain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinasesCirc Res200291121127113412480813
- WangDEffects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouseHypertension2003421889512756220
- HoltwickRPressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-AJ Clin Invest200311191399140712727932
- RubattuSAssociation of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertensionJ Am Coll Cardiol200648349950516875975
- NakayamaTFunctional deletion mutation of the 5’-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the JapaneseCirc Res200086884184510785505
- HussainMBReciprocal regulation of cGMP-mediated vasorelaxation by soluble and particulate guanylate cyclasesAm J Physiol Heart Circ Physiol20012803H1151115911179059
- MadhaniMVascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signallingBr J Pharmacol200313971289129612890708
- SigurdssonASwedbergKThe role of neurohormonal activation in chronic heart failure and postmyocardial infarctionAm Heart J19961321 Pt 2 Su2292348677861
- FowkesRCForrest-OwenWMcArdleCAC-type natriuretic peptide (CNP) effects in anterior pituitary cell lines: evidence for homologous desensitisation of CNP-stimulated cGMP accumulation in alpha T3-1 gonadotroph-derived cellsJ Endocrinol2000166119520310856898
- SakaoSInitial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cellsFASEB J20051991178118015897232
- Taraseviciene-StewartLInhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertensionFASEB J200115242743811156958
- SuenobuNNatriuretic peptides and nitric oxide induce endothelial apoptosis via a cGMP-dependent mechanismArterioscler Thromb Vasc Biol19991911401469888876
- CrowMTThe mitochondrial death pathway and cardiac myocyte apoptosisCirc Res2004951095797015539639
- KatoTAtrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and AktJ Clin Invest2005115102716273016200208
- KoideMAtrial natriuretic peptide accelerates proliferation of chick embryonic cardiomyocytes in vitroDifferentiation19966111118921580
- ChiurchiuVBrain Natriuretic Peptide (BNP) regulates the production of inflammatory mediators in human THP-1 macrophagesRegul Pept200814813263218462818
- IzumiTBlockade of the natriuretic peptide receptor guanylyl cyclase-A inhibits NF-kappaB activation and alleviates myocardial ischemia/reperfusion injuryJ Clin Invest2001108220321311457873
- SuematsuNOxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytesCirculation2003107101418142312642364
- ChandelNSRole of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxinJ Immunol200016521013102110878378
- HoshikawaYGeneration of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxiaJ Appl Physiol20019041299130611247927
- Al-MehdiABEndothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+Circ Res19988377307379758643
- HumbertMIncreased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertensionAm J Respir Crit Care Med19951515162816317735624
- TuderRMVoelkelNFPulmonary hypertension and inflammationJ Lab Clin Med1998132116249665367
- ReynoldsEWNesiritide for the treatment of pulmonary hypertension and cor pulmonale in an infantPediatr Cardiol200728322923317375350
- ZakirRMA comparison of nesiritide vs. epoprostenol in a patient with precapillary pulmonary hypertension due to scleroderma complicated by postcapillary pulmonary hypertensionCongest Heart Fail200511633133416330910
- HolmesSJRenal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal manJ Clin Endocrinol Metab199376191968380606
- JensenKTCarstensJPedersenEBEffect of BNP on renal hemodynamics, tubular function and vasoactive hormones in humansAm J Physiol19982741 Pt 2F63F729458824
- La VillaGCardiovascular and renal effects of low dose brain natriuretic peptide infusion in manJ Clin Endocrinol Metab1994785116611718175974
- La VillaGAcute effects of physiological increments of brain natriuretic peptide in humansHypertension19952646286337558223
- LazzeriCCardiovascular function during brain natriuretic peptide infusion in manCardiology19958653964017585742
- van der ZanderKHemodynamic and renal effects of low-dose brain natriuretic peptide infusion in humans: a randomized, placebo-controlled crossover studyAm J Physiol Heart Circ Physiol20032853H1206121212738624
- YoshimuraMHemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failureCirculation1991844158115881914098
- CargillRILipworthBJAcute effects of ANP and BNP on hypoxic pulmonary vasoconstriction in humansBr J Clin Pharmacol19954065855908703666
- CargillRILipworthBJAtrial natriuretic peptide and brain natriuretic peptide in cor pulmonale. Hemodynamic and endocrine effectsChest19961105122012258915224
- MichealsADMaddenCKJ Hemodynamic effects of intravenous nesiritide in patients with pulmonary hypertension abstractJ Am Coll Cardiol200443Suppl 5A192A
- KlingerJRPulmonary hemodynamic responses to brain natriuretic peptide and sildenafil in patients with pulmonary arterial hypertensionChest2006129241742516478861
- ColucciWSNesiritide for the treatment of decompensated heart failureJ Card Fail2001719210011264555
- ElkayamUNesiritide: a new drug for the treatment of decompensated heart failureJ Cardiovasc Pharmacol Ther20027318119412232567
- DicksteinKPro-atrial natriuretic factor is predictive for the clinical status of patients with heart failureTidsskr Nor Laegeforen199611613156215668685865
- GottliebSSPrognostic importance of atrial natriuretic peptide in patients with chronic heart failureJ Am Coll Cardiol1989137153415392524515
- MotwaniJGPlasma brain natriuretic peptide as an indicator for angiotensin-converting-enzyme inhibition after myocardial infarctionLancet19933418853110911138097802
- StevensTLA functional role for endogenous atrial natriuretic peptide in a canine model of early left ventricular dysfunctionJ Clin Invest1995953110111087883958
- NishikimiTIncreased susceptibility to heart failure in response to volume overload in mice lacking natriuretic peptide receptor-A geneCardiovasc Res20056619410315769452
- ForfiaPRAcute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heartJ Am Coll Cardiol200749101079108817349888
- SupapornTBlunted cGMP response to agonists and enhanced glomerular cyclic 3’,5’-nucleotide phosphodiesterase activities in experimental congestive heart failureKidney Int1996505171817258914042
- LamCSAlternate circulating pro-B-type natriuretic peptide and B-type natriuretic peptide forms in the general populationJ Am Coll Cardiol200749111193120217367664
- BurgerAJEffect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT studyAm Heart J200214461102110812486437
- ColucciWSIntravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study GroupN Engl J Med2000343424625310911006
- MillsRMSustained hemodynamic effects of an infusion of nesiritide (human b-type natriuretic peptide) in heart failure: a randomized, double-blind, placebo-controlled clinical trial. Natrecor Study GroupJ Am Coll Cardiol199934115516210400005
- PeacockWFTObservation unit treatment of heart failure with nesiritide: results from the proaction trialJ Emerg Med200529324325216183441
- Sackner-BernsteinJDShort-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trialsJAMA2005293151900190515840865
- Sackner-BernsteinJDRisk of worsening renal function with nesiritide in patients with acutely decompensated heart failureCirculation2005111121487149115781736
- WilkinsMRSildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) studyAm J Respir Crit Care Med2005171111292129715750042
- KhushKKNesiritide acutely increases pulmonary and systemic levels of nitric oxide in patients with pulmonary hypertensionJ Card Fail200612750751316952783
- ChenHHSubcutaneous administration of brain natriuretic peptide in experimental heart failureJ Am Coll Cardiol20003651706171211079680
- ChenHHMaximizing the renal cyclic 3’-5’-guanosine monophosphate system with type V phosphodiesterase inhibition and exogenous natriuretic peptide: a novel strategy to improve renal function in experimental overt heart failureJ Am Soc Nephrol200617102742274716928803
- ChenHHMaximizing the natriuretic peptide system in experimental heart failure: subcutaneous brain natriuretic peptide and acute vasopeptidase inhibitionCirculation20021058999100311864932
- ChenHHSubcutaneous administration of the cardiac hormone BNP in symptomatic human heart failureJ Card Fail200410211511915101022
- ChenHHIntact acute cardiorenal and humoral responsiveness following chronic subcutaneous administration of the cardiac peptide BNP in experimental heart failureEur J Heart Fail20068768168616459135
- EvgenovOVNO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potentialNat Rev Drug Discov20065975576816955067
- DelieFBlanco-PrietoMJPolymeric particulates to improve oral bioavailability of peptide drugsMolecules2005101658018007277
- CataliottiAOral human brain natriuretic peptide activates cyclic guanosine 3’,5’-monophosphate and decreases mean arterial pressureCirculation2005112683684016061734
- CataliottiAChronic actions of a novel oral B-type natriuretic peptide conjugate in normal dogs and acute actions in angiotensin II-mediated hypertensionCirculation2008118171729173618838565
- CortiRVasopeptidase inhibitors: a new therapeutic concept in cardiovascular disease?Circulation2001104151856186211591626
- KlingerJRNeutral endopeptidase inhibition attenuates development of hypoxic pulmonary hypertension in ratsJ Appl Physiol1993754161516238282611
- GhofraniHAOral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertensionJ Am Coll Cardiol200342115816412849677
- ZhaoLBeneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic Peptide activityCirculation2003107223423712538421
- BarrCSRhodesPStruthersADC-type natriuretic peptidePeptides1996177124312518959763
- AndreassenAKWergelandRSimonsenSGeiranOGuevaraCUelandTN-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertensionAm J Cardiol200698528529