Abstract
N-Palmitoylethanolamine (NAE 16:0) is an endogenous lipid signaling molecule that has limited water solubility, and its action is short-lived due to its rapid metabolism. This poses a problem for use in vivo as oral administration requires a high concentration for significant levels to reach target tissues, and injection of the compound in a dimethyl sulfoxide- or ethanol-based vehicle is usually not desirable during long-term treatment. A depot injection of NAE 16:0 was successfully emulsified in sterile corn oil (10 mg/kg) and administered in young DBA/2 mice in order to elevate baseline levels of NAE 16:0 in target tissues. NAE 16:0 levels were increased in various tissues, particularly in the retina, 24 and 48 hours following injections. Increases ranged between 22% and 215% (above basal levels) in blood serum, heart, brain, and retina and induced an entourage effect by increasing levels of other 18 carbon N-Acylethanolamines (NAEs), which ranged between 31% and 117% above baseline. These results indicate that NAE 16:0 can be used as a depot preparation, avoiding the use of inadequate vehicles, and can provide the basis for designing tissue-specific dosing regimens for therapies involving NAEs and related compounds.
Introduction
N-Acylethanolamines (NAEs) are endogenous lipid signaling molecules involved in numerous physiological functions in mammals, including neurotransmission and cellular protection.Citation1 NAEs, including anandamide (NAE 20:4), an endogenous ligand of cannabinoid receptor 1 (CB1), are substrates of the fatty acid amide hydrolase (FAAH) enzyme.Citation2,Citation3 N-Palmitoylethanolamine (NAE 16:0) is an NAE that has been shown to protect cells from oxidative stressCitation4,Citation5 and activate neuroprotective kinase signaling pathways,Citation4 reduce myocardial infarct volume and neurological behavioral deficits in ischemic rats,Citation6 provide substantial relief of objective and subjective symptoms of atopic eczema,Citation7 decrease melanoma progression,Citation8 and is reported to have anti-inflammatory properties.Citation9 During in vivo studies, NAE 16:0 is rapidly metabolized by FAAH and, therefore, the action of NAE 16:0 is fairly short-lived,Citation1 which poses a problem when administered orally. NAE 16:0 is a fatty acid amide which has very limited water solubilityCitation1,Citation9–Citation11 and this is a major issue for in vitro and in vivo treatments, with dimethyl sulfoxide or ethanol vehicle required to dissolve NAE 16:0 at a rather low concentration of 20–25 mM. Often, then, the NAE 16:0 will precipitate out of solution when diluting into media or buffer if the final concentration is not low enough. This makes it difficult to determine a range for higher concentrations of NAE 16:0 in vitro or in vivo. Saline vehicles containing products such as polyethylene glycol, Tween 80,Citation12 or cyclodextrin in combination with ethanolCitation13 have also been used in order to aid solubility for single-dose in vivo administration of NAE 16:0, but NAE 16:0 has not yet been studied as a long-term dosing regimen.
Corn oil is commonly used as a vehicle to administer lipophilic, water-insoluble agents in vivo via gavage feeding, subcutaneous, intraperitoneal, or intramuscular injections.Citation14 Oil-based depot injections are usually performed as an intramuscular or subcutaneous injection in order to achieve a slow and steady release of the compound. Radd et al performed release rate studies of haloperidol in corn oil and several other oils to test for an appropriate vehicle for depot injections (using fatty acids as solubilizers).Citation15 Corn oil was shown to release haloperidol at a rate of 0.113 mg/cm2/h1/2 and was one of the slower releasing oils that were tested.Citation15 Numerous studies use sterile corn oil for injection administration of highly lipophilic compounds, eg, steroid hormones, such as progesterone,Citation16,Citation17 and estradiol,Citation16,Citation18–Citation20 and drugs such as tamoxifen,Citation16,Citation18–Citation26 an estrogen receptor antagonist. The present study examines the effectiveness of NAE 16:0 as a depot injection, which allows for emulsification of the compound at a higher concentration in a corn oil preparation, to avoid precipitation problems. Furthermore, the depot injection of emulsified NAE 16:0 may represent a “slow-release” preparation for future experiments to study the effects of long-term treatments with lipid-soluble acylethanolamides on disease progression in mouse models of disease. For this study we have used young DBA/2 mice (Charles River Laboratories International, Inc, Wilmington, MA, USA), a mouse model of glaucoma, which are nonglaucomatous, to quantify baseline levels of NAE 16:0 in target tissues, and to measure the levels of NAE 16:0 levels in these tissues 24 and 48 hours after depot injections.
In this study, we show that basal levels of NAE 16:0 differ markedly in different tissues of young, non-glaucomatous DBA/2 mice and are particularly elevated in the retina. We also show that NAE 16:0 can be successfully emulsified into an oil depot injection for in vivo administration; the compound effectively reached the blood serum, heart, brain, and retina, and also induced an elevated entourage effect by increasing other acylethanolamide lipids, namely NAE 18:0, 18:1, 18:2, and 20:4.
Materials and methods
Animals
Ten 6-week-old male DBA/2 mice (16–21 g) were obtained from Charles River Laboratories and the mice were housed with five mice per cage. All animals had unlimited access to food and water and were maintained on a 12 hour light/dark cycle. Animals were monitored on a daily basis with no reports of any weight or behavioral changes or pain or distress from the 24–48 hour treatment. All animal husbandry and experimental procedures were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, performed in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee (University of Missouri – Kansas City Kansas City MO, USA).
NAE 16:0 treatment
Directly before treatment, NAE 16:0 compound (Best West Laboratories Inc, Salt Lake City, UT, USA) was ground using a mortar and pestle and emulsified in sterile corn oil by vortexing and ultrasonification at 37°C for 30 minutes. DBA/2 mice received a 100 μL subcutaneous injection of either sterile corn oil (vehicle, n = 4) or NAE 16:0 (10 mg/kg, based on a previous studyCitation6) and were sacrificed by CO2 asphyxiation at either 24 (n = 3) or 48 (n = 3) hours following injections. Blood serum was collected heart, brain, and retina were dissected snap-frozen in liquid nitrogen and stored at −80°C in preparation for lipid extraction.
Lipid extraction and NAE quantification
Lipid extraction was as described previouslyCitation6 Briefly, the individual tissue samples were weighed (fresh weight [FW]) and homogenized in 2 mL hot 2-propanol (70°C) and internal standards (50 ng each of D4-NAE 16:0 and D4-NAE 20:4 [Cayman Chemical Co, Ann Arbor, MI, USA]) were added. Samples were then incubated in a 70°C water bath for 30 minutes, following which, 1 mL of chloroform was added to each test tube and samples were vortexed and incubated at 4°C for overnight extraction. Monophasic lipid extracts were partitioned with 2 mL of 1 M KCl. The lower organic phase was washed three additional times with 1 M KCl and subsequently dried to completion under argon. The total lipid mass (LM) was estimated gravimetrically and the samples were dissolved in chloroform and stored under argon at −80°C until further purification by solid phase extraction (SPE). Silica SPE cartridges (100 mg, 1.5 mL [Supelco Analytical, Bellefonte, PA, USA]) were conditioned with 2 mL methanol, followed by 4 mL chloroform. Samples dissolved in 1 mL of chloroform were loaded onto SPE cartridges, followed by washing with 2 mL chloroform. NAEs were eluted with 2 mL 1:1 (v/v) ethyl acetate:acetone, evaporated under nitrogen and derivatized in N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA; Thermo Fisher Scientific, Waltham, MA, USA) for 30 minutes at 55°C (12). Derivatized samples were dried under nitrogen and redissolved in 50 μL N-hexane. TMS-ether derivatives of NAEs were identified via selective ion monitoring and quantified against internal standards (D4-NAE 16:0 and D4-NAE 20:4) by gas chromatography/mass spectrometry (model 7890 GC coupled with a 5975C mass selective detector [Agilent Technologies, Santa Clara, CA, USA]).Citation27
Statistical analysis
Data was statistically analyzed using a one-way analysis of variance (ANOVA) with a Dunnett’s or Bonferroni posttest when comparing within treatment groups or control data, respectively. Data are shown as mean ± standard error of the mean.
Results
The highest basal levels of NAE 16:0 (nmol/g FW) were in the retina compared to blood serum (P < 0.001), heart (P < 0.001), and brain (P < 0.001; ). The same was true for metabolic data, that basal levels of NAE 16:0 (nmol/g LM) were highest in the retina compared to blood serum (P < 0.01), heart (P < 0.05), and brain (P < 0.001; ). Brain had the lowest levels of NAE 16:0 per g FW and LM, when compared to retina, heart (P < 0.05), and blood serum (P < 0.01; ).
Figure 1 Comparison of control levels of NAE 16:0 in various tissues of DBA/2 mice.
Abbreviations: FW, fresh weight; LM, lipid mass; NAE 16:0, N-Palmitoyl-ethanolamine.
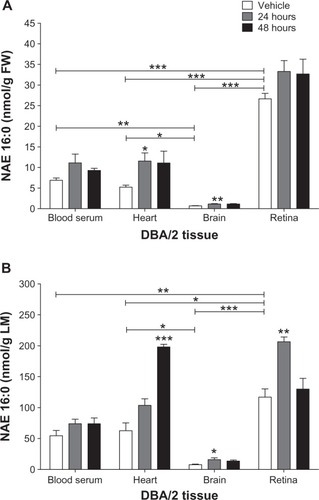
NAE 16:0 levels were increased significantly over baseline in heart on a tissue weight basis after 24 hours (P < 0.05; ) and metabolically on a lipid weight basis after 48 hours (P < 0.001; ). NAE 16:0 per g brain tissue showed a sustained increase over 24 and 48 hours (P < 0.05 and FW, P < 0.05, respectively; ) and was increased per LM after 24 hours (). NAE 16:0 per g LM in retina significantly increased over baseline after 24 hours (P < 0.01; ).
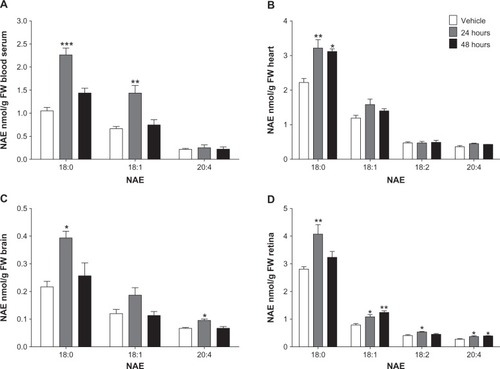
NAE 18:0, 18:1, 18:2, and 20:4 were also increased in some tissues following the NAE 16:0 depot injection (). Levels of NAE 18:0 in blood serum (P < 0.001), heart (P < 0.01), brain (P < 0.05), and retina (P < 0.01) were raised after 24 hours () and sustained in heart after 48 hours (P < 0.05; ). Levels of 18:1 in blood serum (P < 0.01) and retina (P < 0.05) were elevated after 24 hours and levels in the retina further increased after 48 hours (P < 0.01; ). Levels of 18:2 were above detection levels in heart and retina only. Levels of 18:2 in retina were significantly raised after 24 hours (P < 0.05; ). NAE 20:4 levels in brain (P < 0.05) and retina (P < 0.05) were increased after 24 hours and the retina sustained increased levels over 48 hours (P < 0.05; ).
Figure 2 Elevation above control levels of NAE 18:0, 18:1, 18:2, and 20:4, 24 and 48 hours following NAE 16:0 depot injection.
Abbreviations: FW, fresh weight; NAE, N-Acylethanolamine; NAE 16:0, N-Palmitoylethanolamine.
Discussion
NAEs are lipid signaling molecules that are present in the central nervous system (CNS). The present study measures the levels of NAEs in blood serum, heart, brain, and retina of DBA/2 mice in the absence or presence of NAE 16:0, administered as an oil-based depot injection.
DBA/2 mice showed higher levels of all NAEs in blood serum when compared to other mouse strains and species.Citation1,Citation28–Citation30 Levels of NAE 16:0 and 18:1 were higher in the DBA/2 heart when compared to other mouse strains,Citation31,Citation32 however, lower levels of NAE 20:4 were measured. Brain levels of all NAEs were lower in the DBA/2 mice when compared to other mouse strains and species.Citation31,Citation33 Very low basal levels of NAE 18:2 have been reported in the brain at ~2 pmol/g FWCitation34 and were not detected in this study. NAE 16:0 was much higher in the retina of DBA/2 mice compared to human retina,Citation35,Citation36 but NAE 20:4 had similar retina levels to humans.Citation35,Citation36 These variations in values could be due to differences in species or mouse strain, but are also likely due to differences in extraction and detection measures between various studies.
NAE 16:0 was increased above baseline in serum and heart by 61% and 119% after 24 hours, respectively, and, after 48 hours, NAE 16:0 was increased by 215% per total LM in heart tissue. Per LM, NAE 16:0 levels were significantly increased by 106% and 77% in brain and retina after 24 hours, respectively, and a 63%–65% and a 22%–25% increase was sustained over a 48 hour period per g F W, respectively. Additionally, we showed that the NAE 16:0 depot injection induced an entourage effect of other NAEs. Levels of NAE 18:0 were significantly increased by 45%–115% in all tissues after 24 hours, and the heart sustained increased NAE 18:0 levels of 40%–45% over the 48 hour period. NAE 18:1 was increased in blood serum and retina after 24 hours by 117% and 38%, respectively, and levels were further increased by 20% in the retina 48 hours following the depot injection. NAE 18:2 was only detected in heart and retina, and levels were unchanged in heart but increased by 31% in retina after 24 hours. NAE 20:4 was significantly increased in brain after 24 hours, and the retina sustained a 33%–43% increase over 48 hours.
NAE 20:4 and other polyunsaturated NAEs exert biological activity by binding to cannabinoid receptors (CB).Citation37 CB1 is found distributed throughout the CNS and various tissueCitation38 whereas CB2 is mainly found in the immune system, retina, and CNS.Citation39 NAE 20:4 is also a full agonist for the vanilloid receptor 1 (VR1), an ionotropic cation channel expressed in the peripheral sensory system as well as in the CNS, and acts as nociceptor transducer.Citation40 NAEs with 18 carbon atom acyl chains (NAE 18:0, 18:1, and 18:2) are thought to be potential modulators of VR1.Citation41–Citation43 There is some discussion that NAE 16:0 may act directly on an unidentified CB receptor or be a positive allosteric modulator of the VR1 receptor,Citation40,Citation44 or that NAE 16:0-mediated neuroprotection is thought to act by reducing apoptotic and inflammatory pathways, independent of CB and VR1 activation.Citation37,Citation45,Citation46 NAE 16:0 is thought to be an ‘entourage’ compound for NAE 20:4, which enhances its biological actions, possibly by competing with NAE 20:4 for FAAH, thereby reducing anandamide hydrolysis and increasing anandamide levels consequently, increasing CB receptor activation,Citation44 or by increasing the affinity of NAE 20:4 for receptors.Citation44 Long-term treatment with NAE 16:0, in vitro, was shown to downregulate FAAH.Citation40 Therefore, it is possible that long-term NAE 16:0 administration could simulate the downregulation of FAAH, thus increasing endogenous NAEs, including NAE 20:4, to elicit protection from neurodegeneration in chronic diseases, as well as exerting neuroprotective effects through apoptotic and inflammatory pathways.
Conclusion
This study has shown that NAE 16:0 can be successfully emulsified into an oil depot injection for in vivo administration, eliminating the use of gavage feeding and allowing for a safer vehicle to be administered in vivo, by bypassing the solubility difficulties with NAE 16:0 and eliminating the use of vehicles such as ethanol and dimethyl sulfoxide, which are inadequate for long-term studies. Also, depot injections allow for fewer injections and a steady slow-release of the compound, which can be maintained if studying long-term NAE 16:0 dosing and its effect over time. We also have shown that NAE 16:0, administered as a subcutaneous depot injection, effectively reaches the blood serum, heart, brain, and retina and can induce an elevation in the tissue content of other NAEs. Overall, this study allows for the long-term administration of NAE 16:0, which can induce elevated levels of other NAEs, either by inhibiting the FAAH enzyme or via alternative routes, for in vivo experiments targeting the protection of tissues from acute or chronic degeneration such as in neuroprotection.Citation4,Citation39
Acknowledgments
Research reported in this publication was supported in part by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources, and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by a Fight for Sight Post-Doctoral Award (SLG) and the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, the Vision Research Foundation of Kansas City, and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully acknowledged. Acylethanolamide analysis by gas chromatography/mass spectrometry was made possible through a supplemental instrument award to KDC from United States Department of Energy, Office of Basic Energy Sciences (BES grant number DE-FG02-05ER15647).
Disclosure
The authors report no conflicts of interest in this work.
References
- Lambert DM Vandevoorde S Jonsson KO Fowler CJ The palmitoylethanolamide family: a new class of anti-inflammatory agents? Curr Med Chem 2002 9 6 663 674 11945130
- Jian W Edom R Weng N Zannikos P Zhang Z Wang H Validation and application of an LC-MS/MS method for quantitation of three fatty acid ethanolamides as biomarkers for fatty acid hydrolase inhibition in human plasma J Chromatogr B Analyt Technol Biomed Life Sci 2010 878 20 1687 1699
- Day TA Rakhshan F Deutsch DG Barker EL Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide Mol Pharmacol 2001 59 6 1369 1375 11353795
- Duncan RS Chapman KD Koulen P The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line Mol Neurodegener 2009 4 50 20003317
- Lombardi G Miglio G Varsaldi F Minassi A Appendino G Oxyhomologation of the amide bond potentiates neuroprotective effects of the endolipid N-palmitoylethanolamine J Pharmacol Exp Ther 2007 320 2 599 606 17068202
- Kilaru A Tamura P Garg P Changes in N-acylethanolamine Pathway Related Metabolites in a Rat Model of Cerebral Ischemia/Reperfusion J Glycom Lipidom 2010 1 1 101
- Eberlein B Eicke C Reinhardt HW Ring J Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study) J Eur Acad Dermatol Venereol 2008 22 1 73 82 18181976
- Hamtiaux L Masquelier J Muccioli GG The association of N-palmitoylethanolamine with the FAAH inhibitor URB597 impairs melanoma growth through a supra-additive action BMC Cancer 2012 12 92 22429826
- Ueda N Yamanaka K Yamamoto S Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance J Biol Chem 21 2001 276 38 35552 35557 11463796
- Vandevoorde S Jonsson K-O Fowler CJ Lambert DM Modifications of the Ethanolamine Head in N-Palmitoylethanolamine: Synthesis and Evaluation of New Agents Interfering with the Metabolism of Anandamide J Med Chem 2003 46 8 1440 1448 12672243
- Facci L Dal Toso R Romanello S Buriani A Skaper SD Leon A Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide Proc Natl Acad Sci U S A 1995 92 8 3376 3380 7724569
- Citraro R Russo E Scicchitano F Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-α receptor activation in a genetic model of absence epilepsy Neuropharmacology 2013 69 115 126 23206503
- García MdC Adler-Graschinsky E Celuch SM Enhancement of the hypotensive effects of intrathecally injected endocannabinoids by the entourage compound palmitoylethanolamide Eur J Pharmacol 2009 610 1–3 75 80 19289116
- Geng X-C Li B Zhang L Corn oil as a vehicle in drug development exerts a dose-dependent effect on gene expression profiles in rat thymus J Appl Toxicol 2012 32 10 850 857 22760963
- Radd BL Newman AC Fegely BJ Chrzanowski FA Lichten JL Walkling WD Development of haloperidol in oil injection formulations J Parenter Sci Technol 1985 39 1 48 51 3973803
- Lemini C Medina M Avila ME In vivo and in vitro evaluation of the estrogenic properties of the 17beta-(butylamino)-1,3,5(10)-estratrien-3-ol (buame) related to 17beta-estradiol Pharmacol Rep 2012 64 4 940 950 23087146
- Fadem BH Evidence for extended action of gonadal hormones on the organization of sexually dimorphic behavior and morphology in gray short-tailed opossums (Monodelphis domestica) Horm Behav 2001 39 2 113 120 11243739
- Jaccoby S Arnon E Snapir N Robinzon B Effects of estradiol and tamoxifen on feeding, fattiness, and some endocrine criteria in hypothalamic obese hens Pharmacol Biochem Behav 1995 50 1 55 63 7700955
- Castellano-Díaz E Gonzalez-Quijano MI Liminana JM Díaz-Chico BN Tamoxifen decreases the estradiol induced progesterone receptors by interfering with nuclear estrogen receptor accumulation J Steroid Biochem 1989 33 1 133 139 2761261
- Jaccoby S Pinchasov Y Snapir N Robinzon B Hypothalamic obese, functionally castrated hens are hypersensitive to estrogenic modulation of lipid metabolism Physiol Behav 1996 60 3 913 918 8873269
- Hensler M Bardova K Jilkova ZM The inhibition of fat cell proliferation by n-3 fatty acids in dietary obese mice Lipids Health Dis 2011 10 128 21810216
- Reinert RB Kantz J Misfeldt AA Tamoxifen-induced cre-loxp recombination is prolonged in pancreatic islets of adult mice PloS One 2012 7 3 e33529 22470452
- Riopel MM Li J Liu S Leask A Wang R β1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function Lab Invest 2013 93 1 31 40 23069938
- Tong S Chen Q Shan SQ Dewhirst MW Yuan F Quantitative comparison of the inhibitory effects of GW5638 and tamoxifen on angiogenesis in the cornea pocket assay Angiogenesis 2006 9 2 53 58 16622786
- Kim DJ Han BS Ahn B Lee KK Kang JS Tsuda H Promotion potential of tamoxifen on hepatocarcinogenesis in female SD or F344 rats initiated with diethylnitrosamine Cancer Lett 1996 104 1 13 19 8640739
- Gao S Singh J In vitro percutaneous absorption enhancement of a lipophilic drug tamoxifen by terpenes J Control Release 1998 51 2–3 193 199 9685917
- Venables BJ Waggoner CA Chapman KD N-acylethanolamines in seeds of selected legumes Phytochemistry 2005 66 16 1913 1918 16054175
- Hill MN Miller GE Carrier EJ Gorzalka BB Hillard CJ Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress Psychoneuroendocrinology 2009 34 8 1257 1262 19394765
- Caraceni P Viola A Piscitelli F Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis Liver Int 2010 30 6 816 825 19840245
- Sipe JC Scott TM Murray S Biomarkers of endocannabinoid system activation in severe obesity PLoS One 2010 5 1 e8792 20098695
- Kilaru A Isaac G Tamura P Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase Lipids 2010 45 9 863 875 20714818
- Piscitelli F Carta G Bisogno T Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice Nutr Metab (Lond) 2011 8 1 51 21749725
- Maccarrone M Attinà M Bari M Cartoni A Ledent C Finazzi-Agrò A Anandamide degradation and N-acylethanolamines level in wildtype and CB1 cannabinoid receptor knockout mice of different ages J Neurochem 2001 78 2 339 348 11461969
- Artmann A Petersen G Hellgren LI Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine Biochim Biophys Acta 2008 1781 4 200 212 18316044
- Matias I Wang JW Moriello AS Nieves A Woodward DF Di Marzo V Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration Prostaglandins Leukot Essent Fatty Acids 2006 75 6 413 418 17011761
- Chen J Matias I Dinh T Finding of endocannabinoids in human eye tissues: implications for glaucoma Biochem Biophys Res Commun 2005 330 4 1062 1067 15823551
- Berdyshev EV Schmid PC Krebsbach RJ Cannabinoid-receptor-independent cell signalling by N-acylethanolamines Biochem J 2001 360 Pt 1 67 75 11695993
- Yazulla S Studholme KM McIntosh HH Fan SF Cannabinoid receptors on goldfish retinal bipolar cells: electron-microscope immunocytochemistry and whole-cell recordings Vis Neurosci 2000 17 3 391 401 10910107
- Lu Q Straiker A Maguire G Expression of CB2 cannabinoid receptor mRNA in adult rat retina Vis Neurosci 2000 17 1 91 95 10750830
- De Petrocellis L Bisogno T Ligresti A Bifulco M Melck D Di Marzo V Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems Fundam Clin Pharmacol 2002 16 4 297 302 12570018
- Movahed P Jonsson BA Birnir B Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists J Biol Chem 2005 280 46 38496 38504 16081411
- Parolaro D Massi P Rubino T Monti E Endocannabinoids in the immune system and cancer Prostaglandins Leukot Essent Fatty Acids 2002 66 2–3 319 332 12052046
- Zimov S Yazulla S Localization of vanilloid receptor 1 (TRPV1/VR1)-like immunoreactivity in goldfish and zebrafish retinas: restriction to photoreceptor synaptic ribbons J Neurocytol 2004 33 4 441 452 15520529
- Ho WS Barrett DA Randall MD ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors Br J pharmacol 2008 155 6 837 846 18695637
- Garg P Duncan RS Kaja S Koulen P Intracellular mechanisms of N-acylethanolamine-mediated neuroprotection in a rat model of stroke Neuroscience 2010 166 1 252 262 19963043
- Duncan RS Xin H Goad DL Chapman KD Koulen P Protection of Neurons in the Retinal Ganglion Cell Layer against Excitotoxicity by the N-Acylethanolamine, Linoleoylethanolamine Clin Ophthalmol 2011 5 543 548 21573043