?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We report the intercalation and characterization of para-amino salicylic acid (PASA) into zinc/aluminum-layered double hydroxides (ZLDHs) by two methods, direct and indirect, to form nanocomposites: PASA nanocomposite prepared by a direct method (PASA-D) and PASA nanocomposite prepared by an indirect method (PASA-I). Powder X-ray diffraction, Fourier-transform infrared spectroscopy, and thermogravimetric analysis revealed that the PASA drugs were accommodated within the ZLDH interlayers. The anions of the drug were accommodated as an alternate monolayer (along the long-axis orientation) between ZLDH interlayers. Drug loading was estimated to be 22.8% and 16.6% for PASA-D and PASA-I, respectively. The in vitro release properties of the drug were investigated in physiological simulated phosphate-buffered saline solution of pH 7.4 and 4.8. The release followed the pseudo-second-order model for both nanocomposites. Cell viability (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [MTT] assays) was assessed against normal human lung fibroblast MRC-5 and 3T3 mouse fibroblast cells at 24, 48, and 72 hours. The results showed that the nanocomposite formulations did not possess any cytotoxicity, at least up to 72 hours.
Introduction
Layered double hydroxides (LDHs) have a hydrotalcite-like structure in which some of the divalent cations are replaced by trivalent cations, which results in positively charged brucite-like sheets stacked on top of one another, resulting in a layered structure. The positive charge of these brucite-like nanosheets is neutralized by the anions and water molecules.Citation1 A variety of anions can be accommodated between the layers to counter balance the positive charge and this tendency makes them versatile to be used for different applications. The anions can be inorganic, like halides, nitrates, and sulfates, and organic, like drugs, amino acids, dyes, polymers, and DNA etc.Citation1,Citation2 LDHs are represented by the general formula (MII 1–x MIII x [OH]2)(Ax/n n−) · yH2O, in which MII is a divalent metal ion, MIII is a trivalent metal ion, and anions are used to neutralize the positive charge of the sheets. The LDHs have many applications, such as their use for extraction of toxic waste from water,Citation3 in catalysis,Citation4 and as a flame retardant,Citation5 and they have also been used in chiral separation.Citation6 Recently, the most important application of LDHs has been investigated, particularly slow-release drug-delivery systems. Different drugs have been intercalated recently into nanosheets of LDHs, such as anticancer drugs, antihistamines, antidiabetics, and antimicrobials.Citation7,Citation8
The sustained release of the intercalated anions is a tremendous characteristic of LDHs, and is supposed to enhance the therapeutic effects of the drugs. The sustained release can also increase the water solubility of the poorly soluble drugs, which ultimately reduces the side effects of the drugs related to poor water solubility. Low cytotoxicity and ease of LDH preparation make them a suitable candidate for drug-delivery purposes.
Ryu et alCitation9 applied LDHs to the antimicrobial agent cefazolin and conducted in vitro antibacterial studies. They found that the cefazolin-LDH formulation possessed higher antibacterial activity compared to naked cefazolin.Citation9 Hesse et al recently implanted ciprofloxacin-LDHs in the middle ear of rabbits infected with Pseudomonas aeruginosa, and clinical and microbial examination results showed the ciprofloxacin-LDHs possessed sound antimicrobial activity.Citation10
Tuberculosis (TB) is an infectious disease caused by the bacteria Mycobacterium tuberculosis. The treatment duration of TB can last from 3 to 24 months, depending on the type of TB.Citation11 According to the latest facts and figures on TB in the Global Tuberculosis Report 2012, there were about 8.7 million new cases of TB in 2011, and 1.4 million humans lost their lives. TB is one of the top killers of women, with 300,000 deaths among HIV-negative women and 200,000 deaths among HIV-positive women in 2011.Citation12
Para-amino salicylic acid (PASA) is an antituberculosis agent. PASA was found to be active against TB, and was used in clinical trials more than six decades ago – in 1948.Citation13 Initially, it was included in the treatment of drug-susceptible TB, but because of such side effects as anorexia, epigastric distress, nausea, vomiting, and abdominal cramps it is no longer used for drug-susceptible TB, but is still used for multidrug-resistant TB. A controlled-release formulation of PASA would be very useful for overcoming these side effects.Citation14 Different approaches are being developed for efficient and effective drug-delivery systems for the targeted and sustained release of anti-TB drugs, such as plant proteins, inhalable microparticles of polylactic acid, glucan particles and mannitol microparticles. A comprehensive review by Saifullah et alCitation11 discusses many of these delivery systems in detail.
In this article, we report the successful intercalation of PASA into the galleries of zinc/aluminum-layered double hydroxide (ZLDH) with coprecipitation and ion-exchange methods. We also studied the spatial orientation of the drug between the inorganic interlayers, the sustained-release properties, and the kinetics of its release. Furthermore, we investigated the thermal stability of the drug outside and within inorganic interlayers of ZLDH, and we also determined the morphology of resulting nanocomposites. The main objective of this research was drug-delivery applications of these nanocomposites; therefore, we investigated cytotoxic studies on the 3T3 fibroblast mouse cell line and normal human lung cell lines where TB bacteria usually reside.
Materials and methods
All the chemicals used were of analytical reagent grade without any further purification. PASA (99% pure), zinc nitrate hexahydrate, and aluminum nitrate nonahydrate were purchased from Sigma-Aldrich (St Louis, MO, USA). Dimethyl sulfoxide (DDDT) with 0.1% water content, used as a solvent, was purchased from Thermo Fisher Scientific (Ajax Finechem; Sydney, NSW, Australia).
Preparation of Zn/Al-NO3 LDH
A zinc to aluminum solution with a molar ratio of 4:1 was dissolved in 250 mL deionized water and stirred for 15 minutes, and the pH of the solution was raised to 7.0 by adding 2 M NaOH solution. The experiment was conducted under continuous nitrogen flux. Then, the sample was subjected to oil bath-agitation for 18 hours and then washed with deionized water three times and centrifuged.
Preparation of nanocomposite by coprecipitation method
The sample was prepared by the method reported previously by Crepaldi et al.Citation15 Zinc nitrate hexahydrated and aluminum nitrate nonahydrated salts were dissolved in 50 mL deionized water at a molar ratio of 4:1. The solution was stirred for 15 minutes, then 0.4 M PASA prepared in 50 mL DDDT and deionized water in a molar ratio of 1:1 was added dropwise to the salt solution. Then, the pH of the solution was increased to 7.25 by dropwise addition of 0.5 M NaOH aqueous solution under a nitrogen atmosphere. After that, the sample was subjected to agitation in an oil bath at 70°C for 18 hours. Then, the sample was centrifuged and washed with deionized water. The resulting nanocomposite was denoted as PASA-D (PASA nanocomposite prepared by direct method).
Preparation of nanocomposite by ion exchange method
The sample was prepared by adopting the method reported by Lakraimi et al.Citation16 Zinc and aluminum salts at a molar ratio of 1:4 required for 100 mL volume were dissolved in 50 mL deionized water and stirred for 15 minutes. The salt solution was added dropwise to a 250 mL beaker containing 50 mL deionized water. NaOH 1 M solution was simultaneously added to maintain the pH at 7.00. Then, a 0.4 M solution of PASA prepared earlier in DDDT and water at a molar ratio of 1:1 in 50 mL prepared earlier was added to the freshly prepared ZLDH. The solution pH was maintained at 7.00 by simultaneous addition of 1 M NaOH solution. The whole experiment was conducted under a nitrogen atmosphere. The resulting nanocomposite was denoted as PASA-I (PASA nanocomposite prepared by an indirect method).
Controlled-release study
The release of the drug was studied in human body-simulated buffer solutions − 0.1 M at two different pHs, 4.8 and 7.4 – to see the release profiles of PASA from the interlayer galleries of ZLDHs. There are different kinds of anions present in phosphate-buffered saline (PBS), such as mono phosphate H2PO4 −, dibasic phosphate HPO4 2−, and Cl−. About 0.4 mg of the nanocomposites of PASA-D and PASA-I were placed into 3.5 mL of either a pH 4.8 or pH 7.4 PBS. Maximum absorbance of 268 nm was selected in an ultraviolet-visible (UV–vis) absorption spectrophotometer. This was to compare the release of the drug from PASA-D and PASA-I in different physiological environments.
Cell culture
3T3 mouse fibroblast cells and human lung fibroblast MRC-5 cells were obtained from the American Type Culture Collection (Manassas, VA, USA), and the cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) and Roswell Park Memorial Institute 1640 media containing 10% fetal bovine serum. Growth media contained 100 units/mL penicillin and 50 μg/mL streptomycin. Fibroblast cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air.
Cell viability assays
Healthy cells were seeded onto 96-well culture plates at 1 × 104 cells per well and allowed to adhere overnight at 37°C. Then, cultured cells were incubated with medium (100 μL) containing dispersed LDH nanocomposites in various concentration ranges from 0.781 μg/mL to 50 μg/mL. The control cells were not exposed to the nanocomposites. At specific time points of 24, 48, and 72 hours of incubation, the growth medium was removed from 96-well plates and incubated with 100 μL of the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reagent in DMEM for another 3–4 hours at 37°C. The number of viable cells was analyzed by uptake of MTT, and read at 570 nm by an enzyme-linked immunosorbent assay plate reader. Cell viability results are presented as means ± standard deviation.
Characterization
The X-ray diffraction (XRD) studies were done using a Shimadzu (Kyoto, Japan) XRD-6000 diffractometer. Radiation, CuKα at 30 KV and 30 mA for recording the powder XRD patterns in the range of 2–60θ°. Fourier-transform infrared (FTIR) spectra of the materials were recorded over the range of 4,000–400 cm−1 on a PerkinElmer (Waltham, MA, USA) 100 series spectrophotometer by direct sample method. For the analysis of carbon, hydrogen, nitrogen, and sulfur (CHNS), a LECO (St Joseph, MI, USA) CHNS-932 instrument was used. For the thermogravimetric and differential thermogravimetric analyses, a Mettler-Toledo (Greifensee, Switzerland) instrument was used. The morphology of the sample surface was studied with a JEOL (Tokyo, Japan) JSM-6400 scanning electron microscope (SEM). For optical properties, and controlled-release studies, a Shimadzu 1650 series UV-vis spectrophotometer was utilized. Inductively coupled plasma (ICP) was used to determine Zn2+ and Al3+ concentration.
Results and discussion
Powder X-ray diffraction
shows XRD patterns for the resulting nanocomposites synthesized by coprecipitation and ion-exchange methods, labeled as PASA-D and PASA-I, respectively. The ZLDHs with nitrate anion as counter ion have been reported to have basal spacing of 8.9 Å in XRD patterns.Citation18
Figure 1 X-ray diffraction patterns of PASA-D (A), PASA-I (B) and free PASA (C).
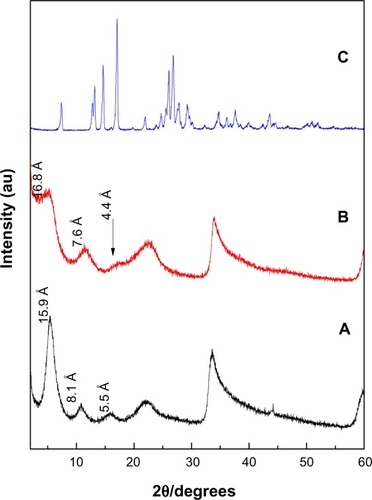
The XRD patterns show that the basal spacings of the resulting nanocomposites PASA-D and PASA-I increased from 8.9 Å to 15.9Å and 16.8Å, respectively. The increase in basal spacing is direct evidence of successful intercalation of drug molecules in the ZLDH interlayers. The expansion is attributed to the intercalation of PASA in its anionic form inside the interlamellae of ZLDH.
Furthermore, a small amount of CO3 2− coexisted between the ZLDH interlayers, partly because it is difficult to avoid contamination from the air completely and partly due to the favorable lattice stabilization enthalpy associated with the small and highly charged CO3 2− anions.Citation19 The intense sharp peaks of PASA-D suggest that the nanocomposite possessed high degree crystallinity. The high crystallinity of PASA-D may be attributed to its long range and well-ordered arrangement of PASA anions in the inorganic interlamellar domain of the nanocomposite, and most probably this was taking place during the aging process. During coprecipitation, possibly better interactions took place between the negatively charged PASA anions with the positively charged ZLDH inorganic layers.Citation20 In the case of the PASA-I (), XRD peaks were wider instead of sharp, some of the hkl reflections were missing, and the XRD reflections were less intense. This suggests that the nanocomposite has a lower degree of crystallinity compared to host LDHs.Citation16 The possible reason for lower crystallinity may be the preparation method, as the host has to be rearranged during the ion-exchange process. The difference in d-spacing of PASA-D and PASA-I nanocomposites may be due to the layer-charge density or content of water between the layers.Citation21
Orientation of PASA in the inorganic interlayers of ZLDH
According to XRD analysis, the interlayer distance (d003) increased to 15.9 Å for PASA-D and 16.8 Å for PASA-I nanocomposites. The thickness of the Zn/Al-LDH layer was 4.8 Å,Citation2 thus the gallery height of Zn/Al-LDH after intercalation can be calculated by the interlayer distance minus the thickness of the Zn/Al-LDH layer, which is 11.1 Å (15.9–4.8) and 12.0 Å (16.8–4.8) for PASA-D and PASA-I nanocomposites, respectively. The long axis, short axis, and molecular thickness of PASA were calculated using ChemOffice software, and they were found to be 9.4, 7.1, and 2.9 Å, respectively ().
Figure 2 Three-dimensional structure of PASA (A) and molecular structural models of PASA intercalated between interlayers of Zn/Al-LDH (B).
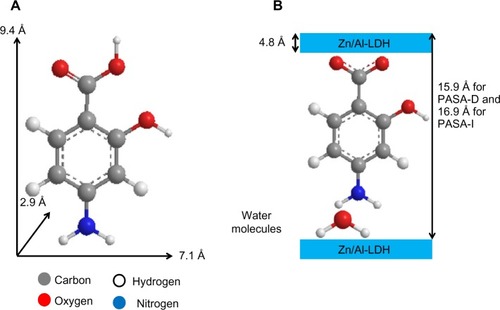
The gallery height of the PASA-D nanocomposite was 11.1 Å, which is near the value of the long axis (9.4 Å); similar is the case of the PASA-I nanocomposite, which had 12.0 Å gallery height. This result suggests that PASA anions are accommodated as an alternate monolayer (along the long-axis orientation) between layers with the carboxyl of adjacent anions attaching to the upper hydroxide layers and the amino group attached to another layer through water molecules ().
Infrared spectroscopy
The intercalation of PASA anions into the interlayers of ZLDH was further confirmed by FTIR spectroscopy (). All the vibrational bands of the PASA anions are observed together with the absorption bands of ZLDH.
Figure 3 Fourier-transform infrared spectra of (A) PASA, (B) PASA-D, and (C) PASA-I.
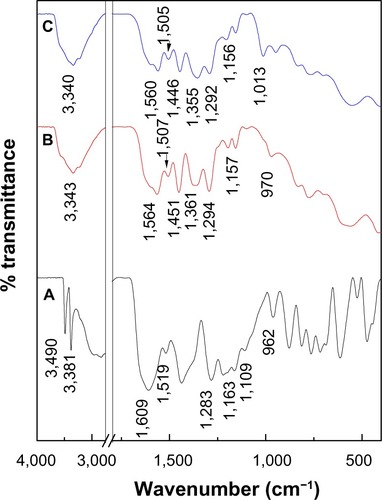
The FTIR spectrum of PASA shows many absorption bands (). In addition to bands at high wave-number values, due to stretching of O–H, = C–H, and N–H moieties,Citation22 a band was recorded at 1,609 cm−1, due to C=O stretching of the carboxylic acid group. Due to C–C stretching of the aromatic ring, bands were recorded at 1,519 and 1,437 cm−1,Citation22 those due to the stretching mode of C–O and O–H bending of the acid and alcohol functions were recorded at 1,296 cm−1 and 1,220 cm−1, respectively, while in-plane and out-plane of C–H bending vibrations were recorded at 1,109 and 813 cm−1, respectively.Citation22,Citation23 A vibrations at 972 cm−1 was due to the ring-breathing mode.Citation24
The FTIR spectra recorded after intercalation of the PASA into the interlayers of ZLDH are shown in and C. The FTIR spectra of PASA-D and PASA-I nanocomposites contained both the characteristic peaks of pure PASA and the typical peak of ZLDH, which indicates that the PASA anions have been intercalated into the interlayer galleries of the ZLDH. Due to the negative charge of the acid group, the band of carboxylic acid (COOH) for free PASA at 1,609 cm−1 had disappeared, while new bands were recorded at 1,564 and 1,361 cm−1 for the PASA-D nanocomposite, which corresponds to the asymmetric and symmetric vibrations, respectively, of the carboxylate group COO−.Citation25 In addition, bands at 1,560 and 1,355 cm−1 for PASA-I nanocomposite can be observed, which corresponds to the asymmetry and symmetry, respectively, of the carboxylate group COO−.Citation25 The band assigned to the nitrate group (1,384 cm−1) was absent from both nanocomposites, and this result supported the view that the NO3 − anion was fully replaced by the drug. The bands below 600 cm−1 are due to metal–oxygen bond-stretching modes in the inorganic layers.Citation17,Citation26 The rest of the FTIR spectra for PASA, PASA-D, and PASA-I nanocomposite bands are listed in .
Table 1 Assignments of FTIR absorption bands of free PASA, PASA-D and PASA-I nanocomposites
Elemental analysis
Elemental analysis was used to determine the PASA and inorganic composition of PASA-D and PASA-I nanocomposites. As predicted, PASA-D and PASA-I nanocomposites showed the presence of both PASA and ZLDH inorganic layer constituents. This result indicated that intercalation had occurred in which PASA was already intercalated into the ZLDH interlayers.
shows the Zn2+/Al3+ molar ratios in PASA-D and PASA-I were 3.8 and 4.1, respectively, compared to 4.0 for the initial molar ratio that was prepared for the mother liquor. The C:N ratio of PASA was 6.2. In addition, the C:N ratios for PASA-D and PASA-I nanocomposites were larger than expected (9.2 and 11.3, respectively). According to FTIR spectra of PASA-D and PASA-I, strong bands at 1,361 and 1,355 cm−1 are due to carbonate and COO− symmetric stretching vibration. This result indicates that a trace amount of carbonate was present in the interlamellae space of the LDH. From the elemental chemical analysis results, the empirical formulas for PASA-I and PASA-D were found to be
and
, respectively.
Table 2 Chemical compositions of PASA, PASA-D, and PASA-I nanocomposites
Thermal analysis
The thermal behavior of the PASA, PASA-D, and PASA-I nanocomposites were examined by thermogravimetric and differential thermogravimetric analysis. The thermograms are shown in –. For free drug (), two major thermal events occurred. The first was observed in the region of 101°C–150°C, and a sharp peak was observed at 145°C, with a 31.4% weight loss corresponding to the dehydration of PASA. The second event showed a strong peak at 227°C with 67.7% weight loss between the region of 151°C–235°C corresponding to the combustion of PASA.Citation27 Thermal behaviors of PASA-D and PASA-I nanocomposites are shown in , respectively. Three events were observed in both PASA-D and PASA-I nanocomposites. The first stage of the event in PASA-D occurred at 67°C with the weight loss of 5.6% because of the physically absorbed water on the surface of the nanocomposite, while the same event occurred in PASA-I at 75°C with a weight loss of 12.5%. The second event occurred at a temperature of 254°C with weight loss of 22.1% in the case of PASA-D, whereas the same event occurred at a temperature of 240°C with weight loss of 21.1% in the case of PASA-I. This event in both nanocomposites was due to the dehydroxylation of inorganic layers and thermal decomposition of PASA.
Figure 4 Thermogravimetric analysis–differential thermogravimetric thermograms of PASA (A), PASA-D (B), and PASA-I (C).
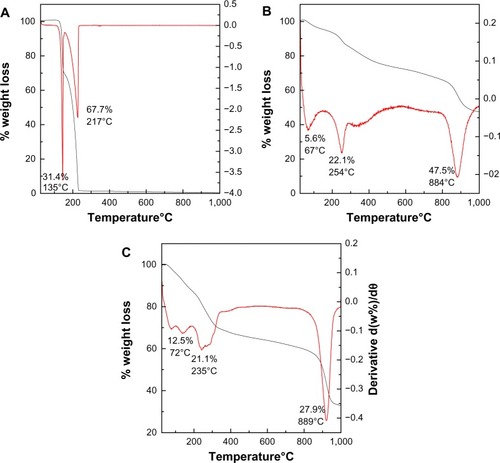
The third event occurred at a temperature of 884°C with weight loss of 47.5% in the case of PASA-D and at a temperature of 920°C with weight loss of 28% in the case of PASA-I, due to the formation of the spinel (ZnAl2O4) phase.Citation28
The thermal stability of PASA was enhanced after intercalation into the ZLDH, as the drug decomposition occurred at 254°C in the case of PASA-D and at 240°C in the case of PASA-I, instead of 227°C. This enhanced thermal stability of PASA can be attributed to the electrostatic interaction between PASA and the inorganic interlayers of ZLDH.
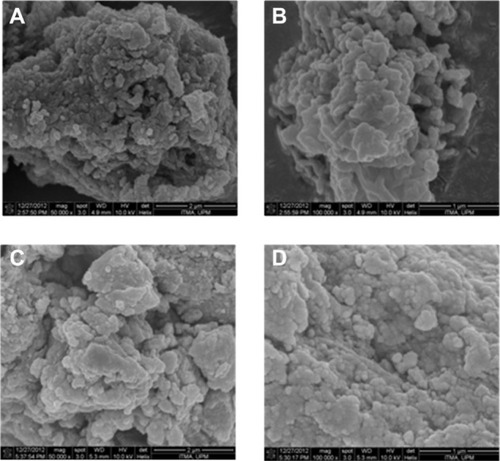
Surface morphology
The surface morphology of PASA-D and PASA-I is shown in –. The micrographs were obtained using an SEM at 50,000× magnifications (A and C) and 100,000× magnifications (B and D). The SEM images for both PASA-D and PASA-I nanocomposites show agglomerates of nonporous, flaky structure without any specific shapes.
Figure 5 Field-emission scanning electron micrographs of PASA-D (A and B) and PASA-I (C and D) nanocomposites at 50,000× and 100,000× magnifications.
In vitro PASA release from PASA-D and PASA-I nanocomposites
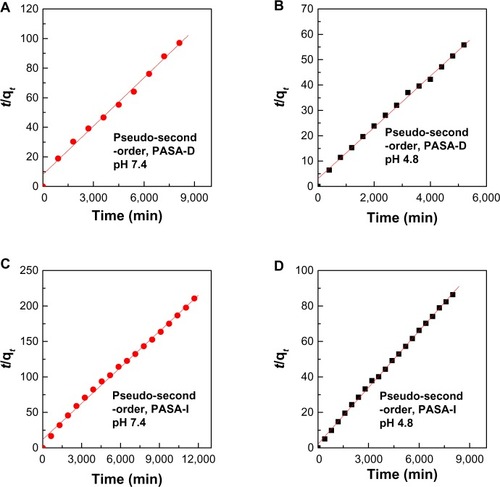
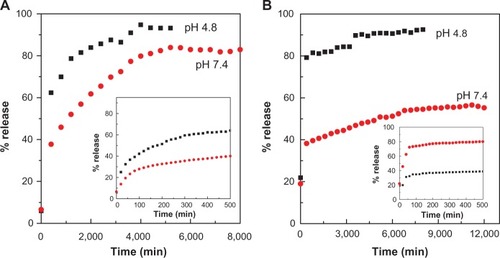
The in vitro release properties of the drug were investigated by adding the PASA-D and PASA-I nanocomposites to PBS solutions at pH 7.4 and 4.8. shows the release profiles of PASA-D and PASA-I nanocomposites in PBS solutions at pH 7.4 and 4.8. For these nanocomposites, the first aliquot revealed a high amount of anion release (burst effect).Citation29 This probably occurred due to the release of the PASA anions adsorbed on the surface of ZLDH. The release behaviors at pH 4.8 were very fast compared to pH 7.4, which can be attributed to the partial dissolution of the ZLDH layer at acidic solutionsCitation30,Citation31 as well as the ion-exchange process. The release of PASA-D and PASA-I nanocomposites was 100% and 93%, respectively. Time taken for the release was found to be 5,400 and 8,369 minutes for PASA-D and PASA-I nanocomposites, respectively. The PASA-D and PASA-I nanocomposites were more stable at pH 7.4, as the release followed the ion-exchange process between the intercalated anions in the interlayer and phosphate anions in the buffer solutions.Citation32 The release of the drug anions at pH 7.4 in PASA-D and PASA-I nanocomposites reached 88% and 57% respectively, within 8,627 and 1,200 minutes. This was probably due to the characteristics of the ion-exchange process that occurs,Citation16,Citation33,Citation34 ie, this is an equilibrium process and the interlayer anions cannot be exchanged completely, unless the released organic species were removed or consumed continuously.
Figure 6 Release profiles of PASA from PASA-D (A) and PASA-I (B) nanocomposites into PBS at pH 7.4 and 4.8. Insets show the release profiles of PASA from nanocomposite at initial 500 minutes time.
Release kinetics of PASA from PASA-D and PASA-I nanocomposites
The kinetic models for the PASA release from PASA-D and PASA-I nanocomposites were analyzed using pseudo-first-order, pseudo-second-order, and parabolic diffusion kinetic equations.
The pseudo-first-order kinetic equation may be represented in a linear form asCitation35:
where q e and q t are the equilibrium release amount and the release amount at any time (t), respectively. The k 1 value can be obtained from the slope by plotting ln (q e − q t) versus t. The pseudo-second-order kinetic equation may be represented in a linear form asCitation36:
The parabolic diffusion kinetic equation may be represented asCitation37:
where, M0 and M t are the drug content remaining in the ZLDH at release time 0 and t, respectively, and b is a constant.
Using these equations for the three kinetic models, it was found that the pseudo-second-order model more satisfactorily described the kinetic release process of PASA from PASA-D and PASA-I nanocomposites. shows plots of the fitting of PASA released from nanocomposites. At pH 4.8, the correlation coefficient (R 2) was 0.9978 and 0.9991 for PASA-D and PASA-I nanocomposites, respectively, with k 2 values of 3.3 × 10−5 and 5.2 × 10−5 mg/minute, respectively, and at pH 7.4, the corresponding values were 0.9946 and 0.9952, respectively, with k 2 values of 1.5 × 10 Citation5 and 1.7 × 10 Citation5 mg/minute, respectively. All of these results are summarized in .
Table 3 Correlation coefficient (R 2), and rate constants (k) obtained by fitting the data of PASA release from PASA-D and PASA-I nanocomposites into PBS solution at pH 4.8 and 7.4
Cytotoxicity study of ZLDHs and PASA, PASA-D, and PASA-I nanocomposites
In vitro toxicological studies of nanomaterials is a developing new field of research these days, aimed at understanding them and finding out their possible effects on living systems. Cell-cytotoxicity testing (MTT assay) is a very common preliminary step in assessing combustion-generated and functionalized nanoparticle-related health hazards. The MTT cytotoxicity assay reveals various cellular metabolic actions by the reduction of the yellow tetrazolium salt MTT to a purple MTT formazan. The nanocomposites PASA-D and PASA-I, at different gradient concentrations and various time points, ie, 0.781, 1.562, 3.125, 6.25, 12.5, 25, and 50 μg/mL for 24 hours, 48 hours, and 72 hours, were exposed to 3T3 mouse fibroblast cells and human lung fibroblast MRC-5 cells. Cell viability was determined by MTT assay ().
Figure 8 Cell viability (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [MTT] assay) of 3T3 mouse fibroblast cells and human lung fibroblast MRC-5 cells exposed to various gradient concentrations at 24, 48, and 72 hours’ exposure time. The data presented are means ± standard deviation of triplicate samples.
![Figure 8 Cell viability (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [MTT] assay) of 3T3 mouse fibroblast cells and human lung fibroblast MRC-5 cells exposed to various gradient concentrations at 24, 48, and 72 hours’ exposure time. The data presented are means ± standard deviation of triplicate samples.](/cms/asset/8b926bc5-e5d6-414b-b9bb-6bd4b385f1c8/dddt_a_50665_f0008_c.jpg)
Neither of the PASA-D and PASA-I nanocomposites showed any reduction in cell viability during 24, 48, and 72 hours of inhibition at concentrations of 0.781–50 μg/mL. Thus, the nanocomposite formulations possess no cytotoxicity and may be considered to be appropriate for drug-delivery purposes. Based on these preliminary toxicological findings, further detailed studies will be conducted for biomedical and translational applications.
Conclusion
A new delivery system for PASA has been developed in this work. The drug was intercalated into ZLDH inorganic galleries by coprecipitation and ion-exchange methods. XRD, FTIR, CHNS, and TGA results indicate the successful intercalation of PASA between the LDH inorganic interlayers. The in vitro release studies show that there was a burst phenomenon occurring at the beginning of release tests at different pHs in PBS solutions, due to the absorption of the drug on the surface of ZLDH. Three kinetic models (pseudo-first-order, pseudo-second-order, and parabolic diffusion kinetic equations) were used to study the kinetics of PASA release from the nanocomposites. The kinetic studies showed the pseudo-second-order model more satisfactorily described the cumulative kinetic release process of PASA from PASA-D and PASA-I nanocomposites. The MTT results showed that the PASA-D and PASA-I nanocomposite formulations did not show any cytotoxicity against the 3T3 cell line or human lung fibroblast MRC-5 cells. All of these results suggest that ZLDH formulations are suitable for TB chemotherapy. Therefore, we suggest that further in vivo studies should be carried out for the validation of these in vitro studies.
Acknowledgments
Funding for this research was provided by the Ministry of Science, Technology and Innovation of Malaysia (MOSTI) under the National Nanotechnology Directorate grant NND/NA/1/TD11-010, (UPM Vot number 5489100) and the Higher Education Commission of Malaysia under the Commonwealth Scholarship and Fellowship Plan (Reference KPT.B.600-6/3, Vol 68) for Bullo Saifullah.
Disclosure
The authors report no conflicts of interest in this work.
References
- Duan X Evans DG Layered Double Hydroxides Heidelberg Springer 2006
- Cavani F Trifirò F Vaccari A Hydrotalcite-type anionic clays: preparation, properties and applications Catal Today 1991 11 2 173 301
- Koilraj P Thakur RS Srinivasan K Solid state structural transformation of tetraborate into monoborate in the interlayer galleries of reconstructed ZnAl layered double hydroxide J Phys Chem C 2013 117 13 6578 6586
- Kuhl S Friedrich M Armbruster M Behrens M Cu, Zn, Al layered double hydroxides as precursors for copper catalysts in methanol steam reforming-pH-controlled synthesis by microemulsion technique J Mater Chem 2012 22 19 9632 9638
- Wang DY Das A Leuteritz A Structural characteristics and flammability of fire retarding EPDM/layered double hydroxide (LDH) nanocomposites RSC Adv 2012 2 9 3927 3933
- Fu Z Xie Y Chen X Jiao F Liu L Preparation and characteristic of chiral separation reagent pillared Zn-Al hydrotalcite-like compound Mater Rev 2010 S2
- Hasan S Al Ali H Al-Qubaisi M Controlled-release formulation of antihistamine based on cetirizine zinc-layered hydroxide nanocomposites and its effect on histamine release from basophilic leukemia (RBL-2H3) cells Int J Nanomedicine 2012 7 3351 3363 22848164
- Hussein-Al-Ali S Al-Qubaisi M El Zowalaty M Hussein MZ Ismail M Antimicrobial activity of hippurate nanocomposite and its cytotoxicity effect in combination with cytarabine against HL-60 J Nanomater 2013 2013 843647
- Ryu SJ Jung H Oh JM Lee JK Choy JH Layered double hydroxide as novel antibacterial drug delivery system J Phys Chem Solids 2010 71 4 685 688
- Hesse D Badar M Bleich A Layered double hydroxides as efficient drug delivery system of ciprofloxacin in the middle ear: an animal study in rabbits J Mater Sci Mater Med 2013 24 1 129 136 23053799
- Saifullah B Hussein MZ Hussein Al Ali SH Controlled-release approaches towards the chemotherapy of tuberculosis Int J Nanomedicine 2012 7 5451 23091386
- World Health Organization Global Tuberculosis Report 2012 Geneva WHO 2012
- Mitchison DA Role of individual drugs in the chemotherapy of tuberculosis Int J Tuberc Lung Dis 2000 4 9 796 806 10985648
- Mitnick C Bayona J Palacios E Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru N Engl J Med 2003 348 2 119 128 12519922
- Crepaldi EL Pavan PC Valim JB Comparative study of the coprecipitation methods for the preparation of layered double hydroxides J Braz Chem Soc 2000 11 1 64 70
- Lakraimi M Legrouri A Barroug A De Roy A Besse JP Preparation of a new stable hybrid material by chloride–2,4-dichlorophenoxyacetate ion exchange into the zinc–aluminium–chloride layered double hydroxide J Mater Chem 2000 10 4 1007 1011
- Rives V Layered Double Hydroxides: Present and Future Hauppauge (NY) Nova Science 2001
- Hussein-Al-Ali SH Al-Qubaisi M Hussein MZ Ismail M Zainal Z Hakim MN In vitro inhibition of histamine release behavior of cetirizine intercalated into Zn/Al- and Mg/Al-layered double hydroxides Int J Mol Sci 2012 13 5 5899 5916 22754339
- Oriakhi CO Farr IV Lerner MM Incorporation of poly(acrylic acid), poly(vinylsulfonate) and poly(styrenesulfonate) within layered double hydroxides J Mater Chem 1996 6 1 103 107
- Crepaldi EL Pavan PC Valim JB Anion exchange in layered double hydroxides by surfactant salt formation J Mater Chem 2000 10 6 1337 1343
- Newman SP Williams SJ Coveney PV Jones W Interlayer arrangement of hydrated MgAl layered double hydroxides containing guest terephthalate anions: comparison of simulation and measurement J Phys Chem B 1998 102 35 6710 6719
- Akkaya Y Akyuz S Infrared and Raman spectra, ab initio calculations vibrational assignment of 4-aminosalicylic acid Vib Spectrosc 2006 42 2 292 301
- Li F Zhang L Evans DG Forano C Duan X Structure and thermal evolution of Mg-Al layered double hydroxide containing interlayer organic glyphosate anions Thermochim Acta 2004 424 1–2 15 23
- Panicker CY Varghese HT John A Philip D Istvan K Keresztury G FT-IR, FT-Raman and FT-SERS spectra of 4-aminosalicylic acid sodium salt dihydrate Spectrochim Acta A Mol Biomol Spectrosc 2002 58 2 281 287 11808734
- Silion M Hritcu D Popa MI Intercalation of salicylic acid into ZnAl layered double hydroxides by ion-exchange and coprecipitation method J Optoelectron Adv Mater 2009 11 4 528 534
- Kloprogge JT Frost RL Fourier transform infrared and Raman spectroscopic study of the local structure of Mg-, Ni-, and Co-hydrotalcites J Solid State Chem 1999 146 2 506 515
- Rotich MK Brown ME Glass BD Thermal studies on mixtures of aminosalicylic acids with cyclodextrins J Therm Anal Calorim 2003 73 2 687 706
- Cheng X Huang X Wang X Sun D Influence of calcination on the adsorptive removal of phosphate by Zn-Al layered double hydroxides from excess sludge liquor J Hazard Mater 2010 177 1 516 523 20060217
- Huang X Brazel CS On the importance and mechanisms of burst release in matrix-controlled drug delivery systems J Control Release 2001 73 2 121 136 11516493
- Khan AI O’Hare D Intercalation chemistry of layered double hydroxides: recent developments and applications J Mater Chem 2002 12 11 3191 3198
- bin Hussein MZ Zainal Z Yahaya AH Foo DWV Controlled release of a plant growth regulator, alpha-naphthaleneacetate from the lamella of Zn-Al-layered double hydroxide nanocomposite J Control Release 2002 82 2 417 427 12175754
- Zhang H Zou K Sun H Duan X A magnetic organic-inorganic composite: synthesis and characterization of magnetic 5-aminosalicylic acid intercalated layered double hydroxides J Solid State Chem 2005 178 11 3485 3493
- Fogg AM Dunn JS Shyu SG Cary DR O’Hare D Selective ion-exchange intercalation of isomeric dicarboxylate anions into the layered double hydroxide [LiAl2(OH)6]Cl · H2O Chem Mater 1998 10 1 351 355
- Fogg AM Dunn JS O’Hare D Formation of second-stage intermediates in anion-exchange intercalation reactions of the layered double hydroxide [LiAl2(OH)6]Cl · H2O as observed by time-resolved, in situ X-ray diffraction Chem Mater 1998 10 1 356 360
- Dong L Yan L Hou WG Liu SJ Synthesis and release behavior of composites of camptothecin and layered double hydroxide J Solid State Chem 2010 183 8 1811 1816
- Ho YS Ofomaja AE Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber J Hazard Mater 2006 129 1 137 142 16188379
- Kong X Shi S Han J Zhu F Wei M Duan X Preparation of glycy-L-tyrosine intercalated layered double hydroxide film and its in vitro release behavior Chem Eng J 2010 157 2 598 604