Abstract
The objective of this work was to characterize the in vitro (Plasmodium falciparum) and in vivo (Plasmodium berghei) activity profile of the recently discovered lead compound SSJ-183. The molecule showed in vitro a fast and strong inhibitory effect on growth of all P. falciparum blood stages, with a tendency to a more pronounced stage-specific action on ring forms at low concentrations. Furthermore, the compound appeared to be equally efficacious on drug-resistant and drug-sensitive parasite strains. In vivo, SSJ-183 showed a rapid onset of action, comparable to that seen for the antimalarial drug artesunate. SSJ-183 exhibited a half-life of about 10 hours and no significant differences in absorption or exposure between noninfected and infected mice. SSJ-183 appears to be a promising new lead compound with an attractive antimalarial profile.
Introduction
In 2010, 216 million people were infected with malaria, and an estimated 665,000 were killed by this devastating parasitic disease, according to World Health Organization (WHO) reports.Citation1 Of the nearly 1,400 drugs registered worldwide in the last quarter of the 20th century, only four were antimalarials.Citation2 The high costs of available antimalarials and the spread of resistance dramatically minimize the choice of effective medicines in endemic countries; this situation demands renewed efforts toward development of new therapeutic agents.
The recently discovered SSJ-183 is a benzo[α]phenoxazine-based antimalarial ().Citation3 Two-dimensional gel electrophoresis studies investigating the mechanism of action of this compound were performed with the rodent malaria parasite SSJ-183 Plasmodium berghei and indicated a significant change of the expression levels of nine proteins that represent potential therapeutic targets.Citation4 The reported in vitro half-maximal inhibitory concentration (IC50) values of 3 ng/mL against the parasite strain K1 and 22,000 ng/mL against L6 myoblasts in a cytotoxicity test indicate a specific antimalarial potency of the compound (selectivity index of >7,000).Citation3 The selectivity was further supported by the ≥1,000-fold higher IC50 values of SSJ-183 against three other protozoal parasites (Trypanosoma brucei rhodesiense, Trypanosoma cruzi and Leishmania donovani) as compared with K1. In vivo data were obtained from NMRI mice infected with the P. berghei ANKA strain; the infected mice were cured by oral doses of 100 mg/kg given for 3 consecutive days (3 × 100 mg/kg).Citation3 Three × oral doses of 30 mg/kg cured 80% of the mice, whereas 3 × 10 mg/kg was entirely ineffective.
The objectives of the present study were to further assess the potential of SSJ-183 for malaria chemotherapy. Such information is needed to assess whether the molecule could fit into the target product profiles of, for example, the nonprofit organization Medicines for Malaria Venture (MMV).Citation5
Materials and methods
Chemicals and materials
Antimalarial compounds were dissolved in dimethyl sulfoxide (DDDT; 10 mg/mL) (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) and stored at 4°C. The following compounds were tested: pyrimethamine, proguanil, and mefloquine (F Hoffmann-La Roche Ltd, Basel, Switzerland); chloroquine, amodiaquine and artesunate (Sigma-Aldrich Chemie GmbH); SSJ-183 (M Ihara, Hoshi University, Japan); artemisinin (Chongqing Holley Wuling Mountain Pharmaceuticals Co, Ltd, Huawu, People’s Republic of China); piperaquine (Sigma Tau, Pomezia, Italy); pyronaridine (AvaChem Scientific, San Antonio, TX, USA); and atovaquone (GlaxoSmithKline, Tres Cantos, Spain). [3H]hypoxanthine and nonradioactive hypoxanthine were purchased from ANAWA Biomedical Services and Products (Zürich, Switzerland) and Fluka Analytical, (Sigma-Aldrich Chemie GmbH), respectively.
Parasite cultivation
The drug-sensitive P. falciparum strain NF54 (airport strain from The Netherlands) was provided by F. Hoffmann-La Roche Ltd; the chloroquine (CQ)- and pyrimethamine-resistant strain K1 (Thailand) was obtained from the Malaria Research and Reference Reagent Resource Center (MR4; Manassas, VA, USA) (MRA-159). Apart from these two, five additional isolates were used in cross-resistance tests, namely 7G8 (Brazil), TM90C2A (Thailand), D6 (Sierra Leone), W2 (Indochina) and V1/S (Vietnam), all from MR4 (MRA-154, -202, -285, -157, -176). All strains except for D6 share CQ- and pyrimethamine-resistance with K1; TM90C2A is additionally resistant to mefloquine. No resistance is known for D6. The parasites were cultivated as has been described previously.Citation6
[3H]hypoxanthine incorporation assay
Parasite growth in the presence of antimalarial compounds was assessed using the [3H]hypoxanthine incorporation assay and expressed by IC50 values.Citation6 The total incubation time per assay was 72 hours; radioactive hypoxanthine was added for the last 24 hours. The respective IC50 values in ng/mL against unsynchronized NF54 parasites were 3.0 (pyrimethamine), 440 (proguanil),Citation6 4.1 (mefloquine), 1.5 (amodiaquine), 3.5 (artesunate), 3.2 (SSJ-183), 3.0 (artemisinin), 7.8 (piperaquine), 2.6 (pyronaridine) and 0.38 (atovaquone).Citation6
In vitro assessment of stage-specific activity
Cultures were synchronized twice with 5% D-sorbitol as described previously.Citation7,Citation8 The second treatment was 6–8 hours after the first. This procedure provided a parasite culture containing ≥80% young trophozoites (up to 20 hours old). The same procedure, but with another 16 hours of cultivation after the second sorbitol treatment, was carried out separately to obtain early schizonts (up to 36 hours old).
To obtain ring forms, the two synchronizing treatments were divided by a cultivation period of 34 hours rather than 6–8 hours, yielding a parasite culture with >80% rings (up to 6 hours old). Two 96-well microtiter plates for each of the three synchronous stages were incubated with two-fold serial dilutions of SSJ-183 and pyrimethamine, one for 6 hours and the second for 24 hours (final concentration ranges of both antimalarials were 250–7.81 ng/mL). For a concentration of 250 ng/mL the SSJ-183 stock solution (10 mg/mL) was first diluted 1/50 in DDDT, followed by a 1/100 dilution and another 1/2 dilution in the hypoxanthine-free version of the medium as described by Maerki et al.Citation9 The DDDT concentration in the experiments (<0.5%) had no inhibitory effect on parasite cultures. The washing procedures and further processing of the plates were carried out according to published procedures.Citation8,Citation9
In vitro drug–drug interactions
In vitro interactions of 1:1, 1:3 and 3:1 ratios of SSJ-183 with amodiaquine, artemisinin, mefloquine, piperaquine, or pyronaridine were investigated using a modified fixed-ratio isobologram method and the NF54 and K1 parasite strain.Citation6,Citation10 As previously recommended interactions were classified as synergistic or antagonistic with sums of fractional inhibitory concentrations (ΣFICs) of ≤ 0.8 and > 1.4, respectively, with any value in between indicating no interaction.Citation6 Cutoff ranges were determined by mixing the same drug at various ratios and accounting for experimental variation.
In vitro cross-resistance with various strains of P. falciparum
The sensitivity of the P. falciparum isolates NF54, K1, W2, 7G8, TM90C2A, D6, and V1/S to SSJ-183 was assessed by comparison of the respective IC50 values. The ratio between the maximal and minimal obtained value among the results of all tested strains was taken as a means to assess possible cross-resistance. SSJ-183 stock solution (10 mg/mL) was directly diluted in the hypoxanthine-free medium, resulting in an IC50 of 21 ng/mL against unsynchronized NF54 parasites. As mentioned previously, in the experiments where stage-specific action was assessed the SSJ-183 stock solution (10 mg/mL) was first diluted 1/50 in DDDT, which resulted in an IC50 value that was ~7 times lower (3.2 ng/mL).
Statistical analysis
The data for the pharmacodynamic profile of SSJ-183 are expressed as the mean ± standard deviation (SD) of 3–4 independent experiments. To evaluate statistical significance of stage-dependent differences in drug-sensitivity at a level of 5%, a one-way ANOVA followed by the Tukey Honestly Significant Difference (HSD) test was performed using the software available online from VassarStats (http://www.vassarstats.net/).
Similarly, the mean ΣFIC values are the means of 3 independent experiments +SD. Cross-resistance data were based on the averaged results of 2–3 independent experiments.
In vivo onset of action
In vivo efficacy was conducted as previously described using P. berghei-infected mice.Citation11 SSJ-183 and the comparator drug artesunate were dissolved or suspended in a vehicle consisting of 0.5% (w/v) hydroxypropyl methyl cellulose, 0.5% (v/v) benzyl alcohol, 0.4% (v/v) Tween® 80 (Sigma-Aldrich, St Louis, MO, USA), and 0.9% (w/v) sodium chloride in water. The onset of action was determined after a single oral dose of 100 mg/kg of compounds to five female NMRI mice (20–22 g) on day 3 postinfection, resulting in a high initial parasitemia (~40%) to allow the onset of action to be assessed. The reduction in parasitemia and the time of recrudescence was assessed by daily blood analysis (standard flow cytometry techniques) for 7 days followed by intermittent assessment up to 11 days.
Pharmacokinetic properties
The pharmacokinetics properties of SSJ-183 maleate were studied in parallel groups of mice, including noninfected NMRI mice and P. berghei-infected NMRI mice with dosing on day 1 postinfection. SSJ-183 was administered at a nominal dose of 20 mg/kg by oral gavage of a 2 mg/mL suspension (10 mL/kg) prepared in an aqueous vehicle containing 0.5% (w/v) hydroxypropyl methyl cellulose, 0.5% (v/v) benzyl alcohol and 0.4% (v/v) Tween® 80. Blood samples were collected from the tail vein at 0.5, 1, 2, 4, 8, 24, 32, and 48 hours (n=3 mice per time point). Blood was collected into calibrated and heparinized capillaries, transferred to Eppendorf tubes, placed on ice immediately and centrifuged (5,000 rpm for 5 min) as soon as possible (within 15 minutes of collection). The supernatant plasma was removed and stored deep-frozen (−70°C) in the dark until analysis.
Plasma concentrations of SSJ-183 and its metabolites, N-deethyl SSJ-183 and bis-N,N-deethyl SSJ-183, were determined by liquid chromatography-mass spectrometry. Calibration standards were prepared by spiking blank mouse plasma with SSJ-183 and each of the metabolites. Following the addition of internal standard (diazepam), proteins were precipitated by the addition of acetonitrile at a ratio of 3:1 acetonitrile:plasma. Samples were vortexed centrifuged and the supernatant (5 μL) injected directly onto the column (Supelco Ascentis® Express RP-Amide column [Sigma-Aldrich], 2.7 μm particle size, 50 × 2.1 mm in diameter equipped with a Phenomenex Security Guard cartridge with a Synergi Polar RP insert [Phenomenex, Torrance, CA, USA] and maintained at 40°C). Chromatography was conducted using a Waters Acquity UPLC® (Waters Corporation, Milford MA, USA) with a mobile phase of 0.005 M ammonium formate in water (A) and 0.005 M ammonium formate in methanol (B), delivered at 0.4 mL/min, and mixed using a linear gradient from 10 to 95% B over 3.5 min, followed by a re-equilibration for 0.5 min prior to the next injection. Detection was conducted using a Waters Xevo TQ (Waters Corporation) triple quadrupole instrument operated in positive mode electrospray ionization with a capillary voltage and detector multiplier voltage of 3.5 kV and 500 V, respectively, and source block and desolvation temperature of 120°C and 650°C; the analyte elution was monitored using multiple reaction monitoring (MRM). Metabolite identity was confirmed by comparing the MS/MS spectrum and chromatographic retention characteristics to those for the authentic standards. Plasma concentrations of the analytes were quantified by an internal standard method using the system software, Quanlynx 4.1 (Micromass, Fort Myers, FL, USA). Accuracy, precision, and linearity for each of the analytes were assessed over the range of 0.5–10,000 ng/mL; the analytical lower limit of quantitation (LLQ) was typically 0.5 ng/mL for SSJ-183, and 1 ng/mL for N-deethyl SSJ-183 and bis-N,N-deethyl SSJ-183. Plasma concentration versus time profiles were analyzed by noncompartmental methods (WinNonlin v 5.2.1, Pharsight Corp, Mountain View, CA, USA), using mean concentrations at each time point.
Results
In vitro assessment of stage-specific activity
The time- and concentration-related stage-specificity of SSJ-183 was assessed in vitro using the [3H]hypoxanthine incorporation assay. Pyrimethamine served as the control drug, and similar to earlier findings,Citation8 it was found to be ineffective against ring and trophozoite stages (data not shown). The inhibitory effects on parasite growth at 6 and 24 hours of incubation with SSJ-183 at six different concentrations (250, 125, 62.5, 31.3, 15.6, and 7.81 ng/mL) are shown in . SSJ-183 was highly active and impaired growth of all three intraerythrocytic parasite stages by >99% at the highest concentration (250 ng/mL) with both incubation times, and at the intermediate concentration (62.5 ng/mL) after 24 hours. After 6 hours at the lowest concentration (7.81 ng/mL), the ring forms were significantly more susceptible than schizonts (P<0.05), with a residual growth of 35%, followed by trophozoites (62% growth), and schizonts (100% growth). The same tendency was observed at the intermediate concentration (62.5 ng/mL), where rings and trophozoites showed <3%, and schizonts 32%, growth. Similarly, the schizonts were significantly (P<0.05) less susceptible than both of the other two stages at concentrations of 31.3 and 15.6 ng/mL. After 24 hours of incubation, the rings were again found to be the most susceptible parasite stage for all applied concentrations ≤31.3 ng/mL, although this finding was not statistically significant.
Figure 2 Stage-dependent effects of SSJ-183 on [3H]hypoxanthine incorporation in synchronous cultures of Plasmodium falciparum strain NF54.
Abbreviations: SD, standard deviation; n, number; h, hours.
![Figure 2 Stage-dependent effects of SSJ-183 on [3H]hypoxanthine incorporation in synchronous cultures of Plasmodium falciparum strain NF54.](/cms/asset/b2f24e41-5c4e-4ae1-8109-d7f222bd5198/dddt_a_51298_f0002_b.jpg)
In vitro drug–drug interactions
SSJ-183 was combined with a panel of five currently used antimalarials to assess potential drug interactions at three different ratios (1:3, 1:1, and 3:1) using the fixed-ratio isobologram method.Citation10 The results are summarized in . The previously described synergism of the control combination of atovaquone + proguanil was confirmed by our data (mean ΣFICs in the range 0.23–0.31).Citation6 We tested SSJ-183 in combination with artemisinin. The mean range of the ΣFICs (0.69–0.79) indicated synergism. Combinations with mefloquine or piperaquine showed additive action (mean ΣFICs for K1/NF54 were 1.2/1.1 and 1.4/1.3.), and amodiaquine or pyronaridine combinations appeared antagonistic (mean ΣFICs for K1/NF54 were 1.5/1.4 and 1.7/1.7).
Table 1 In vitro interaction of drug combinations against two Plasmodium falciparum strains
In vitro cross-resistance with various strains of P. falciparum
SSJ-183 was found to potently inhibit growth of all seven strains tested (IC50 values from 17 to 50 ng/mL) irrespective of the drug-sensitivity status of the parasites (). In contrast, five out of seven strains showed considerable resistance against the control drugs chloroquine and pyrimethamine (IC50 values 5.7–237 ng/mL and 1.3–5,456 ng/mL, respectively). Notably, the activity of SSJ-183 and its lack of cross-resistance was comparable to that of the clinically used comparator drug artesunate for every strain tested (IC50 values for artesunate: 2.1–5.0 ng/mL).
Table 2 In vitro cross-resistance experiments with various sensitive and resistant Plasmodium falciparum strains (IC50 values are the mean of 2 to 3 independent experiments)
In vivo onset of action
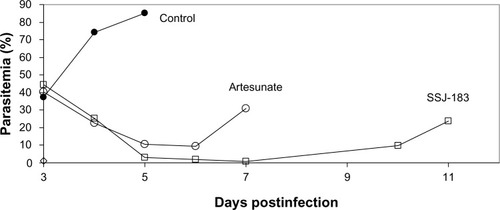
Single oral doses of 100 mg/kg SSJ-183 or artesunate were administered to P. berghei-infected mice on day 3 postinfection. SSJ-183 had a rapid onset of action against P. berghei comparable to that seen for artesunate (). Recrudescence for both compounds was also comparable, becoming evident by days 7–10 postinfection. The single oral dose where 90% reduction of parasitemia compared to untreated control animals was achieved (ED90) was found to be 20 mg/kg (data not shown).
Pharmacokinetic properties
The plasma concentration versus time profiles for noninfected and infected mice are shown in , and the corresponding pharmacokinetic data are presented in . Following oral administration of SSJ-183 at the ED90 (20 mg/kg), absorption was relatively fast with maximum plasma concentrations (Cmax) observed 1 hour postdose in both noninfected and infected groups. The terminal elimination phase was prolonged and the terminal half-life was approximately 10 hours in both groups. There was no significant difference in absorption or exposure between the two groups.
Table 3 Pharmacokinetic parameters for SSJ-183, N-deethyl SSJ-183 and bis-N,N-deethyl SSJ-183 after oral administration (20 mg/kg) of SSJ-183 to noninfected and Plasmodium berghei-infected NMRI mice
Figure 4 Plasma concentration versus time profiles for (A) SSJ-183, (B) N-deethyl SSJ-183, and (C) bis-N,N-deethyl SSJ-183 following oral administration of SSJ-183 (20 mg/kg) to noninfected (filled circles) and Plasmodium berghei-infected (open triangles) NMRI mice.
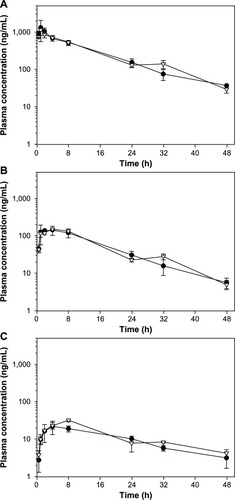
The metabolites N-deethyl SSJ-183 and bis-N,N-deethyl SSJ-183 showed similar in vitro potency when compared to SSJ-183 (data not shown) and were detected in the plasma of all mice throughout the postdose sampling period, with maximum concentrations observed 4–8 hours postdose, followed by prolonged terminal elimination phases. As for the parent compound, differences were minimal between the noninfected versus infected groups.
Discussion
The susceptibility of ring forms, trophozoites, and schizonts of P. falciparum to the lead compound SSJ-183 were determined. At 24 hours incubation time and the highest concentration, SSJ-183 impaired the growth of all stages by >99%. For most of the lower concentrations, the ring forms showed the highest susceptibility. This observation is similar to findings reported for methylene blue, a molecule related to SSJ-183.Citation3 After 6 hours of incubation with SSJ-183, schizont growth was affected significantly less than that of both rings and trophozoites.
In the P. berghei mouse model, a single oral dose of 100 mg/kg of SSJ-183 demonstrated a prompt onset of action, which was comparable to that of the artemisinin derivative artemether.Citation11 This is significant, as the artemisinin derivatives are considered to be the most potent and fast-acting class of antimalarials currently available. Following oral administration to mice, SSJ-183 also demonstrated a long half-life, with plasma concentrations maintained above the IC50 for longer than 48 hours at the 20 mg/kg oral dose.
Drug combinations are an essential strategy to prevent or delay the emergence and spread of resistance. The current first-line treatments for both uncomplicated and severe malaria, as recommended by the World Health Organization (WHO), are combination therapies. SSJ-183 and artemisinin showed synergistic interactions at the 1:3 and 1:1 ratios (mean ΣFICs, 0.60–0.73), whereas the combination ratio of 3:1 was additive (mean ΣFICs, 0.80–0.97). Although the synergistic action of SSJ-183/artemisinin was less pronounced compared with atovaquone/proguanil, the data fits well with earlier findings describing synergistic interactions between artemisinin derivatives and methylene blue.Citation12 The artemisinins are administered as artemisininbased combination therapies (ACTs) and are the recommended first-line therapy to clear P. falciparum infections. Although the clinical and parasitological efficacy of ACTs remains high in most settings,Citation1 prolonged parasite clearance times have been reported in Western Cambodia.Citation13–Citation15 Laboratory studies and mathematical modeling suggest that these changes in clearance time of parasites mainly result from decreased susceptibility of ring-stage parasites. Therefore, if the partner drug of an ACT acts specifically on the trophozoite or schizont stage, as is the case for most of the drugs, the artemisinin portion of the combination is virtually administered as a monotherapy in respect to the parasiticidal effect on ring-forms. The tendency of SSJ-183 to act at low concentrations specifically on rings might thus help to protect this more vulnerable phase in the life-saving treatment with ACTs.
Assessing cross-resistance against antimalarials is a challenge, as there are problems to concisely define drug-resistant malaria, especially in vitro.Citation16 To account for the baseline assay-to-assay variation, artesunate served as a reference in our studies. The finding that SSJ-183 inhibited parasite growth of all tested P. falciparum strains in an IC50 range similar to that of artesunate irrespective of the sensitivity status of the strain suggests that cross-resistance may not be a concern for SSJ-183.
In conclusion, SSJ-183 has an attractive antimalarial profile and could possibly be combined with a variety of different drugs without losing its efficacy. The activity of SSJ-183 remains unaffected by cross-resistance against several antimalarials, and it shows a reasonable half-life and no significant differences in absorption or exposure in noninfected versus infected animals. These findings support the new molecule as a promising candidate for further profiling studies, such as the verification of its safety and its ability to be able to reduce transmission and to prevent relapse of dormant parasite forms.
Acknowledgments
In vivo efficacy studies in mice were conducted at the Swiss Tropical and Public Health Institute (Basel) and approved by the Veterinary Office of Canton Basel-Stadt. Part of this work was sponsored by Medicines for Malaria Venture (www.mmv.org).
Disclosure
The authors declare no conflicts of interest in this study.
References
- World Health Organization World Malaria Report 2011 Summary and Keypoints. Available from: http://www.who.int/malaria/world_malaria_report_2011/wmr2011_summary_keypoints.pdf Accessed September 8, 2012
- Olliaro PL Taylor WR Antimalarial compounds: from bench to bedside J Exp Biol 2003 206 Pt 21 3753 3759 14506210
- Ge J-F Arai C Yang M Discovery of novel benzo[a]phenoxazine SSJ-183 as a drug candidate for malaria ACS Med Chem Lett 2010 1 7 360 364
- Lu J Arai C Md AB Ihara M Plasmodium berghei proteome changes in response to SSJ-183 treatment Bioorg Med Chem 2011 19 13 4144 4147 21622001
- Burrows JN Hooft van Huijsduijnen R Möhrle JJ Oeuvray C Wells TN Designing the next generation of medicines for malaria control and eradication Malar J 2013 12 187 23742293
- Snyder C Chollet J Santo-Tomas J In vitro and in vivo interaction of synthetic peroxide RBx11160 (OZ277) with piperaquine in Plasmodium models Exp Parasitol 2007 115 3 296 300 17087929
- Lambros C Vanderberger JP Synchronization of Plasmodium falciparum erythrocytic stages in culture J Parasitol 1979 65 418 420 383936
- Hofer S Brun R Maerki S Matile H Scheurer C Wittlin S In vitro assessment of the pharmcodynamic properties of DB75, piperaquine, OZ277 and OZ401 in cultures of Plasmodium falciparum J Antimicrob Chemother 2008 62 1061 1064 18669520
- Maerki S Brun R Charman S Dorn A Matile H Wittlin S In vitro assessment of the pharmacodynamic properties and the partitioning of OZ277/RBx-11160 in cultures of Plasmodium falciparum J Antimicrob Chemother 2006 58 52 58 16735432
- Fivelman QL Adagu IS Warhurst DC Fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum Antimicrob Agents Chemother 2004 48 11 4097 4102 15504827
- Charman SA Arbe-Barnes S Bathurst IC Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria Proc Natl Acad Sci U S A 2011 108 11 4400 4405 21300861
- Akoachere M Buchholz K Fischer E In vitro assessment of methylene blue on chloroquine-sensitive and -resistant Plasmodium falciparum strains reveals synergistic action with artemisinins Antimicrob Agents Chemother 2005 49 11 4592 4597 16251300
- White NJ Qinghaosu (artemisinin): the price of success Science 2008 320 5874 330 334 18420924
- Saralamba S Pan-Ngum W Maude RJ Intrahost modeling of artemisinin resistance in Plasmodium falciparum Proc Natl Acad Sci U S A 2011 108 1 397 402 21173254
- Dondorp AM Yeung S White L Artemisinin resistance: current status and scenarios for containment Nat Rev Microbiol 2010 8 4 272 280 20208550
- Witkowski B Khim N Chim P Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia Antimicrob Agents Chemother 2013 57 2 914 923 23208708
- Brunner R Aissaoui H Boss C Identification of a new chemical class of antimalarials J Infect Dis 2012 206 5 735 743 22732921