Abstract
The survival rate of patients with metastatic colorectal cancer (mCRC) has significantly improved with applications of molecularly targeted drugs, such as bevacizumab, and led to a substantial improvement in the overall survival rate. These drugs are capable of specifically targeting the inherent abnormal pathways in cancer cells, which are potentially less toxic than traditional nonselective chemotherapeutics. In this review, the recent clinical information about molecularly targeted therapy for mCRC is summarized, with specific focus on several of the US Food and Drug Administration-approved molecularly targeted drugs for the treatment of mCRC in the clinic. Progression-free and overall survival in patients with mCRC was improved greatly by the addition of bevacizumab and/or cetuximab to standard chemotherapy, in either first- or second-line treatment. Aflibercept has been used in combination with folinic acid (leucovorin)–fluorouracil–irinotecan (FOLFIRI) chemotherapy in mCRC patients and among patients with mCRC with wild-type KRAS, the outcomes were significantly improved by panitumumab in combination with folinic acid (leucovorin)–fluorouracil–oxaliplatin (FOLFOX) or FOLFIRI. Because of the new preliminary studies, it has been recommended that regorafenib be used with FOLFOX or FOLFIRI as first- or second-line treatment of mCRC chemotherapy. In summary, an era of new opportunities has been opened for treatment of mCRC and/or other malignancies, resulting from the discovery of new selective targeting drugs.
Introduction
Colorectal cancer (CRC) is one of the most malignant types of cancers. In the US and Europe, CRC is the second most frequent cancer that leads to death, which ranks below only lung cancer.Citation1 The incidence of CRC in the People’s Republic of China has been reported to increase annually and will continue to rise in the next few years.Citation2 Currently, there are approximately 1.25 million patients diagnosed with CRC, and more than 600,000 patients will die from this disease every year.Citation3 Metastases develop in at least 50% of CRC patients, and most of these patients have unresectable tumors.Citation4 When tumor lesions are not fully resectable or become metastatic, the first treatment option is chemotherapy. Since the 1990s, fluorouracil (5FU)-based chemotherapy has improved the survival rate of patients with metastatic CRC (mCRC) to an overall survival (OS) of 12 months, and the addition of oxaliplatin and irinotecan increased the OS to approximately 18 months.Citation5–Citation8 The addition of molecularly targeted drugs, such as bevacizumab, led to a substantial jump in OS, which approached 30 months in some studies.Citation9 In 2007, monoclonal antibody (mAb) therapy was first recommended for mCRC.Citation10,Citation11 According to the European and US guidelines, the combination of chemotherapy and a mAb is recommended for the first-line treatment of mCRC, and the second-line treatment depends on the first-line regimen used. For patients with chemoresistant mCRC with wild-type KRAS, monotherapy with cetuximab or panitumumab is recommended.Citation12 In addition, regorafenib was recently approved by the US Food and Drug Administration (FDA) for the treatment of mCRC patients who have been treated with chemotherapy previously, which is used in combination with an anti-vascular endothelial growth factor (VEGF) therapy, or with an anti-epidermal growth factor receptor (EGFR) therapy in case of wild-type KRAS.Citation13 In recent years, significant advances have been made in the integration of targeted therapies in the treatment of mCRC and a plethora of new data have been published shedding light on the efficacy of targeted drugs in treatment of mCRC. However, these data have sometimes been inconsistent, resulting in a challenging environment in which physicians are required to make treatment choices. This review focuses on several FDA-approved molecularly targeted drugs that are being used regularly in the treatment of mCRC.
Bevacizumab, an angiogenesis inhibitor
Bevacizumab (Avastin; Genentech/Roche, San Francisco, CA, USA) is a humanized mAb that inhibits the growth of new blood vessels. As the first clinically available inhibitor of angiogenesis in the US, bevacizumab has been licensed to treat various cancers, including breast (outside the US),Citation14–Citation16 glioblastoma (US only),Citation17,Citation18 lung,Citation19,Citation20 kidney,Citation21,Citation22 ovarianCitation23–Citation25 and CRC.Citation26–Citation28 Mechanically, bevacizumab inhibits VEGF-A,Citation29 a chemical signal that mediates angiogenesis, which is required for the development of cancer.Citation30,Citation31 shows how the VEGF-A signaling pathway is linked to its main biological functions. VEGF-A can bind VEGF receptor (VEGFR)-2 dimer. Neuropilin (NRP)-1 and -2 are co-receptors that stabilize the VEGFR-2 dimer.Citation32 Upon ligand binding to VEGFR-2 dimer, several signaling pathways can be activated, affecting diverse biological processes in endothelial and cancer cells. Anti-VEGF-A mAb, such as bevacizumab, can bind VEGF-A and block its function. While it has been clearly demonstrated that bevacizumab has antitumor efficacy in various cancers, especially in combination with conventional chemotherapy, its exact mechanism of action remains not fully understood. Continued VEGF-A inhibition with bevacizumab can play an important role in improving the overall success of therapy for patients who have mCRC.Citation9 However, many patients inevitably relapsed due to the newly acquired resistance, even though the progression-free survival (PFS) was statistically increased.Citation33,Citation34
Figure 1 Schematic representation of how the VEGF-A signaling pathway is linked to its main biological functions.
Abbreviations: mAb, monoclonal antibody; NRP, neuropilin; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; PKC, protein kinase C; PLC, phospholipase C; FAK, focal adhesion protein; TSAd-Src, T cell-specific adaptor protein containing an Src homology.
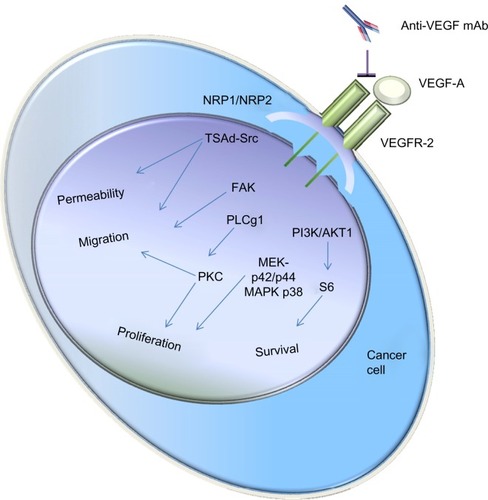
When combined with 5FU-based chemotherapy, bevacizumab was found to significantly prolong the OS and PFS of patients with mCRC. For this reason, bevacizumab was first approved in 2004 for the combined use with standard chemotherapy for the treatment of mCRC.Citation35,Citation36 In 2005, the European Medicines Agency also approved bevacizumab in combination with irinotecan, a chemotherapeutic drug that prevents DNA from unwinding by inhibition of topoisomerase 1 for first-line treatment of mCRC.Citation37 Bevacizumab was further approved for mCRC in combination with standard fluoropyrimidine-based chemotherapy after its benefits were demonstrated by randomized studies.Citation38,Citation39 More recently, on January 23, 2013, bevacizumab was approved to be used in combination with fluoropyrimidine-based irinotecan or oxaliplatin chemotherapy for the treatment of mCRC.Citation40 The approval allows patients who received the first-line treatment with bevacizumab plus an irinotecan- or oxaliplatin-containing chemotherapy to continue to receive bevacizumab plus a different irinotecan- or oxaliplatin-containing chemotherapy as the second-line treatment after their cancer worsens.Citation40
The use of bevacizumab with standard chemotherapy has improved PFS and OS for the treatment of mCRC in both first- and second-line treatment; however, the clinical significance of maintenance bevacizumab remains controversial. In a Phase III trial to compare the efficacy and safety of bevacizumab alone with bevacizumab and capecitabine plus oxaliplatin (XELOX) as maintenance treatment following induction chemotherapy with XELOX plus bevacizumab in the first-line treatment of patients with mCRC, Díaz-Rubio et al did not find statistically significant differences in the median PFS or OS times between bevacizumab-treated versus XELOX plus bevacizumab-treated patients.Citation41 The results of a recent retrospective study indicate that the maintenance therapy with bevacizumab is a safe and valuable option in mCRC patients, especially in those who achieve an objective response after first-line chemotherapy.Citation42 In addition, two reports from the 2013 American Society of Clinical Oncology (ASCO) annual meeting support the use of bevacizumab in maintenance treatment of mCRC.Citation43,Citation44
In summary, PFS and OS have been significantly improved by the addition of bevacizumab to standard chemotherapy in patients with mCRC in both first- and second-line treatment. Still, the role of bevacizumab as maintenance treatment of mCRC needs further studies.
Aflibercept, a fusion protein
Aflibercept (ZALTRAP; co-developed by Sanofi-Aventis and Regeneron Pharmaceuticals, Inc, Tarrytown, NY, USA) is a fusion protein for the treatment of wet macular degenerationCitation45,Citation46 and mCRC.Citation47 The mechanism of aflibercept action is to target VEGF-A, VEGF-B, and placental growth factor (PIGF) by blocking angiogenesis. It also prevents the activation of VEGFR-1 and VEGFR-2 by these ligands.Citation47,Citation48 As a member of the VEGF family, PIGF specifically binds to VEGFR-1 and enhances VEGF-A expression, which plays an important role in angiogenesis.Citation49 PIGF expression is also upregulated by anti-VEGF therapy in cancer patients, indicating that PIGF may also play a role in the resistance to anti-VEGF treatment.Citation50,Citation51 In addition, aflibercept was shown to inhibit tumor proliferation, angiogenesis, and metastases in tumor-bearing mouse model.Citation52,Citation53
On August 3, 2012, aflibercept was approved by the FDA for patients who were previously treated with mCRC.Citation47 The approval stipulates that aflibercept can be used in combination with folinic acid (leucovorin)–5FU–irinotecan (FOLFIRI) chemotherapy for mCRC patients whose cancer has progressed or demonstrated resistance to oxaliplatin (Eloxatin; Sanofi-Aventis)-based chemotherapy. The benefit of aflibercept in combination with FOLFIRI was confirmed in the Phase III VELOUR trial.Citation54 In this study, aflibercept or placebo combined with FOLFIRI was administered to patients with mCRC at 4 mg/kg intravenously every 2 weeks. The results demonstrate that the aflibercept-containing group had better PFS (6.9 versus 4.67 months; hazard ratio [HR] 0.758; P<0.0001) and OS (13.5 versus 12.06 months; HR 0.817; P=0.0032) compared to the control group. In the aflibercept arm, the overall response rate was 19.8%, whereas in the placebo arm, the overall response rate was 11.1% (P=0.0001).Citation54,Citation55
It seems that the benefit achieved by aflibercept and bevacizumab are comparable in second-line settings. As shown in a clinical trial, a median OS improvement of 1.4 months (HR 0.81; 95% confidence interval [CI]: 0.69–0.94; P=0.0062) was achieved when bevacizumab was continued in the second-line while switching the cytotoxic chemotherapy.Citation56 Meanwhile, in the VELOUR trial, the addition of aflibercept to FOLFIRI resulted in a comparable median OS survival improvement of 1.44 months (HR 0.817; 95.34% CI: 0.713–0.937; P=0.0032).Citation54 However, compared to bevacizumab, aflibercept appeared to have a higher frequency of vascular-related adverse events. Therefore, aflibercept is not recommended for routine use in mCRC patients who progress on oxaliplatin-containing treatment.
Cetuximab, a chimeric monoclonal antibody
Cetuximab (Erbitux), a chimeric (mouse/human) monoclonal antibody, is manufactured and distributed by Bristol-Myers Squibb (New York, NY, USA) and Eli Lilly and Company (Indianapolis, IN, USA). This drug is specific to the EGFR,Citation57,Citation58 which is administered by intravenous infusion.Citation59 Cetuximab is able to induce various proapoptotic factors, such as Bax, leading to the activation of caspases and thus triggering apoptosis.Citation60,Citation61 In addition, cetuximab can also recruit immune cells to tumor cells, thereby inducing antibody-dependent cellular cytotoxicity in vivo.Citation62,Citation63 shows the EGFR pathway and its main downstream effectors, PI3K/AKT and KRAS/BRAF/MEK/MERK. Activated AKT and MEK/MERK can induce cancer cell proliferation and invasion. In addition, activated AKT can induce cancer stem cell renewal and differentiation.Citation64,Citation65 Anti-EGFR mAbs, such as cetuximab, can bind EGFR and block its function.
Figure 2 An overview of the EGFR pathway and its main downstream effectors, PI3K/AKT and KRAS/BRAF/MEK/MERK.
Abbreviations: EGFR, epidermal growth factor receptor; mAb, monoclonal antibody; CSC, cancer stem cell.
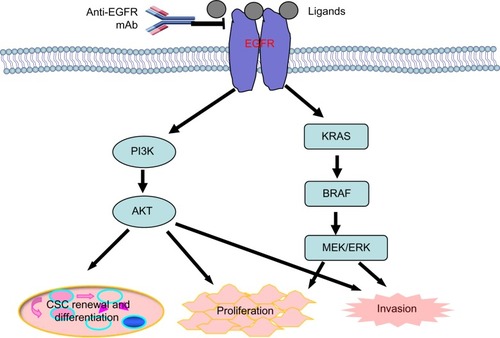
Initially, cetuximab was used in the palliative treatment of tumor. This drug, either as monotherapy or in combination with chemotherapy and/or radiation, particularly in the settings of mCRC, has shown positive antitumor activity in clinical trials.Citation66–Citation69 In 2004, cetuximab was approved to be used for patients with EGFR-expressing mCRC who are refractory to irinotecan-based chemotherapy.Citation70 Subsequently, there have been several clinical trials supporting the use of cetuximab for the treatment of mCRC. On July 6, 2012, the FDA approved the combination of cetuximab with FOLFIRI as the first-line treatment for patients with mutation-negative (wild-type) K-ras and EGFR-expressing mCRC.Citation71–Citation73 Concurrent with this approval, the therascreen® KRAS RGQ PCR Kit (QIAGEN Manchester, Ltd) was also approved by the FDA for determining the K-ras mutations. The approval of cetuximab and KRAS mutation kit was based on the results of the CRYSTAL trial and two supportive studies, CA225025 and EMR 62 202-047 (OPUS), which made retrospective analyses of tumor samples from a large number of patients in accordance with K-ras mutation status.Citation74,Citation75 The addition of cetuximab to chemotherapy has significantly improved the OS, PFS, and the overall response rates in patients with K-ras wild-type tumors. However, no benefit, or even potential harm, has been observed in patients with K-ras mutant tumors.
The efficacy of down-staging programs in mCRC patients can be improved by the addition of cetuximab to conventional chemotherapy regimens, which may offer patients potential curative resection. However, it is well recognized that both intrinsic and acquired resistance have been developed despite the clinical gains arising from use of cetuximab.Citation76 Therefore, there remains the need to develop more efficient antibody-based anti-EGFR therapies.
Panitumumab, a fully human monoclonal antibody
Panitumumab (Vectibix; Amgen Inc, Thousand Oaks, CA, USA) specifically blocks the EGFR extracellular domain. As a fully human monoclonal antibody, it can be used as a single drug in patients who are chemotherapy refractory or in different combinations. In either setting, panitumumab has been shown to be well tolerated and efficacious.Citation77,Citation78
Panitumumab was first approved in September 2006 in the US and in 2007 in Europe as a monotherapy for the treatment of mCRC that is EGFR-expressing and refractory to fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. However, in patients with KRAS mutations in codons 12 or 13, use of panitumumab is not recommended.Citation79,Citation80 Recently, clinical studies have been conducted focusing on the potential benefits of treatment of mCRC with panitumumab in combination with chemotherapy. The results of these studies support that the clinical outcomes of patients with wild-type KRAS are improved by the addition of panitumumab to chemotherapy.Citation81–Citation84 In another study, PFS was significantly improved by the addition of panitumumab to first-line folinic acid (leucovorin)–5FU–oxaliplatin (FOLFOX)4, which was 9.6 versus 8.0 months compared with FOLFOX4 alone (HR 0.80; 95% CI: 0.66–0.97; P=0.02). However, addition of panitumumab to FOLFOX4 did not significantly improve the median OS in the patients with wild-type KRAS tumors (23.9 versus 19.7 months compared to FOLFOX4 alone; HR 0.83; 95% CI: 0.67–1.02; P=0.072).Citation81 In case of failure of initial treatment for mCRC with one prior chemotherapy regimen, the addition of panitumumab to FOLFIRI significantly improves the PFS in the wild-type KRAS subpopulation, with 5.9 months for panitumumab–FOLFIRI versus 3.9 months for FOLFIRI alone (HR 0.73; 95% CI: 0.59–0.90; P=0.004).Citation82 A trend toward increased OS was also observed, although it was not statistically significant. The median OS was improved from 12.5 to 14.5 months, and the response rate increased from 10% to 35% with the addition of panitumumab compared to FOLFIRI alone.Citation82 However, no difference in efficacy was observed in patients with mutant KRAS.Citation82 Additional reports also demonstrate that, for patients with wild-type KRAS tumors, the objective response rate, PFS, and OS are numerically improved by panitumumab plus FOLFIRI.Citation83,Citation84
Overall, the results of the above studies support that panitumumab in combination with FOLFOX or FOLFIRI improves the outcomes among patients with mCRC with wild-type KRAS.
Regorafenib, a multikinase inhibitor
As an oral multikinase inhibitor, regorafenib ([BAY 73-4506] Stivarga; Bayer AG, Leverkusen, Germany) targets angio-genic, stromal, oncogenic receptor tyrosine kinase (RTK) as well as tumor microenvironment (platelet-derived growth factor receptor [PDGFR] and fibroblast growth factor receptor [FGFR]).Citation85 Multiple membrane-bound and intracellular kinases are inhibited by regorafenib and its active metabolites. Therefore, multiple normal cellular functions and pathologic processes are inhibited, including the RET, VEGFR1, VEGFR2, VEGFR3, KIT, PDGFR-alpha, PDGFR-beta, FGFR1, FGFR2, TIE2, DDR2, Trk2A, Eph2A, RAF-1, BRAF, BRAFV600E, SAPK2, PTK5, and Abl pathways.Citation85,Citation86 It was further reported that regorafenib is effective in inhibiting angiogenesis, tumorigenesis, and metastasis in a highly aggressive metastatic colon cancer murine model, indicating its potential in the treatment of advanced CRCs.Citation87
On September 27, 2012, the FDA approved regorafenib to be used in combination with an anti-VEGF therapy (or an anti-EGFR therapy, if wild-type KRAS), for the treatment of mCRC that has been treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.Citation13 In an international, randomized (2:1), double-blind, and placebo-controlled trial (study 14387) conducted in 760 patients with previously treated mCRC, regorafenib treatment resulted in a statistically significant prolongation in OS [HR 0.77; 95% CI: 0.64–0.94; P=0.0102], with 6.4 months (95% CI: 5.8–7.3) of median survival time in the regorafenib group versus 5.0 months (95% CI: 4.4–5.8) in the placebo group. A statistically significant improvement in PFS was also demonstrated in patients who were treated with regorafenib [HR 0.49; 95% CI: 0.42 –0.58; P<0.0001]. Specifically, the median PFS in patients receiving regorafenib was 2.0 months (95% CI: 1.9–2.3), whereas, in the placebo group, the median PFS was 1.7 months (95% CI: 1.7–1.8).Citation13
As shown in a recent preliminary study, regorafenib in combination with FOLFOX or FOLFIRI has an acceptable tolerability as first- or second-line treatment of mCRC chemotherapy.Citation88 In other tumor types, regorafenib has also shown exciting potential, especially in gastrointestinal stromal tumors.Citation86,Citation89
Conclusion
With the discovery of a plethora of molecular cellular targets, a large number of selective targeting drugs have been generated, and this has opened a new era for cancer therapy. These drugs specifically target the inherent abnormalities of cancer cells, which is potentially less toxic than traditional nonselective cytotoxic drugs. Addition of these drugs, such as bevacizumab and/or cetuximab, to standard chemotherapy has resulted in improved PFS and OS in patients with mCRC, either in first- or second-line treatment. In patients with mCRC that has progressed on or is resistant to oxaliplatin (Eloxatin)-based chemotherapy, aflibercept has been used in combination with FOLFIRI chemotherapy. Among patients with mCRC with wild-type KRAS, the outcomes are improved when panitumumab is added to FOLFOX or FOLFIRI chemotherapy. Regorafenib can be used in combination with FOLFOX or FOLFIRI as first- or second-line treatment of mCRC chemotherapy.
Disclosure
The authors report no conflicts of interest in this work. The authors retained full editorial control over the content of the manuscript and received no compensation from any party for their work.
References
- Jemal A Center MM DeSantis C Ward EM Global patterns of cancer incidence and mortality rates and trends Cancer Epidemiol Biomarkers Prev 2010 19 8 1893 1907 20647400
- Dai Z Zheng RS Zou XN Analysis and prediction of colorectal cancer incidence trend in China Zhonghua Yu Fang Yi Xue Za Zhi 2012 46 598 603 Chinese 22943913
- Globocan 2008 cancer fact sheet [webpage on the Internet] Lyon IARC 2010 Available from: http://globocan.iarc.fr/factsheets/cancers/colorectal.asp Accessed August 15, 2013
- Van Cutsem E Nordlinger B Cervantes A ESMO Guidelines Working Group Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment Ann Oncol 2010 21 Suppl 5 v93 v97 20555112
- André T Louvet C Maindrault-Goebel F CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR Eur J Cancer 1999 35 1343 1347 10658525
- Cheeseman SL Joel SP Chester JD A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer Br J Cancer 2002 87 393 399 12177775
- Goldberg RM Sargent DJ Morton RF A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer J Clin Oncol 2004 22 23 30 14665611
- Grothey A Sargent D Goldberg RM Schmoll HJ Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment J Clin Oncol 2004 22 1209 1214 15051767
- Grothey A Sugrue MM Purdie DM Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol 2008 26 5326 5334 18854571
- Scott LJ Bevacizumab: in first-line treatment of metastatic breast cancer Drugs 2007 67 1793 1799 17683175
- Zhu Z Targeted cancer therapies based on antibodies directed against epidermal growth factor receptor: status and perspectives Acta Pharmacol Sin 2007 28 1476 1493 17723181
- Edwards MS Chadda SD Zhao Z Barber BL Sykes DP A systematic review of treatment guidelines for metastatic colorectal cancer Colorectal Dis 2012 14 e31 e47 21848897
- Regorafenib FAf Previously Treated Metastatic Colorectal Cancer http://en.wikipedia.org/wiki/RegorafenibAccessed October 15, 2013
- Stevenson CE Nagahashi M Ramachandran S Yamada A Bear HD Takabe K Bevacizumab and breast cancer: what does the future hold? Future Oncol 2012 8 403 414 22515444
- Rodgers M Soares M Epstein D Yang H Fox D Eastwood A Bevacizumab in combination with a taxane for the first-line treatment of HER2-negative metastatic breast cancer Health Technol Assess 2011 15 Suppl 1 1 12 21609648
- Miles D Zielinski C Martin M Vrdoljak E Robert N Combining capecitabine and bevacizumab in metastatic breast cancer: a comprehensive review Eur J Cancer 2012 48 482 491 22257791
- Narita Y Drug review: safety and efficacy of bevacizumab for glioblastoma and other brain tumors Jpn J Clin Oncol 2013 43 587 595 23585688
- Rahmathulla G Hovey EJ Hashemi-Sadraei N Ahluwalia MS Bevacizumab in high-grade gliomas: a review of its uses, toxicity assessment, and future treatment challenges Onco Targets Ther 2013 6 371 389 23620671
- Soria JC Mauguen A Reck M meta-analysis of bevacizumab in advanced NSCLC collaborative group Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer Ann Oncol 2013 24 20 30 23180113
- Planchard D Bevacizumab in non-small-cell lung cancer: a review Expert Rev Anticancer Ther 2011 11 1163 1179 21916570
- Melichar B Procházková-Študentová H Vitásková D Bevacizumab in combination with IFN-alpha in metastatic renal cell carcinoma: the AVOREN trial Expert Rev Anticancer Ther 2012 12 1253 1261 23136836
- Di Lorenzo G Porta C Bellmunt J Toxicities of targeted therapy and their management in kidney cancer Eur Urol 2011 59 526 540 21277078
- Garcia A Singh H Bevacizumab and ovarian cancer Ther Adv Med Oncol 2013 5 133 141 23450196
- Miyake TM Sood AK Coleman RL Contemporary use of bevacizumab in ovarian cancer Expert Opin Biol Ther 2013 13 283 294 23190436
- Eskander RN Randall LM Bevacizumab in the treatment of ovarian cancer Biologics 2011 5 1 5 21383911
- Yeung Y Tebbutt NC Bevacizumab in colorectal cancer: current and future directions Expert Rev Anticancer Ther 2012 12 1263 1273 23113577
- Whyte S Pandor A Stevenson M Bevacizumab for metastatic colorectal cancer: a NICE single technology appraisal Pharmacoeconomics 2012 30 1119 1132 23058097
- Strickler JH Hurwitz HI Bevacizumab-based therapies in the first-line treatment of metastatic colorectal cancer Oncologist 2012 17 513 524 22477726
- Grothey A Galanis E Targeting angiogenesis: progress with anti-VEGF treatment with large molecules Nat Rev Clin Oncol 2009 6 507 518 19636328
- Shibuya M Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases J Biochem 2013 153 13 19 23172303
- Giacca M Zacchigna S VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond Gene Ther 2012 19 622 629 22378343
- Wang L Zeng H Wang P Soker S Mukhopadhyay D Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration J Biol Chem 2003 278 48848 48860 14514674
- Giuliano S Pages G Mechanisms of resistance to anti-angiogenesis therapies Biochimie 2013 95 1110 1119 23507428
- Moreno Garcia V Basu B Molife LR Kaye SB Combining antiangiogenics to overcome resistance: rationale and clinical experience Clin Cancer Res 2012 18 3750 3761 22547772
- Olszewski AJ Grossbard ML Kozuch PS The horizon of antiangiogenic therapy for colorectal cancer Oncology (Williston Park) 2005 19 297 306 discussion 306, 308, 317–333 15828549
- Ellis LM Bevacizumab Nat Rev Drug Discov 2005 Suppl S8 S9 15962523
- Rougier P Lepere C Second-line treatment of patients with metastatic colorectal cancer Semin Oncol 2005 32 S48 S54 16399432
- Giantonio BJ Catalano PJ Meropol NJ Eastern Cooperative Oncology Group Study E3200 Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200 J Clin Oncol 2007 25 1539 1544 17442997
- Saltz LB Clarke S Díaz-Rubio E Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study J Clin Oncol 2008 26 2013 2019 18421054
- FDA approval for bevacizumab [webpage on the Internet] Bethesda, MD National Cancer Institute Available from: http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab Accessed August 15, 2013
- Díaz-Rubio E Gómez-España A Massutí B Spanish Cooperative Group for the Treatment of Digestive Tumors First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colo-rectal cancer: the phase III MACRO TTD study Oncologist 2012 17 15 25 22234633
- Moscetti L Nelli F Fabbri MA Maintenance single-agent bevacizumab or observation after first-line chemotherapy in patients with metastatic colorectal cancer: a multicenter retrospective study Invest New Drugs 2013 31 4 1035 1043 23417697
- Greil R Von Moos R Bennouna J Bevacizumab plus chemotherapy continued beyond first disease progression in patients with metastatic colorectal cancer previously treated with bevacizumab-based therapy: patterns of disease progression and outcomes based on extent of disease in the ML18147 study. 2013 ASCO Annual Meeting J Clin Oncol 2013 31 (Suppl; abstr 3604)
- Koopman M Simkens LHJ Ten Tije AJ Maintenance treatment with capecitabine and bevacizumab versus observation after induction treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (mCRC): the phase III CAIRO3 study of the Dutch Colorectal Cancer Group (DCCG). 2013 ASCO Annual Meeting J Clin Oncol 2013 31 (Suppl; abstr 3502)
- Evoy KE Abel SR Aflibercept: newly approved for the treatment of macular edema following central retinal vein occlusion Ann Pharmacother 2013 47 819 827 23673531
- Thomas M Mousa SS Mousa SA Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration Clin Ophthalmol 2013 7 495 501 23503202
- Sharma T Dhingra R Singh S Aflibercept: a novel VEGF targeted agent to explore the future perspectives of anti-angiogenic therapy for the treatment of multiple tumors Mini Rev Med Chem 2013 13 530 540 23317499
- Chu QS Aflibercept (AVE0005): an alternative strategy for inhibiting tumour angiogenesis by vascular endothelial growth factors Expert Opin Biol Ther 2009 9 263 271 19236257
- Roy H Bhardwaj S Babu M Adenovirus-mediated gene transfer of placental growth factor to perivascular tissue induces angiogenesis via upregulation of the expression of endogenous vascular endothelial growth factor-A Hum Gene Ther 2005 16 1422 1428 16390273
- Fischer C Mazzone M Jonckx B Carmeliet P FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 2008 8 942 956 19029957
- Rini BI Michaelson MD Rosenberg JE Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma J Clin Oncol 2008 26 3743 3748 18669461
- Gaya A Tse V A preclinical and clinical review of aflibercept for the management of cancer Cancer Treat Rev 2012 38 484 493 22264850
- Fetterly GJ Aras U Lal D Murphy M Meholick PD Wang ES Development of a preclinical PK/PD model to assess antitumor response of a sequential aflibercept and doxorubicin-dosing strategy in acute myeloid leukemia AAPS J 2013 15 3 662 673 23550025
- Van Cutsem E Tabernero J Lakomy R Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen J Clin Oncol 2012 30 3499 3506 22949147
- Sun W Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy J Hematol Oncol 2012 5 63 23057939
- Bennouna J Sastre J Arnold D ML18147 Study Investigators Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial Lancet Oncol 2013 14 29 37 23168366
- Bou-Assaly W Mukherji S Cetuximab (erbitux) AJNR Am J Neuroradiol 2010 31 626 627 20167650
- Vincenzi B Zoccoli A Pantano F Venditti O Galluzzo S Cetuximab: from bench to bedside Curr Cancer Drug Targets 2010 10 80 95 20088790
- Khoukaz T Administration of anti-EGFR therapy: a practical review Semin Oncol Nurs 2006 22 20 27 16616283
- Tortora G Caputo R Pomatico G Cooperative inhibitory effect of novel mixed backbone oligonucleotide targeting protein kinase A in combination with docetaxel and anti-epidermal growth factor-receptor antibody on human breast cancer cell growth Clin Cancer Res 1999 5 875 881 10213224
- Mandal M Adam L Mendelsohn J Kumar R Nuclear targeting of Bax during apoptosis in human colorectal cancer cells Oncogene 1998 17 999 1007 9747879
- Kurai J Chikumi H Hashimoto K Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines Clin Cancer Res 2007 13 1552 1561 17332301
- Kimura H Sakai K Arao T Shimoyama T Tamura T Nishio K Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor Cancer Sci 2007 98 1275 1280 17498200
- Bose D Zimmerman LJ Pierobon M Chemoresistant colorectal cancer cells and cancer stem cells mediate growth and survival of bystander cells Br J Cancer 2011 105 1759 1767 22045189
- Feng Y Dai X Li X EGF signalling pathway regulates colon cancer stem cell proliferation and apoptosis Cell Prolif 2012 45 413 419 22925500
- Chung KY Shia J Kemeny NE Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry J Clin Oncol 2005 23 1803 1810 15677699
- Jonker DJ O’Callaghan CJ Karapetis CS Cetuximab for the treatment of colorectal cancer N Engl J Med 2007 357 2040 2048 18003960
- Sobrero AF Maurel J Fehrenbacher L EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer J Clin Oncol 2008 26 2311 2319 18390971
- Borner M Koeberle D Von Moos R Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK Ann Oncol 2008 19 1288 1292 18349029
- Cunningham D Humblet Y Siena S Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer N Engl J Med 2004 351 337 345 15269313
- Cersosimo RJ Management of advanced colorectal cancer, part 1 Am J Health Syst Pharm 2013 70 395 406 23413162
- Cersosimo RJ Management of advanced colorectal cancer, part 2 Am J Health Syst Pharm 2013 70 491 506 23456402
- Hubbard JM Alberts SR Alternate dosing of cetuximab for patients with metastatic colorectal cancer Gastrointest Cancer Res 2013 6 47 55 23745159
- Bokemeyer C Van Cutsem E Rougier P Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic col-orectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials Eur J Cancer 2012 48 1466 1475 22446022
- Tejpar S Celik I Schlichting M Sartorius U Bokemeyer C Van Cutsem E Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab J Clin Oncol 2012 30 3570 3577 22734028
- Brand TM Iida M Wheeler DL Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab Cancer Biol Ther 2011 11 777 792 21293176
- Kopper L Panitumumab: an arrow on target Pathol Oncol Res 2010 16 143 148 20432075
- Cartenì G Fiorentino R Vecchione L Chiurazzi B Battista C Panitumumab a novel drug in cancer treatment Ann Oncol 2007 18 Suppl 6 vi16 vi21 17591813
- Vectibix®(panitumumab) [package insert] Thousand Oaks, CA Amgen Inc 2010
- Agency EM Vectibix European Public Assessment Report, Summary of Product Characteristics 2010 Available from: http://www.emaeuropa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000741/WC500047710pdf Accessed August 15, 2013
- Douillard JY Siena S Cassidy J Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study J Clin Oncol 2010 28 4697 4705 20921465
- Peeters M Price TJ Cervantes A Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer J Clin Oncol 2010 28 4706 4713 20921462
- Cohn AL Shumaker GC Khandelwal P An open-label, single-arm, phase 2 trial of panitumumab plus FOLFIRI as second-line therapy in patients with metastatic colorectal cancer Clin Colorectal Cancer 2011 10 171 177 21855038
- Kohne CH Hofheinz R Mineur L First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer J Cancer Res Clin Oncol 2012 138 65 72 21960318
- Wilhelm SM Dumas J Adnane L Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity Int J Cancer 2011 129 245 255 21170960
- Aprile G Macerelli M Giuliani F Regorafenib for gastrointestinal malignancies: from preclinical data to clinical results of a novel multi-target inhibitor BioDrugs 2013 27 213 224 23435872
- Abou-Elkacem L Arns S Brix G Regorafenib inhibits growth, angiogenesis and metastasis in a highly aggressive, orthotopic colon cancer model Mol Cancer Ther 2013 12 7 1322 1331 23619301
- Schultheis B Folprecht G Kuhlmann J Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study Ann Oncol 2013 24 1560 1567 23493136
- George S Wang Q Heinrich MC Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial J Clin Oncol 2012 30 2401 2407 22614970