Abstract
Background
Modified-release tacrolimus (TAC) is a new, once-daily oral formulation of the established immunosuppressive agent TAC. This study evaluated long-term patient adherence, as well as safety and efficacy, in stable patients after heart transplantation (HTx) who switched from a conventional twice daily calcineurin inhibitor-based regimen (TAC or cyclosporine A [CsA]) to a once-daily modified-release TAC regimen.
Methods
Stable patients were switched from conventional TAC or CsA (twice-daily dosing) to modified-release TAC (once-daily dosing) according to manufacturer’s recommendations using a pre-experimental design. Self-reported adherence was assessed at baseline and 8 months after the switch with the Basel Assessment of Adherence with Immunosuppressive Medication Scale (BAASIS). Additionally, routine laboratory values were analyzed 8 months after switch.
Results
Of 76 patients (58 male, 18 female) initially included, 72 were available for statistical analysis, as modified-release TAC was discontinued due to diarrhea in one patient and gastrointestinal discomfort in three patients. Overall nonadherence at baseline for any of the four BAASIS items was 75.0% versus 40.3% after 8 months (P<0.0001). After 8 months, adherence was improved in 41 patients (56.9%), unchanged in 27 (37.5%), and reduced in four patients (5.6%). The BAASIS visual analog scale score improved significantly from 87.0% ± 13.5% to 97.5% ± 5.7% (P<0.0001). No significant changes were observed for hematological, renal, or liver function parameters after 8 months (all P=not significant).
Conclusion
To our knowledge, this is the first study in stable patients after HTx to demonstrate a significant improvement in long-term (ie, 8-month) patient adherence after the switch to modified-release TAC. Modified-release TAC was generally well tolerated. Further studies are currently underway to investigate long-term safety after HTx of various calcineurin inhibitors for prevention of rejection and occurrence of side effects.
Background
Modified-release tacrolimus (TAC) is a recently developed, once-daily oral formulation of the well-established immunosuppressive agent. Previous studies in patients treated with modified-release TAC after kidney or liver transplantation demonstrated comparable or favorable rates of postconversion patient and graft survivals, incidences of biopsy-proven acute rejection, multiple rejections, and safety profiles relative to conventional twice-daily TAC.Citation1–Citation9 The modified and conventional TAC formulations show similar exposure (area under the curve) and trough levels (minimum concentration), with a reduced peak level (maximum concentration).Citation5 Long-term data on modified-release TAC in patients after heart transplant (HTx) are limited, however. Because lifelong immunosuppressive therapy is a necessity in this distinct patient cohort, the long-term cardiovascular side effects of immunosuppressive therapy cannot be underestimated.Citation10 Additionally, simplification of dose regimens has been associated with improved adherence,Citation11 yet to our knowledge only short-term studies have evaluated the impact on adherence of switching patients from twice-daily to once-daily regimens after HTx.Citation12
The aim of this follow-up analysis was to evaluate adherence levels after 8 months in stable patients post-HTx who were switched from a conventional twice-daily TAC or cyclosporine A (CsA) regimen to a once-daily modified-release TAC regimen. The interval of 8 months was chosen due to the routine follow-up protocol at the Heidelberg HTx center. A secondary aim was to evaluate tolerability, efficacy, and safety of modified-release TAC after the switch.
Methods
Design and sampling methods
Using a pre-experimental design, we included a convenience sample of patients seen at the Department of Cardiology, Heidelberg University Hospital, Germany between April 2009 and June 2012. Patients had to be at least 6 months post-HTx and free from acute infection or rejection for 4 months prior to study inclusion. Patients also had to be on stable doses of conventional TAC or CsA for 4 months preceding study entry (as part of a dual immunosuppressive regimen). Written informed consent was obtained prior to study entry. All human studies were reviewed and approved by the ethics committee of the University of Heidelberg. The setting in which this study was conducted has a compulsory health insurance system, and immunosuppressive medications are covered by the standard health insurance.
Intervention
Stable HTx recipients were switched from a twice-daily calcineurin inhibitor (conventional TAC/CsA) regimen to a single morning dose of modified-release TAC. Conversion to modified-release TAC was made from conventional TAC on a milligram-for-milligram basis and from CsA according to manufacturer’s recommendations (Astellas Pharma GmbH, Munich, Germany). The oral dose of conventional or modified-release TAC was adjusted to reach target trough levels of 3–10 μg/L, depending on the amount of time since HTx. Alternatively, the oral dose of CsA was titrated to reach target trough levels of 50–140 μg/L, depending on the amount of time post-HTx.
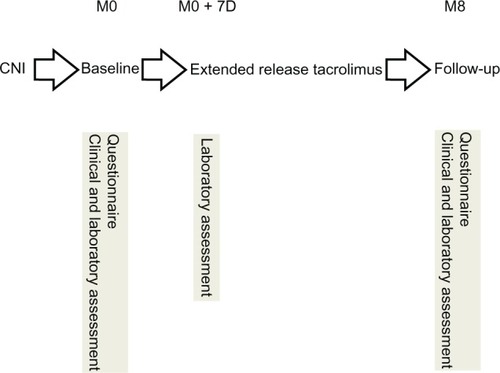
Determinations of immunosuppressive medication trough levels were completed at entry (baseline visit), on day 7 postconversion, and thereafter every month postconversion or whenever clinically indicated (). We applied the same therapeutic monitoring and patient-care techniques currently used with our CsA/TAC patients.
Variables and measurement
Demographic and clinical variables () were retrieved from the patient’s medical file. At baseline and the follow-up visit, routine laboratory (including doses and levels of immunosuppressive medication, blood count, and renal and liver function tests) and clinical assessments were obtained (according to the routine HTx follow-up protocol of the Department of Cardiology, Heidelberg University Hospital, Germany). Patients were asked (open question) to comment on the occurrence of side effects. Adherence with modified-release TAC was assessed by self-report using a validated adherence questionnaire, the Basel Assessment of Adherence to Immunosuppressive Medication Scale (BAASIS).Citation13,Citation14 The BAASIS consists of four items measuring the taking and timing dimensions of medication taking, the presence of drug holidays, and dose reductions, along with a rating of overall adherence on a visual analog scale ranging from 0 (never took medications as prescribed) to 100 (always took medications as prescribed). The BAASIS is applied as an interview in which patients are asked in a nonaccusatory, information-intensive approach about their medication-taking behaviors. Self-reported adherence as measured by BAASIS was assessed at baseline and at 8 months after the switch to modified-release TAC. Nonadherence was defined as any self-reported nonadherence on any of the four items. The presence of drug holidays or dose reductions was scored dichotomously (yes/no). The BAASIS’ predictive validity has been shown in the HIV population with regard to viral rebound.Citation15 Furthermore, the instrument showed fair diagnostic values, with sensitivity of 87.5% and specificity of 78.6%, when compared with prospective 1-year virologic failure in a sample of 133 patients with HIV.Citation16
Table 1 Patient characteristics (n=76)
Statistical analysis
Variables were described using frequencies and mean ± standard deviation as appropriate, on the basis of the measurement level and distribution. In order to compare the level of adherence before and after the switch, paired comparisons were performed using the McNemar test, paired t test, and Wilcoxon signed rank test for dichotomous, continuous normally distributed, and continuous not normally distributed variables, respectively. The level of significance was set at P<0.05. Statistical analyses were performed in SPSS (version 14.0, IBM Corporation, Amonk, NY, USA).
Results
Of 76 patients included in the study, 18 were women and 58 were men. The baseline immunosuppressive regimen for all 76 patients consisted of a calcineurin inhibitor (TAC [n=57] or CsA [n=19]) in combination with either mycophenolate mofetil (n=67), mTOR inhibitor (everolimus [n=7]), or azathioprine (n=2). The 57 patients receiving conventional TAC were converted to modified-release TAC on a milligram-for-milligram (ie, 1:1) basis; the 19 patients receiving CsA were converted according to manufacturer’s recommendations. Patient flow through the study is shown in . Seventy-two patients were available for statistical analysis; four patients (5.3% of all patients included) were reinitiated on conventional TAC (n=3) or CsA (n=1) due to gastrointestinal discomfort (n=3) or diarrhea (n=1) and were not included in the analyses. shows the demographic and clinical characteristics of the sample at baseline (n=72). Mean patient age was 46.0 ± 14.4 years. Initial examination occurred 4.8 ± 4.4 years after HTx (range 0.5–17.0 years post-HTx).
Adherence
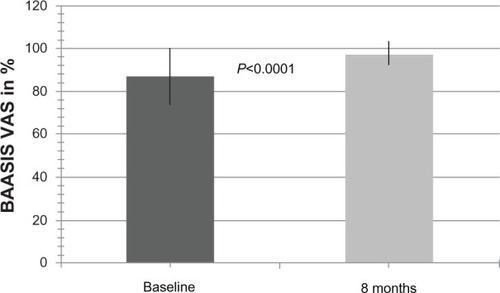
Overall nonadherence at baseline for any of the four BAASIS items was 75.0%. For the subdimensions of taking and timing as well as the occurrence of drug holidays and dose reductions, the nonadherence levels were 33.3%, 69.4%, 0.0%, and 5.6%, respectively. After 8 months, overall nonadherence for any of the four items was 40.3% (P<0.0001). For the subdimensions of taking and timing as well as the occurrence of drug holidays and dose reductions, the nonadherence levels were 13.9% (P=0.0004), 30.6% (P<0.0001), 1.4% (P=NS), and 4.2% (P=NS), respectively. Overall nonadherence at baseline and after 8 months is depicted in . After 8 months, adherence was improved in 41 patients (56.9% of total), unchanged in 27 patients (37.5% of total), and impaired in four patients (5.6% of total). Similarly, the BAASIS visual analog scale score improved significantly from 87.0% ± 13.5% to 97.5% ± 5.7% (P<0.0001, ).
Figure 2 Overall self-reported adherence at baseline and 8 months after conversion to modified-release tacrolimus (BAASIS).
Abbreviation: Baasis, Basel assessment of adherence with immunosuppressive Medication scale.
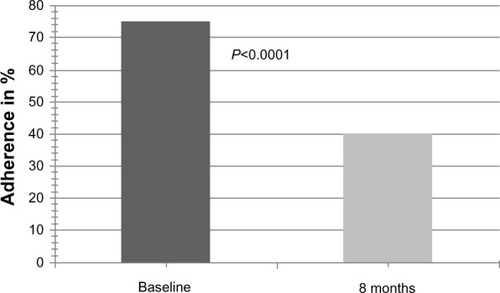
Figure 3 Overall self-reported adherence at baseline and 8 months after conversion to modified-release tacrolimus (BAASIS-VAS scale).
Abbreviations: Baasis, Basel Assessment of Adherence with Immunosuppressive Medication Scale; VAS, visual analog scale.
Safety and tolerability
TAC doses/levels
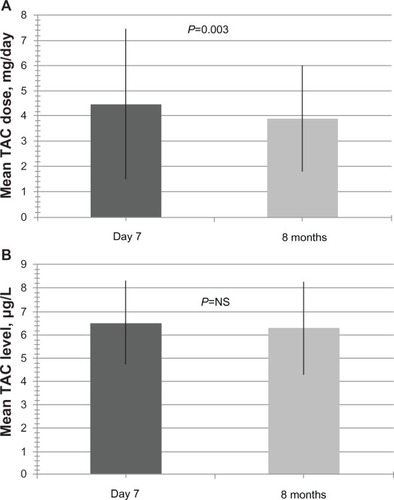
Due to the time-dependent decrease in TAC target levels post-HTx, mean modified-release TAC dose was 3.9 ± 2.1 mg/day 8 months after the switch compared with 4.5 ± 3.0 mg/day on day 7 (P=0.003, ). Similarly, 8 months after the switch, mean modified-release TAC level was 6.3 ± 2.0 μg/L compared to 6.5 ± 1.8 μg/L on day 7 (P=NS, ). After 7 days, 14 of 72 patients (19.4% of total) were not within the range of target levels. Of these 14 patients, only six patients (8.3% of total) had a TAC trough level differing more than 1 μg/L from target level. These patients included one patient initially on CsA and five patients on TAC.
Figure 4 Modified-release tacrolimus doses and levels at day 7 and month 8 after conversion to modified-release tacrolimus.
Abbreviation: TAC, tacrolimus.
Routine laboratory parameters
Serum creatinine levels remained stable after conversion to modified-release TAC. The baseline value for serum creatinine was 1.6 ± 1.0 mg/dL, and the value at month 8 was 1.4 ± 0.4 mg/dL (P=not significant [NS]; ). In addition, no significant changes were observed in hematologic and liver function parameters (all P=NS, ). No cases of acute rejection were found during the study period.
Side effects
Four patients (5.3% of all patients included) were reinitiated on conventional TAC (n=3) or CsA (n=1) due to gastrointestinal discomfort (n=3) and diarrhea (n=1). Symptoms were self limiting after switch, serologic parameters were within normal limits, and microbiological testing was negative.
Discussion
Adherence
In patients after HTx, our follow-up study showed a significant adherence improvement after switch to a single daily dose of modified-release TAC, which can be attributed to more timely immunosuppression and fewer skipped single doses. No significant changes in occurrence of independent reduction of doses was observed, which is not surprising due to the vital importance of immunosuppression after HTx. However, a slight increase in the occurrence of multiple skipped doses (drug holidays) was observed but did not reach the level of statistical significance. Admittedly, given the single-center study design, these results need to be interpreted conservatively.
Safety and tolerability
TAC has gained widespread acceptance as part of a dual immunosuppressive regimen and has proven to be effective in preventing allograft rejection after HTx.Citation17,Citation18 Modified-release TAC is very similar to conventional TAC in terms of bioavailability, but due to its prolonged-release formula with one single daily dose it has several advantages. Generally, the switch to modified-release TAC was feasible, effective, and safe. After 7 days, most patients were within target levels, irrespective of the initial calcineurin inhibitor. Modified-release TAC was generally well tolerated. No serious adverse events were noticed during the study. In four patients the initial calcineurin inhibitor had to be reinitiated, mainly due to gastrointestinal symptoms, which were generally mild and self-limiting.
No statistically significant changes in hematologic, liver, or renal function parameters were observed after the switch to modified-release TAC. Our study showed that mean TAC trough levels decreased during the course of the study. However, as several patients were included during the first year post-HTx, this is not unexpected, as TAC target levels were continuously adapted after HTx according to our center’s routine regimen. Patients maintained effective immunosuppression during the entire study period; there were no cases of biopsy-confirmed acute rejection during the treatment period.
Limitations of the study
To our knowledge, this is the first study reporting on improved adherence in patients after heart transplant 8 months after the switch from a conventional calcineurin inhibitor (twice-daily TAC or CsA) to once-daily modified-release TAC. Admittedly using a pre-experimental design, this study nevertheless provides important information on relevant clinical parameters related to adherence, safety, and the tolerability of switching patients, and it confirms findings from the literature that simplification of dosing regimen does result in improved adherence.Citation19
Conclusion
Therapeutic regimens for transplant recipients are often complex and become a limiting factor for medication adherence. This study in stable patients after HTx suggests a significant improvement in patient adherence after switch to modified-release TAC, in line with previously published short-term data.Citation12 Modified-release TAC was generally well tolerated. On the basis of the given data, we conclude that switching to modified-release TAC from a conventional calcineurin inhibitor is generally feasible, effective, and safe. Further studies are currently underway to investigate long-term safety and side effects in patients taking conventional TAC, extended-release TAC, or CsA after HTx.
Acknowledgments
This research project would not have been possible without the support of Sabina De Geest, PhD, RN, from the University of Basel, Switzerland, regarding provision of the BAASIS questionnaire and expert advice on data analysis.
Disclosure
Andreas O Doesch has a research grant from Astellas Pharma GmbH, Munich, Germany. The authors report no other conflicts of interest in this work.
References
- Abecassis MM Seifeldin R Riordan ME Patient outcomes and economics of once-daily tacrolimus in renal transplant patients: results of a modeling analysis Transplant Proc 2008 40 5 1443 1445 18589126
- Alloway R Steinberg S Khalil K Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen Transplant Proc 2005 37 2 867 870 15848559
- Alloway R Steinberg S Khalil K Two years postconversion from a prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients Transplantation 2007 83 12 1648 1651 17589351
- Diez Ojea B Alonso Alvarez M Aguado Fernández S Baños Gallardo M García Melendreras S Gómez Huertas E Three-month experience with tacrolimus once-daily regimen in stable renal allografts Transplant Proc 2009 41 6 2323 2325 19715908
- First MR Fitzsimmons WE Modified release tacrolimus Yonsei Med J 2004 45 6 1127 1131 15627307
- Florman S Alloway R Kalayoglu M Conversion of stable liver transplant recipients from a twice-daily Prograf-based regimen to a once-daily modified release tacrolimus-based regimen Transplant Proc 2005 37 2 1211 1213 15848672
- Florman S Alloway R Kalayoglu M Once-daily tacrolimus extended release formulation: experience at 2 years postconversion from a Prograf-based regimen in stable liver transplant recipients Transplantation 2007 83 12 1639 1642 17589349
- Heffron TG Pescovitz MD Florman S Once-daily tacrolimus extended-release formulation: 1-year post-conversion in stable pediatric liver transplant recipients Am J Transplant 2007 7 6 1609 1615 17511684
- Silva HT Yang HC Abouljoud M One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients Am J Transplant 2007 7 3 595 608 17217442
- Gasparyan AY Ayvazyan L Cocco G Kitas GD Adverse cardiovascular effects of antirheumatic drugs: implications for clinical practice and research Curr Pharm Des 2012 18 11 1543 1555 22364138
- Claxton AJ Cramer J Pierce C A systematic review of the associations between dose regimens and medication compliance Clin Ther 2001 23 8 1296 1310 11558866
- Doesch AO Mueller S Konstandin M Increased adherence after switch from twice daily calcineurin inhibitor based treatment to once daily modified released tacrolimus in heart transplantation: a pre-experimental study Transplant Proc 2010 42 10 4238 4242 21168673
- De Geest S Abraham I Dunbar-Jacob J Measuring transplant patients’ compliance with immunosuppressive therapy West J Nurs Res 1996 18 5 595 605 8918210
- Dobbels F Berben L De Geest S Transplant 360 Task Force The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review Transplantation 2010 90 2 205 219 20531073
- Glass TR De Geest S Hirschel B Swiss HIV Cohort Study Self-reported non-adherence to antiretroviral therapy repeatedly assessed by two questions predicts treatment failure in virologically suppressed patients Antivir Ther (Lond) 2008 13 1 77 85 18389901
- Deschamps AE De Geest S Vandamme AM Bobbaers H Peetermans WE Van Wijngaerden E Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards AIDS Patient Care STDS 2008 22 9 735 743 18754705
- Kofler S Bigdeli AK Kaczmarek I Long-term outcomes after 1000 heart transplantations in six different eras of innovation in a single center Transpl Int 2009 22 12 1140 1150 19891043
- Dösch AO Ehlermann P Koch A Remppis A Katus HA Dengler TJ A comparison of measured trough levels and abbreviated AUC estimation by limited sampling strategies for monitoring mycophenolic acid exposure in stable heart transplant patients receiving cyclosporin A-containing and cyclosporin A-free immunosuppressive regimens Clin Ther 2006 28 6 893 905 16860172
- Haynes RB Ackloo E Sahota N McDonald HP Yao X Interventions for enhancing medication adherence Cochrane Database Syst Rev 2008 2 CD000011 18425859