Abstract
Most patients with non-small-cell lung cancer (NSCLC) present with advanced disease and their long-term prognosis remains poor. Epidermal growth factor receptor (EGFR)-targeted therapies, such as gefitinib, have been subjected to comprehensive clinical development. Several phase II and III trials evaluated the clinical efficacy of gefitinib as monotherapy in pretreated patients with advanced NSCLC, as well as both monotherapy and combined with chemotherapy in chemotherapy-naive patients. A phase III trial (ISEL) in heavily pretreated advanced NSCLC patients demonstrated some improvement in survival with gefitinib compared with placebo; however, the difference was not statistically significant within the overall population. A large phase III trial in pretreated patients (INTEREST) demonstrated the non-inferiority of gefitinib in comparison with docetaxel for overall survival, together with an improved quality of life and tolerability profiles. In a large phase III trial (IPASS) in Asian chemotherapy-naive, never or former light-smoker patients with adenocarcinoma, gefitinib was more effective than carboplatin–paclitaxel in prolonging progression-free survival, particularly in patients harboring EGFR gene mutations. Gefitinib was a generally well tolerated treatment, with skin rash and diarrhea being the most common treatment adverse events. As a result, gefitinib is expected to have a large impact on the management of patients with advanced NSCLC, in particular in EGFR mutated patients.
Keywords:
Introduction
Lung cancer is still the main cause of cancer deaths in the world.Citation1 Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers and can be divided into three principal subtypes: adenocarcinoma, squamous-cell carcinoma and large cell carcinoma.Citation2
At the time of diagnosis, about 75% of NSCLC patients present with locally advanced or metastatic disease.Citation1 For most of these patients the only therapeutic option is based on chemotherapy alone. The aim of this treatment is to slow down the progression of the disease, to relieve the patients from the lung cancer symptoms and, whenever possible, to increase the overall survival.
A meta-analysis, published in 1995 and updated in 2008, affirmed the efficacy of first-line platinum-based chemotherapy in improving overall survival compared with best supportive care (BSC) in advanced NSCLC.Citation3,Citation4 Subsequently, survival differences were not demonstrated among the various platinum-based doublets, including a third-generation drug, such as paclitaxel, docetaxel, vinorelbine or gemcitabine.Citation5–Citation7
Recently, after the publication of the ECOG 4599 and AVAIL trial results, bevacizumab has been approved for the first-line treatment of non-squamous NSCLC in association with platinum-based chemotherapy.Citation8,Citation9 Moreover, pemetrexed has been recently registered for the first-line treatment of non-squamous NSCLC in association with cisplatin, after the results obtained by Scagliotti and colleagues in a trial demonstrating the non-inferiority of this new combination compared to standard cisplatin-gemcitabine.Citation10
For second-line therapy, docetaxel and pemetrexed have been approved for this indication.Citation11 The recent ASCO guidelines for the treatment of advanced NSCLC recommend also the use of gefitinib or erlotinib, epidermal growth factor receptor (EGFR)-tyrosine-kinase inhibitors (TKIs), in second-or third-line settings.Citation12
Aiming to obtain an improvement in NSCLC management and prognosis, researchers have investigated the role of molecular-targeted agents, such as inhibitors of specific cellular growth pathways, like that of EGFR and of vascular endothelial growth factor receptor (VEGFR).
EGFR is the cell-surface receptor for the epidermal growth factor family proteins. The interaction between the receptor and its ligands activates signal transduction pathways involved in cell proliferation and survival. Increased expression of EGFR has been found in 40%–80% of NSCLC.Citation13 Therefore, different approaches have been developed in order to inhibit EGFR, such as competition for the extracellular domain by monoclonal antibodies (cetuximab) or the inhibition of EGFR tyrosine-kinase activity by small-molecules interacting with the intracellular domain (erlotinib and gefitinib). In this paper, we will review the phase II-III trial results obtained with gefitinib in the treatment of NSCLC.
Mode of action and pharmacokinetic of gefitinib
Gefitinib is an orally administered low-molecular-weight anilinoquinazoline that inhibits the phosphorylation and tyrosine-kinase activity of the intracellular ATP-binding domain of EGFR through competitive binding to this site. The inhibition of the receptor and its related downstream process is achieved at dosages of 250 mg/day, while the maximal tolerable dosage, assessed in phase I trials, is 700 mg/day.Citation14 Pharmacokinetic studies found that gefitinib is adsorbed slowly and it reaches peak plasma concentration after 3–7 hours. Because of its biological half-life of about 28 hours, gefitinib is administered once in a day.Citation15,Citation16
Others trials were conducted in order to establish the tumor penetration of gefitinib. In one of these, gefitinib was administered orally for 28 days in patients with early NSCLC and, subsequently, the drug concentrations in surgically resected tumor samples and plasma were compared; the drug concentrations were higher in the tissues than in the plasma, proving that gefitinib is able to penetrate into tumor tissue efficiently.Citation17
Gefitinib is metabolized principally by cytochrome P4503A4, while CYP3A5 and CYP2D6 are less involved. This is the reason why gefitinib metabolism can differ from patient to patient; ie, in consideration of inter-individual variability of CYP3A4 expression and activity. Therefore, inducers or inhibitors of this cytochrome can also influence the pharmacokinetics of this drug.Citation18
Some studies demonstrated that gefitinib blocks selectively EGFR tyrosine-kinase (if compared with tyrosine-kinases of different receptors) and that it does not inhibit serine-threonine-kinases.Citation19 Its activity determines an upregulation of a cell cycle inhibitor (p27) and a downregulation of a transcription factor (c-fos), resulting in arresting the cell cycle in G1 phase.Citation20 EGFR works through two different downstream signaling pathways: MAP kinase cascade, that activates different genes linked to cell proliferation and survival, and PI3K-AKT cascade, in which phosphorylated AKT (p-AKT) inactivates proapoptotic proteins. In some studies, gefitinib was found to be more active on tumors with enhanced basal p-AKT activity.Citation21 Moreover; gefitinib decreases levels of important angiogenesis factors, like VEGF.Citation22
Gefitinib as second- or third-line therapy
IDEAL (IRESSA Dose Evaluation in Advanced Lung Cancer) 1 and 2 trials
According to results of four phase I studies,Citation16,Citation23–Citation25 two large, dose-randomized, double-blind multicenter phase II trials (IDEAL-1 and IDEAL-2) were conducted to evaluate the activity of gefitinib, 250 mg/day versus 500 mg/day, in pretreated patients with advanced NSCLC.Citation26,Citation27
The IDEAL-1 study was conducted in Europe and others countries (Japan, South Africa, Australia) and recruited 210 patients, while IDEAL-2 enrolled 221 patients in US.Citation26,Citation27 In the first study, activity and efficacy were similar between 250 mg and 500 mg.Citation26 The objective response rates (RR) were of 18.4% and 19%, respectively, with a disease control rates (DCR) of 54.4% versus 51.4%; median progression-free survival (PFS) was of 2.7 versus 2.8 months and median overall survival (OS) of 7.6 versus 8.0 months, for 250 and 500 mg, respectively. The symptom improvement rate was 40.3% for the 250 mg group and 37% for the 500 mg group.
Similar results were obtained in the IDEAL-2 trial, in which there was no significant difference in patients who received either 250 or 500 mg dose of gefitinib in terms of symptom improvement (43% versus 35%), RR (12% versus 9%) and OS (7 versus 6 months).Citation27
In both IDEAL-1 and IDEAL-2, in >70% of responding patients, the response occurred within the first 4 weeks. The response rates were durable, with a median duration of 13 months and 7 months for patients in IDEAL-1 and -2, respectively.Citation26,Citation27
Adverse events, such as skin reactions and diarrhea, were generally mild, reversible and manageable with a greater number of dose modifications or withdrawals in patients receiving gefitinib at dose of 500 mg.Citation26,Citation27
From these trials emerged the first evidence about the major efficacy of gefitinib in some specific subgroups of patients, such as female gender, adenocarcinoma histological subtype and Asian ethnicity. In particular, in IDEAL-2 the RR was greater in adenocarcinoma than in other histologies (13% versus 4%, P = 0.046) and in females compared with males (19% versus 3%, P = 0.001).Citation27 In IDEAL-1, the odds of responders was almost 3.5 times higher for patients with adenocarcinoma than for patients with other tumor histologies (odds ratio, OR = 3.45, P = 0.021) and 2.5 times higher for females than males (OR = 2.65, P = 0.017). In this trial, moreover, the RR was higher for Japanese than non-Japanese patients (27.5% versus 10.4%, OR = 3.27, P = 0.0023).Citation26
Therefore, gefitinib showed in these trials meaningful anti-tumor activity associated with rapid symptom relief and improvement of quality of life in pretreated patients with advanced NSCLC. Concomitantly, the 250 mg/day was safer and more tolerated than 500 mg/day dose. So, the 250 mg once daily dose was chosen for subsequent studies and gefitinib was registered in US and Japan for patients with advanced NSCLC as a second- or third-line treatment.
Gefitinib versus placebo: ISEL (IRESSA Survival Evaluation in Lung Cancer) trial
ISEL is a randomized, placebo-controlled, international multicenter phase III study designed to investigate the impact on survival of gefitinib versus best supportive care (BSC) as a second- or third-line treatment in patients with advanced NSCLC.Citation28 In this trial 1,692 patients, who were refractory or intolerant to previous chemotherapy, were enrolled and assigned in a ratio of 2:1 to either gefitinib 250 mg/day (1,129) or placebo (563) plus BSC. The primary endpoint was survival in the overall population and in patients with adenocarcinoma subtype.
Differences in the median survival did not reach a statistical significance, either in the overall population and in patients with adenocarcinoma histology (). However, patients treated with gefitinib had a significantly higher RR and longer time to treatment failure ().
Table 1 Results of the ISEL trialCitation28,Citation31
On preplanned subgroup analyses, a longer survival time was observed for patients treated with gefitinib who were never-smokers (P = 0.012) and of Asian origin (P = 0.01) (). To highlight the possible role of Asian ethnicity as predictive factor of response to gefitinib, a subset analysis including patients of Asian origin (about 20% of treated population) demonstrated that patients treated with gefitinib had a significant improvement in survival rate (9.5 versus 5.5 months; P = 0.010), time to treatment failure (4.4 versus 2.2 months, P = 0.0084) and tumor response (12.4 versus 2.1%).Citation29 Gefitinib was well tolerated (see below; “Safety and tolerability”).
Therefore, the results of the ISEL trial show that treatment with gefitinib was not associated with a significant increase in overall survival either in the overall population or in adenocarcinoma co-primary population. This result was disappointing given the finding of the phase III erlotinib study (NCIC-BR.21) which showed a 2-month increase in survival in previously treated patients with NSCLC.Citation30 Several explanations have been considered for the different results of the two trials. Different patient populations were enrolled in the two studies, due to differences in the eligibility criteria. The ISEL trial required patients to have progressed on the previous line of chemotherapy during treatment or within 90 days, while the BR.21 trial did not. This may have unintentionally selected a patient population in the ISEL trial that was more refractory to treatment and less likely to benefit from additional treatment. Another potential explanation is that a suboptimal dose of gefitinib was investigated in the ISEL trial. The decision to proceed with the 250 mg daily dose was based on a higher rate of toxicity with the 500 mg daily dose and the similar response rates for the two doses. In contrast, the BR.21 trial investigated erlotinib at the MTD (maximum tolerated dose). It is possible that cases that had limited sensitivity or were partially dependent on the EGFR pathway may have had a greater clinical benefit from the MTD dose of erlotinib than gefitinib, which was given at approximately 40% of the MTD. There has also been extensive investigation into molecular markers that explain the significant difference in clinical outcomes associated with EGFR-TKI therapy. A difference in the prevalence of one or more of the biomarkers associated with the response or resistance to EGFR-TKI therapy between the two trials may have contributed to the difference in the results.
A biological ISEL sub-study was performed including the assessment of EGFR gene copy number by fluorescent in situ hybridization (FISH), EGFR and p-AKT protein expression by immunohistochemistry (IHC), EGFR, K-RAS and B-RAF mutational status ().Citation31 It showed that a high EGFR gene copy number in patients treated with gefitinib represents a predictive factor of survival benefit when compared with placebo (HR: 0.61 versus 1.16 for high and low gene copy number, respectively; interaction test, P = 0.045), such as EGFR expression (HR: 0.77 versus 1.57 for positive and negative protein expression, respectively; interaction test, P = 0.049) (). Data on survival from the ISEL trial are consistent with the results from biological sub-study of BR.21 trial.Citation32,Citation33
Figure 1 Progression-free survival (PFS) and overall survival (OS) results (months) obtained with gefitinib in EGFR biomarker subgroups of ISEL and INTEREST trials.Citation31,Citation38
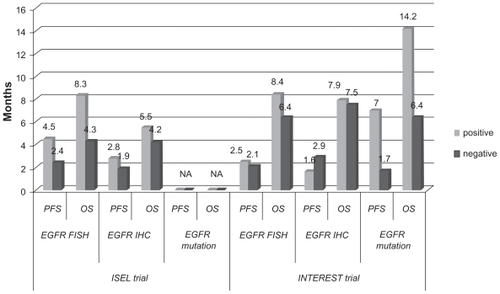
In addition, patients with EGFR mutations obtained higher RR than wild-type patients (37.5% versus 2.6%). According to p-AKT, no correlation was observed in terms of survival; although there was a better RR in p-AKT positive patients treated with gefitinib when compared to p-AKT negative ones (10.1% versus 6.3%, respectively). Of 12 patients with a K-RAS mutation, 6 were treated with gefitinib and no responses were seen, whereas no B-RAF mutations were detected.
Gefitinib versus chemotherapy
Phase II study: SIGN (second line indication of gefitinib in NSCLC) trial
The SIGN was a phase II open-label randomized study, comparing gefitinib 250 mg/day (n = 60 patients) with docetaxel 75 mg/m2 every 3 weeks (n = 73 patients) in advanced pretreated NSCLC.Citation34 The trial was not designed to test for a statistical difference between treatments on any endpoint its primary objective was symptom improvement using the FACT-L questionnaire (see below “Quality of life”). The results suggest that gefitinib and docetaxel have similar activity and efficacy; (symptom improvement rates of 36.8% and 26%; RR of 13.2% and 13.7%; median PFS of 3 and 3.4 months; median OS of 7.5 and 7.1 months, with quality of life improvement rates of 33.8% and 26%, for gefitinib and docetaxel, respectively), with a more favorable tolerability profile for gefitinib (adverse events of all grades: 51.5% and 78.9%, of grade 3–4 8.8% and 25.4%)
Phase II studies: INTEREST, V-15-32 and ISTANA trials
Recently, the results of three phase III trials comparing gefitinib versus docetaxel in this setting have been published ().Citation35–Citation37
Table 2 Phase III trials of gefitinib versus chemotherapy as second-line treatment
INTEREST (Iressa NSCLC trial evaluating response and survival versus taxotere) trial
The INTEREST trial compared gefitinib with docetaxel as second-or third-line therapy in 1,466 patients with advanced NSCLC treated with prior platinum-based chemotherapy. Citation35 The patients were randomly assigned on a 1:1 basis to receive either gefitinib 250 mg/day or docetaxel 75 mg/m2 every 3 weeks. The primary endpoints were the non-inferiority of gefitinib in comparison with docetaxel, in terms of overall survival in the total population and superiority in patients who expressed a high EGFR gene copy number.
In the overall population, median OS was 7.6 months in the gefitinib group versus 8.0 months in the docetaxel group, with a 1-year survival of 32% versus 34%, respectively, demonstrating the non-inferiority of gefitinib with respect to docetaxel (HR, 1.020, 95% CI, 0.905–1.150, with the upper confidence limit less than the non-inferiority limit of 1.154) (). Nevertheless, the survival superiority of gefitinib in the subgroup with high EGFR gene copy number was not proven (8.4 versus 7.5 months; HR, 1.09, 95% CI, 0.78–1.51, P = 0.62). Survival results were consistent across preplanned subgroups.
The median PFS and RR were similar in both treatment groups (), although gefitinib afforded a statistically significant higher rate of improvement in quality of life (see below “Quality of life”).
Moreover, gefitinib had a better tolerability profile; the most common gefitinib adverse events were skin reactions and diarrhea, whereas hematologic disorders (neutropenia grade 3–4 and febrile neutropenia), asthenia and alopecia were more likely to occur with docetaxel.
Recently, the results of a preplanned analysis of molecular predictors from the INTEREST trial have been published.Citation38 The biomarkers considered were EGFR gene copy number by FISH, EGFR protein expression by IHC, EGFR and K-RAS mutational status (). Data obtained showed no statistically significant impact of these biomarkers in terms of OS. EGFR mutation positive patients had longer PFS (HR, 0.16, 95% CI, 0.05–0.49, P = 0.001) and higher RR (42.1% versus 21.1%, P = 0.04) and patients with high EGFR copy number had higher RR (13% versus 7.4%, P = 0.04) with gefitinib versus docetaxel. These biomarkers do not appear to be factors for differential survival between gefitinib and docetaxel in this setting; however, subsequent treatments may have influenced the survival results. There was no statistically significant difference between gefitinib and docetaxel in biomarker-negative patients. This suggests that gefitinib can provide similar overall survival to docetaxel in patients across a broad range of clinical subgroups and that EGFR biomarkers such as mutation status may additionally identify which patients are likely to gain the greatest PFS and RR benefit from gefitinib.
V-15-32 trial
This phase III study compared gefitinib 250 mg/day with docetaxel 60 mg/m2 every 3 weeks in 489 Japanese patients with advanced NSCLC, who were treated with one or two prior chemotherapy regimens.Citation36 Of the 489 patients enrolled, approximately 78% had adenocarcinoma histology, 38% were female and 32% were never-smokers.
The primary endpoint was the non-inferiority of gefitinib in comparison with docetaxel in overall survival; the upper limit of the CI was required to be ≤1.25 in order to demonstrate non-inferiority. This trial demonstrated similar efficacy between gefitinib and docetaxel; however, it did not meet the primary endpoint of demonstrating non-inferiority (HR, 1.12, 95.24% CI, 0.89–1.40, P = 0.330) (). This result could be due to the small number of patients and to post-study cross-over (36% of patients treated with gefitinib received subsequent docetaxel and 53% of docetaxel treated patients received subsequent gefitinib). In addition, the median PFS was 2.0 months in both treatment groups, whereas gefitinib was statistically superior to docetaxel in terms of RR (). Gefitinib also significantly improved the quality of life as compared to docetaxel. The disease control rates and symptom improvement were similar for the two treatments. Gefitinib was also better tolerated than docetaxel (see below “Safety and tolerability”).
ISTANA (IRESSA as second-line therapy in advanced NSCLC) trial
This was a phase III trial conducted in Korea that compared gefitinib with docetaxel as a second-line treatment in 161 patients with advanced NSCLC.Citation37 Its primary endpoint was PFS.
PFS was found to be longer on gefitinib when compared with docetaxel; the PFS HR for gefitinib derived from the primary unadjusted model was 0.729 (90% CI, 0.533–0.988, one-sided P = 0.0441) and from the supportive adjusted model was 0.634 (90% CI, 0.459–0.875, one-sided P = 0.0134). Median PFS was 3.3 months in the gefitinib group and 3.4 months in the docetaxel group; the 6-month PFS rates were 32% and 13%, respectively. In terms of RR, gefitinib was statistically superior to docetaxel (). In the final analysis of OS, the HR was 0.870 (95% CI, 0.613–1.236, P = 0.437). No significant differences were seen in the quality of life or symptom improvement rates between the two treatment groups. Gefitinib was well tolerated, was consistent with previous data and had fewer adverse events than docetaxel.
Therefore, gefitinib showed in this trial an advantage over docetaxel in terms of PFS and RR as a second-line treatment; however, it is necessary underline its limited sample size, smaller than other similar studies, and the patient selection (only Korean or Asian ethnicity patients with a high proportion of never smokers and patients with adenocarcinoma histology).
Gefitinib versus docetaxel: a meta-analysis of four clinical trials
At the last American Society of Clinical Oncology (ASCO) Annual Meeting, Shepherd and colleagues presented a meta-analysis of the four previously reported randomized trials, evaluating gefitinib versus docetaxel in unselected pretreated patients with advanced NSCLC (SIGN, INTER-EST, V-15–32, and ISTANA trials).Citation39 In this meta-analysis gefitinib showed similar OS (HR, 1.03, 95% CI, 0.93–1.13, P = 0.5773) and PFS (HR, 0.96, 95% CI, 0.87–1.05, P = 0.3784), with a statistically significant increase in the RR (13.6% versus 9%, OR, 1.65, 95% CI, 1.24–2.21, P = 0.0007) when compared with docetaxel. This meta-analysis adds to the weight of evidence that gefitinib and docetaxel show similar efficacy in pretreated patients with advanced NSCLC and further contributes to defining the risk-benefit profile of each treatment, which also considers tolerability and ease of administration.
Gefitinib as first-line therapy
Gefitinib in combination with chemotherapy: the failing experience of INTACT-1 and INTACT-2 trials
The results obtained with gefitinib as a single agent in IDEAL trialsCitation26,Citation27 led some authors to test the drug in association with chemotherapy as front-line treatment in two randomized phase III trials, INTACT-1 and INTACT-2 (Iressa NSCLC Trial Assessing Combination Treatment).Citation40,Citation41
The rationale to test the efficacy of the gefitinib-chemotherapy association was given by evidence, in preclinical studies, of interaction between gefitinib and cytotoxic drugs (in particular with cisplatin); ie, a different mechanism of action and favorable safety profile of gefitinib.Citation42
Unfortunately, both studies failed to demonstrate a survival advantage when gefitinib was associated with chemotherapy (). INTACT-1 was a three arm trial in which 1,093 patients were randomized to receive cisplatin and gemcitabine for 6 cycles plus gefitinib 500 mg/day, 250 mg/day or placebo.Citation40 Gefitinib was continued after chemotherapy until progression or unacceptable toxicity. Most of the patients were enrolled in European (74%) and North American (12%) centers. The primary endpoint was overall survival. The results were quite disappointing; the gefitinib arms showed no differences in OS as compared with the placebo arm. No differences were observed for the time to progression (TTP) and RR (). Subgroup analysis for sex, histology and time on chemotherapy also did not show any survival difference.
Table 3 Phase III trials of gefitinib in combination with chemotherapy as first-line treatment of advanced NSCLC (INTACT-1 and 2)
INTACT-2 was a three arms trial in which 1,037 patients, mostly in the US, were randomized to receive carboplatin and paclitaxel for 6 cycles plus gefitinib 500 mg/day, 250 mg/day or placebo.Citation41 As in previous study the primary endpoint was OS. The results of this trial did not show any differences from those of INTACT-1 trial (). The subgroup analysis did not show any statistical difference in survival except for a slight trend toward improved survival for patients with adenocarcinoma; these patients received chemotherapy for more than 90 days in the gefitinib 250 mg/day arm, suggesting a possible effect of gefitinib monotherapy as maintenance therapy. However, this result was not observed in INTACT-1 study.
Two similar phase III trials assessing the efficacy of erlotinib in association with chemotherapy (TRIBUTE and TALENT trials) were conducted at the same time as the INTACT studies and showed similar results, confirming the absence of any benefit in the addition of TKIs to chemotherapy as first-line treatment of advanced NSCLC.Citation43,Citation44
The two gefitinib trials, INTACT 1 and 2, were well designed, adequately powered, and well conducted. The conclusion that concomitant gefitinib administration does not add clinical benefit to conventional chemotherapy in NSCLC seems, therefore, irrefutable. Different theories have been formulated to explain this lack of efficacy. One hypothesis is that each agent works against the same cell subpopulation so that the effect is redundant. Another hypothesis is that the activity of one agent results in the loss of an intermediary molecule, which is essential to the function of the other agent. At present, the strongest hypothesis about the failure of the two studies seems to be that the patients were not selected for any of the known criteria that has later been discovered to be associated with a sensitivity to gefitinib, so that the population who was most likely to receive a real benefit from the agent (EGFR mutation) did not amount enough to statistically change the results obtained.
Gefitinib in elderly and poor-performance patients: INVITE and INSTEP trials
Considering its good toxicity profile, gefitinib has recently been tested as an alternative to single agent monotherapy in elderly and poor performance status (PS) NSCLC patients.
INVITE (IRESSA in NSCLC versus Vinorelbine Investigation in the Elderly) trial is the first phase II study designed to test gefitinib in untreated elderly NSCLC patients compared with a single agent, vinorelbine.Citation45 In this study 196 unselected patients aged >70 years were randomly assigned to receive gefitinib 250 mg/day until progression or vinorelbine for up to 6 cycles. The primary endpoint was PFS; this trial was designed to determine the superiority of gefitinib as compared with vinorelbine. The results showed no statistical difference in PFS (2.7 versus 2.9 months, HR 1.19, 95% CI, 0.85–1.65, P = 0.310), OS (5.9 versus 8.0 months; HR 0.98, 95% CI, 0.66–1.47), RR (3.1% versus 5.1%) and disease control rates (43.3% versus 53.5%) for gefitinib and vinorelbine, respectively. Overall, the quality of life improvement and pulmonary symptom improvement rates were in favor of gefitinib. As expected, gefitinib showed better tolerability profile than vinorelbine.
Although the present study was not designed to show equivalence between the two drugs, there was no statistical difference between the two treatments, suggesting that gefitinib could represent an alternative to single-agent chemotherapy in elderly patients. It is important to underline that the totality of the population enrolled in this trial was unselected for the features that confer sensitivity to gefitinib; in fact, most of them were male (77%), smokers (82%) and with squamous cell carcinoma (48%) and this might explain the low percentage of responders. Most patients were analyzed for EGFR gene copy number by FISH; surprisingly, the 54 patients who were EGFR FISH positive benefited more from vinorelbine than from gefitinib (HR, 3.13, 95% CI, 1.45–6.76 for PFS and HR, 2.88, 95% CI, 1.21–6.83 for OS). This finding was unexpected and in contrast to previous observations.
The INSTEP (IRESSA NSCLC Trial Evaluating Poor PS Patients) trial was a phase II study, comparing gefitinib to BSC in untreated patients with PS ≥ 2, not eligible for chemotherapy.Citation46 In this study, 201 patients were randomized to receive gefitinib 250 mg/day until progression or unacceptable toxicity or BSC. Primary endpoint was PFS. The results showed no statistical difference in outcome for patients treated with gefitinib, even though there was a small trend toward improved PFS, OS and RR in favor of gefitinib. HRs were 0.82 (95% CI, 0.60–1.12, P = 0.217) and 0.84 (95% CI, 0.62–1.15, P = 0.272), for PFS and OS, respectively. RR was 6% for gefitinib and 1% for placebo. No statistical difference was seen in the quality of life. In the subgroup of EGFR FISH positive patients (n = 32), gefitinib improved significantly PFS (HR, 0.29) and there was a trend toward an increase in OS. This trial failed to demonstrate a statistically significant benefit for first-line gefitinib compared with BSC in unfit patients; however, a number of reports from gefitinib expanded access program (EAP) suggest that gefitinib may have utility as first-line treatment in patients with poor PS or unwilling to receive chemotherapy.Citation47
Gefitinib in selected patients
As indicated above, gefitinib, when used in unselected patients, allows only a modest response rate ranging from 10% up to 20%. Nevertheless, it appears that a higher benefit can be obtained in some patient subgroups, such as females, never smokers, Asians and patients with adenocarcinoma histology. Although clinical characteristics may identify candidates for EGFR-TKIs, the ideal patient selection should mostly rely on biological tumor features, in particular in the presence of EGFR gene mutations. The most common mutations are exon 19 deletions and exon 21 point mutation (L858R), which can be found in approximately 10%–20% of NSCLC patients, more frequently in never smokers, women, Asians and with adenocarcinoma.Citation48–Citation50 These alterations in structure of the self-phosphorylating domain enhance EGFR activation and also favor binding of TKIs to their site of action. Patients who harbor these mutations experience response rates higher than 65% and median survival of 20–30 months, as demonstrated in several retrospective studies.Citation51–Citation55 Such results led investigators to test gefitinib as a first-line therapy in EGFR mutated patients in prospective trials.
Phase II studies
Several phase II trials investigated the efficacy of gefitinib as a first-line treatment in highly selected patient populations, based on the presence of activating EGFR gene mutations.Citation56–Citation60
In a phase II trial, Asahina and colleagues obtained a RR of 75% and median PFS of 8.9 months.Citation56 Inoue and colleagues evidenced similar results in 16 patients with EGFR mutated, identified among 75 chemonaive patients (RR of 75%, with a DCR of 88% and a median PFS time of 9.7).Citation57 Yang and colleagues enrolled 106 patients selected by clinical features and determined their EGFR mutation status in 90 of these patients. Exon 19 deletions and L858R mutations were present in 43 patients; the RR and median time to treatment failure were 95% and 8.9 months, respectively, for exon 19 deletions, and 73.9% and 9.1 months for L858R mutation.Citation58
Similar results were obtained also in Caucasian patients. Sequist and colleagues (the iTARGET trial) selected chemonaive patients with non-squamous histology who had one or more clinical characteristics associated with activating EGFR mutations (low or never smoking history, adenocarcinoma histology, female gender and East Asian ethnicity).Citation59 In this clinically enriched patient population, mutations were identified in 35% of patients, which is higher than the rate of 10%–15% seen in previous studies of Western populations.Citation58 Thirty-one patients received gefitinib: RR was 55%, median PFS was 9.2 months, OS 17.5 months, with 1-year survival of 73%. Two patients with classic activating mutations exhibited de novo gefitinib resistance and had concurrent genetic anomalies usually associated with acquired TKI resistance, specifically the T790M EGFR mutation and MET amplification. This study has demonstrated that genotype-directed EGFR-TKI therapy with gefitinib for patients with previously untreated NSCLC is feasible in a Western population.
Considering the favorable safety profile, Inoue and colleagues tested gefitinib in a phase II trial in NSCLC patients with poor PS harboring EGFR mutations, not eligible for chemotherapy.Citation60 Thirty patients with NSCLC and poor PS, including 22 patients with PS 3 to 4, were enrolled. The overall RR was 66%, with a DCR of 90%. PS improvement rate was 79%. The median PFS and OS were 6.5 and 17.8 months, respectively. Despite the fact that most of these patients had aggressive disease, treatment with gefitinib in this setting yielded a median survival three- to four-fold higher than that generally observed with conventional cytotoxics. This is the first report indicating that EGFR mutation-positive patients with extremely poor PS benefit from first-line gefitinib. Because there has previously been no standard treatment for these patients with short-life expectancy, other than BSC, examination of EGFR mutations as a biomarker should be recommended in this patient population.
All these studies demonstrated an advantage in the use of gefitinib as first-line therapy in selected patients harboring EGFR activating mutations, achieving outcome results, which are higher than any other treatment used in NSCLC. Other prospective trials showed similar results in EGFR mutated NSCLC populations in further lines of treatment.Citation61–Citation65
Additional trials have selected patients based on a combination of clinical, pathological or molecular features. The ONCOBELL trial selected patients who were never smokers or who had evidence of a high gene copy on FISH and were p-AKT positive.Citation66 Of the 183 patients who were evaluated, 42 patients were enrolled in the trial and treated with gefitinib. The RR observed was 47.6%, the median TTP was 6.4 months and the 1-year survival rate was 64.3%. EGFR mutations were detected in 24 patients (66.8%) and the RR observed in those patients was 62.5%. The Southwest Oncology Group performed a phase II trial for patients with bronchioalveolar carcinoma.Citation67 This trial included previously treated (n = 22) and untreated (n = 69) patients that received gefitinib at a dose of 500 mg daily. The RR in the previously treated and untreated patients was 9% and 17%, respectively, and the PFS times were 3 and 4 months, respectively. Another area of investigation is the selection of patients based on the clinical history of non-smoking. A phase II trial investigated the activity of gefitinib (250 mg daily) in 37 chemotherapy-naive Korean patients with adenocarcinoma histology and a never smoking history.Citation68 The observed RR was 69%, with a DCR of 81%. The median PFS time and 1-year survival rate observed were 33 weeks and 73%, respectively.
Phase III studies: IPASS, First-SIGNAL, WJTOG3405 and NEJ002 trials
According to the results obtained with gefitinib as a first-line treatment in phase II trials performed in selected populations, four Asian randomized phase III trials (IPASS, First-SIGNAL, WJTOG3405 and NEJ002) were conducted to assess whether gefitinib could represent a valid alternative to chemotherapy in this setting of disease.Citation69–Citation72
IPASS (Iressa Pan-Asia Study) trial
The IPASS trial was a randomized phase III study where previously untreated patients in East Asia who had advanced pulmonary adenocarcinoma and who were non-smokers or former light smokers were randomized to receive gefitinib or carboplatin-paclitaxel.Citation69
Eligible patients were chemotherapy-naive with NSCLC with adenocarcinoma histology, never (<100 cigarettes in lifetime) or light ex-smokers (stopped ≥ 15 years ago and smoked ≤ 10 pack years) and with a performance status of 0 to 2. A total of 1,217 patients were randomized to receive either gefitinib (250 mg/day; n = 609) until disease progression or other criteria for discontinuation or carboplatin (AUC 5 or 6) and paclitaxel (200 mg/m2 every 3 weeks) (n = 608) for a maximum of 6 cycles or until disease progression or other criteria for discontinuation.
The primary objective was to assess the non-inferiority of gefitinib versus carboplatin-paclitaxel for PFS. Exploratory objectives were to evaluate the efficacy outcomes in biomarker subgroups defined by EGFR mutation status, EGFR gene copy number by FISH and EGFR IHC expression.
The study exceeded the primary objective and demonstrated superiority of gefitinib relative to carboplatinpaclitaxel in terms of PFS in the intent-to-treat population (). The HR was 0.74 (95% CI, 0.65–0.85, P < 0.001). Median PFS was 5.7 versus 5.8 months in gefitinib and chemotherapy group, respectively, with 12-month rates of PFS of 24.9% versus 6.7%. Therefore, the risk of progression was reduced by 26% on gefitinib compared with carboplatinpaclitaxel; however, the hazard ratio was not constant over time. Because of the crossing of the curves, the median PFS is similar on both treatments, although clearly it is not a good reflection of the treatment effect in this study. In fact, the pattern of the 4, 6 and 12-month progression-free rates favor carboplatin-paclitaxel for the first 6 months and gefitinib for the remaining 16 months. The PFS treatment effect was consistent with the overall population in all clinical subgroups.
Table 4 Phase III trials of gefitinib versus chemotherapy as first-line treatment in clinically selected patients
At the time of data cut-off, for the primary analysis (14 April 2008), the data were immature as there were only 450/1217 deaths (37%). Follow-up for survival is ongoing. OS was similar between the gefitinib and carboplatinpaclitaxel arms and may be influenced by the large amount of subsequent therapy received in this study, making these data difficult to interpret. Objective RR was significantly higher with gefitinib (43.0%) than with carboplatin-paclitaxel (32.2%) (OR, 1.59, 95% CI, 1.25–2.01, P < 0.001).
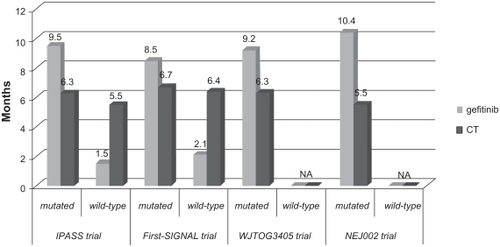
Another important finding of the study was the result obtained in the subgroup of patients that were positive for EGFR mutation. In IPASS, 261 patients (59.7% of those with a known status) were mutation positive, the largest group ever studied in a randomized controlled trial of an EGFR-TKI to date, reflecting the clinical selection of the patients in this study (). Some striking differences in PFS outcome by EGFR mutation status were seen (interaction test, P < 0.0001). PFS was significantly longer for gefitinib than carboplatin-paclitaxel in mutation positive patients (HR, 0.48, 95% CI, 0.36–0.64, P < 0.001), while it was significantly longer for chemotherapy in mutation negative patients (HR, 2.85, 95% CI, 2.05–3.98, P < 0.001). Within these subgroups, the treatment effect appears to be constant over time, unlike in the overall study population. Among the EGFR mutation negative patients, over half of those receiving gefitinib had progressed by the time of the first scheduled scan at 6 weeks and this is likely to be driving the initial disadvantage for gefitinib in the overall population curves, with the later advantage being driven by the very long PFS for gefitinib in EGFR mutation positive patients. The EGFR mutation positive benefit outweighs the EGFR mutation negative deficit, leading to overall superiority for gefitinib.
Figure 2 Progression-free survival (PFS) results (months) in EGFR mutated subgroups in IPASS, First-SIGNAL, WJTOG3405 and NEJ002 trials.Citation69–Citation72
A post-hoc analysis of overall survival by mutation status was also performed, acknowledging that there would only be a small number of events in the analysis and hence limited power (only 37% of patients had died). The hazard ratio was numerically in favor of gefitinib in the EGFR mutation positive patients (based on 81 events; HR, 0.78, 95% CI, 0.50–1.20) and numerically in favor of carboplatinpaclitaxel in EGFR mutation negative patients (based on 94 events; HR, 1.38, 95% CI, 0.92–2.09). However, no statistically significant differences were seen, possibly because the number of events was small.
In the EGFR mutation positive subgroup, RR was significantly higher with gefitinib (71.2%) than with carboplatin-paclitaxel (47.3%) (P < 0.001), while in the EGFR mutation negative subgroup, RR was significantly higher with carboplatin-paclitaxel (23.5%) than with gefitinib (1.1%) (P = 0.001).
About other biomarkers, a possibly related trend was observed with EGFR gene copy number (interaction test, P = 0.0437), with a significant advantage for gefitinib over carboplatin-paclitaxel in patients with high EGFR gene copy number tumors (HR, 0.66, P = 0.005), while in patients with low gene copy number there was a numerical advantage for carboplatin-paclitaxel (HR, 1.24, P = 0.237). Post-hoc analysis suggests this effect was driven by the overlap of high EGFR gene copy number with a positive EGFR mutation status. Objective RR was also significantly higher with gefitinib in the subgroup of patients with high EGFR gene copy number (58.9% versus 44.8% P = 0.024). In the EGFR expression positive subgroup, objective response rate tended to be higher with gefitinib than with carboplatin-paclitaxel, but the difference was not statistically significant (51.5% versus 41.8%, P = 0.109).
Quality of life improvement rates were significantly higher with gefitinib than carboplatin-paclitaxel; while similar proportions of patients on both treatments experienced an improvement in lung cancer symptoms (see below “Quality of life”). As expected, gefitinib was much better tolerated than chemotherapy (see below “Safety and tolerability”).
First-SIGNAL (First-line single agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung) trial
Recently, results from a similar trial, comparing gefitinib with cisplatin-gemcitabine as first-line treatment in Asian never-smokers, with advanced adenocarcinoma, have been reported.Citation70 Three hundred and nine patients, mostly women (89%), were randomly allocated 1:1 to gefitinib 250 mg/day (n = 159) until disease progression or other criteria for discontinuation, or cisplatin-gemcitabine (n = 150) for a maximum of 6 cycles or until disease progression or other criteria for discontinuation. The primary endpoint was OS ().
In the overall population, RR was 53.5% for gefitinib and 45.3% for chemotherapy (OR, 1.385, 95% CI, 0.885–2.167, P = 0.153). The median OS and PFS were nearly identical, with a 1-year PFS rate of 20.3% versus 5.0%. The PFS survival curves were very similar to IPASS curves, with analogue crossing. Similarly to other studies, gefitinib improved quality of life with a better toxicity profile than chemotherapy.
The authors also conducted a subgroup study for EGFR mutations (). Over 30% of patients were analyzed for mutation status, giving an overall EGFR mutation rate of 43.8% (42 out of 96 patients). RR was 84.6% versus 37.5% (P = 0.002) for gefitinib and chemotherapy respectively in mutation positive patients, and 29.9% versus 51.9% in mutation negative (P = 0.051). There was no difference in OS by mutation status; however, there was some difference in PFS favoring gefitinib in mutation positive patients (8.5 versus 6.7 months; HR, 0.613, 95% CI, 0.308–1.221, P = 0.0849).
The absence of difference in OS, both in the overall and EGFR mutated populations, is most likely due to the post-study use of EGFR-TKIs in 80.7% of chemotherapy arm. Even if the study failed to reach its endpoint gefitinib allowed the achievement of a favorable response rate and disease control in Asian, non-smoker patients; especially in those who carry the EGFR mutations, so it could represent a reasonable first-line therapy for this group of patients.
These two studies highlight the importance of a selection of the patients, who are candidates for receiving gefitinib therapy. Some clinical features are related to a high rate of EGFR mutation, so it is reasonable to consider the research of these mutations in patients with these characteristics to evaluate the appropriate timing for treatment with gefitinib. Two phase III Japanese studies have been performed specifically in patients EGFR mutated to compare the efficacy of gefitinib versus chemotherapy in the first-line treatment of NSCLC (WJTOG3405 and NEJ002 trials).Citation71,Citation72
WJTOG3405 (West Japan Thoracic Oncology Group3405) and NEJ002 (North East Japan Gefitinib Study Group002) trials
In the WJTOG3405 trial, 172 EGFR mutated patients were randomly assigned to receive gefitinib or chemotherapy with cisplatin-docetaxel ().Citation71 The primary endpoint was PFS. The study met its endpoint, showing a median PFS of 9.2 months in the gefitinib group and 6.3 months in the chemotherapy group (HR, 0.489, 95% CI, 0.336–0.710, P < 0.0001). In the IPASS trial, PFS curves were similar in the gefitinib and the chemotherapy groups during the first 6 months of treatment, while in the present study the curves favor gefitinib at any time of treatment. RR was 62.1% and 32.2% with gefitinib and chemotherapy, respectively (P < 0.0001). The OS data were not available at the time of publication. A subgroup study on mutation-type-specific survival showed no statistical difference between 19 deletions and exon 21 mutations.
The WJTOG3405 trial results confirm once more gefitinib to be superior to chemotherapy in terms of RR and PFS in patients with EGFR mutations. Another prospective phase III study, comparing gefitinib to chemotherapy (carboplatin-paclitaxel) as first-line treatment in advanced NSCLC patients selected for EGFR mutation, was presented by Kobayashi and colleagues at the ASCO meeting 2009.Citation72 At present, only data on PFS and RR from an interim analysis are available. Median PFS resulted of 10.4 months in the gefitinib arm (n = 98) and 5.5 months in the chemotherapy arm (n = 96) (HR, 0.357, 95% CI, 0.25–0.51, P < 0.001). Also significantly higher RR was obtained in gefitinib arm (74.5% versus 29%, P < 0.001).
Gefitinib as maintenance therapy
Due to its efficacy in advanced pretreated NSCLC patients and its mild toxicity profile, gefitinib has also been considered as maintenance therapy. Two trials tested gefitinib in unselected patients subsequently to chemotherapy (WJTOG0203) and to chemo-radiotherapy (SWOG S0023).Citation73,Citation74
In the West Japan Thoracic Oncology Group Trial 0203 trial, 604 patients were randomly assigned to receive a platinum-doublet chemotherapy for up to 6 cycles or the same doublet for 3 cycles followed by gefitinib as maintenance therapy until progression.Citation73 The trial failed to meet the primary endpoint of improving OS, as there was no statistical difference between the two arms (HR, 0.86, 95% CI, 0.72–1.03, P = 0.11). A small but significant improvement was seen in PFS (4.6 versus 4.3 months in gefitinib and chemotherapy arm, respectively (HR, 0.68, 95% CI, 0.57–0.80, P < 0.001). In a subset analysis, a small significant difference in OS was found in the group of adenocarcinomas (HR, 0.79, 95% CI, 0.65–0.98, P = 0.03). The explanation of these results could be related to the absence of any biological or clinical patient selection. It might be expected that there would be a greater efficacy in patients with EGFR mutation; nevertheless, it is uncertain whether there would be any benefit, administering gefitinib right after chemotherapy or at the time of disease progression.
The South Western Oncology Group study evaluated the efficacy of a sequential therapy with gefitinib following chemo-radiotherapy in unresectable locally advanced NSCLC.Citation74 Patients were randomized to receive gefitinib or placebo after chemotherapy with cisplatin-etoposide for 2 cycles with concomitant radiotherapy followed by 3 cycles of docetaxel consolidation. The primary endpoint was to achieve a 33% increase in median survival time rate. The study was closed after an unplanned interim analysis which showed that the planned objective was ruled out with a P = 0.0015.
At the time of the publication, coincident with a median time of follow up of 27 months, the median OS of gefitinib arm (n = 118) was 25 months compared to 32 months of placebo arm (n = 125) (P = 0.013). These results surprisingly showed gefitinib to be detrimental in locally advanced NSCLC patients after a standard chemo-radiotherapy. It is hard to understand the reasons of these findings. A higher rate of toxic events was reported in the experimental arm, although the moderate intensity of adverse events cannot explain the detrimental effect. Furthermore, toxicity related deaths rate was only 2% in experimental arm. The lack of selection might have contributed to the poor efficacy of gefitinib and partially explains the results, although not the reduction in OS. Another hypothesis is the potential interaction between EGFR inhibitors and radiotherapy. It is known that the concomitant use of these agents with radiotherapy enhance radiation efficacy, and it is also known that some people previously treated with radiotherapy have shown an impaired response to gefitinib.Citation75,Citation76 However, this hypothesis cannot completely explain the results of this study and the reasons of this detrimental effect remain unknown.
At present gefitinib has no indication as maintenance therapy in patients treated with standard therapy. It is possible that EGFR mutated patients could benefit from a maintenance therapy with gefitinib, although further trials on selected patients are required.
Safety and tolerability
Gefitinib is generally well tolerated, particularly in elderly and/or poor PS patients; it is responsible for relatively few severe side effects, as compared with conventional chemotherapeutic agents. The most common side effects are skin rash and diarrhea; less common are nausea, vomiting and anorexia. Another common toxicity is the elevation of AST/ALT, which usually regresses after discontinuation of therapy ().
Table 5 Toxicity data of ISEL, INTEREST and IPASS trialsCitation28,Citation35,Citation69
Interstitial lung disease (ILD) is a rare and potentially life-threatening side effect of gefitinib, which has been reported in some studies. The incidence of gefitinib-induced ILD is consistently higher in Japan (1.6%–3.5%) as compared with other parts of the world (0.3%), although the reason for this geographic difference is unclear.Citation76,Citation77 A retrospective analysis of 112 patients with NSCLC treated with gefitinib found preexisting pulmonary fibrosis to be an important risk factor for developing fatal ILD.Citation76 Another retrospective analysis of 1,976 Japanese patients with NSCLC treated with gefitinib showed positive smoking history, male gender and the coincidence of interstitial pneumonia to be significantly associated with gefitinib-induced ILD.Citation77
The studies reported in this article have shown evidence of tolerable toxicity profile for gefitinib (in we report the toxicity data of the three largest studies: ISEL, INTEREST and IPASS trials).Citation28,Citation35,Citation69
In the ISEL trial, the most common adverse events in the gefitinib group were of grade 1–2, whereas those of grade 3–4 were similar for gefitinib and placebo (30% versus 27%), with the same rate of ILD being just 1%.Citation28 Only 5% of patients treated with gefitinib experienced adverse events leading to withdrawal.
The INTEREST trial showed serious adverse events in 4% of patients receiving gefitinib and 18% receiving docetaxel; this led to a lower rate of drug discontinuation for gefitinib (4% versus 11%) and a lower rate of adverse events leading to death (1% versus 2%).Citation35 The most common toxicities seen in the docetaxel group were hematological events: neutropenia (in 73.7% of cases with 10.1% of febrile neutropenia); asthenia (46.7%); alopecia (35.5%); neurotoxicity (23.9%); and fluid retention (15.7%). Skin rash and diarrhea were the main adverse effects seen in the gefitinib group which occurred in 49.4% and 35%, respectively. Interstitial lung disease was 1% in both arms.
In the similar Japanese study (V-15–32) adverse events were consistent with those previously described; 76.2% of patients receiving gefitinib experienced rash of all grades (with 0.4% of grade 3–4) and 51.6% experienced diarrhea of all grades (with 2% of grade 3–4).Citation36 ILD events were described in 5.7% of patients receiving gefitinib compared to 2.9% in the docetaxel group.
In the IPASS trial, the rate of grade 3–4 toxicities was 28.7% for gefitinib and 61% for chemotherapy.Citation69 This led to a lower rate of dose modification (16.1% versus 35.2%) and discontinuation of treatment (6.9% versus 13.6%). Treatment related deaths were 3.8% in the gefitinib arm and 2.7% in the chemotherapy arm; 16 (2.6%) patients treated with gefitinib developed ILD versus 8 (1.4%) treated with chemotherapy. The most common adverse events were skin rash (in 66.2% of patients) and diarrhea (46.6%) in the gefitinib group and neurotoxic effects (69.9%), neutropenia (67.1%) and alopecia (58.4%) in the carboplatin-paclitaxel group.
The favorable tolerance is particularly important for elderly patients or patients with comorbidities and poor PS. The INVITE trial showed a better safety profile for gefitinib as compared to vinorelbine in elderly patients.Citation45 Only 9.6% had a dose interruption instead of the 21.9% in the chemo-therapy group; with 21% of gefitinib patients that had a dose reduction versus 47.9% of vinorelbine arm. No toxicity related death occurred in patients treated with gefitinib. In the INSTEP trial, patients with poor performance status treated with gefitinib experienced diarrhea (51%) and skin rash (34%), but no toxicity lead to death.Citation46 The treatment discontinuation rate was low (14%).
Quality of life
The aim of every treatment for advanced NSCLC is purely palliative, set to achieve a prolongation in survival (whenever possible) and, above all, relief from disease symptoms without additive side effects. This rationale led investigators to consider quality of life (QoL) as an important parameter and endpoint in the trials.
The first phase II trials, such as IDEAL-1 and 2, showed that gefitinib administration improved the QoL of treated patients, as demonstrated by the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire used to assess it. In fact, there was a demonstration of symptom improvement rates of 40.3% and 43.1% in IDEAL-1 and 2, respectively.Citation26,Citation27 Symptom improvement was rapid and correlated with tumor response and survival. In IDEAL-2, at the recommended gefitinib dose of 250 mg/day, median overall survival times were 13.6 and 4.6 months for patients with and without symptom improvement, respectively, and 9.7 months for patients with symptom improvement without tumor response.Citation78 Among patients with stable disease or disease progression, those with symptom improvement had significantly better overall survival than those without improvement.
These data were confirmed by following trials, such as SIGN, INTEREST and V-15–32 studies.Citation34–Citation36 In the SIGN trial, in which symptom improvement was a primary endpoint, QoL and symptom improvement, evaluated by FACT-L, were greater with gefitinib than docetaxel (33.8% versus 26% and 36.8% versus 26%, respectively).Citation34 In the INTEREST trial more patients treated with gefitinib obtained a statistically significant higher rate of improvement in QoL. In fact, FACT-L total score was 25.1% versus 14.7% (P < 0.0001) and FACT-L TOI (Trial Outcome Index) 17.3% versus 10.3% (P = 0.0026), for gefitinib and docetaxel, respectively. Similar proportion of patients improved their lung cancer symptoms (evaluated by FACT-L Lung Cancer Subscale) with gefitinib and docetaxel (20.4% versus 16.8%, respectively).Citation35 Finally, the V-15–32 trial showed a statistically significant improvement rate in terms of QoL in patients treated with gefitinib when compared with docetaxel.Citation36,Citation79 FACT-L total score was 23.4% versus 13.9% (P = 0.023) and TOI was 20.5% versus 8.7% (P = 0.002) for gefitinib and docetaxel, respectively. There were no significant differences between treatments in LCS improvement rates (23% versus 20%, P = 0.562).
In the IPASS trial, QoL was one of the secondary endpoints. Citation69 Significantly more patients in the gefitinib group than those in the carboplatin-paclitaxel group had a clinically relevant improvement in QoL, assessed by scores on the FACT-L questionnaire (48% versus 40%; OR, 1.34, 95% CI, 1.06–1.69, P = 0.01) and by scores on the TOI (46.4% versus 32.8%; OR, 1.78, 95% CI, 1.40–2.26, P < 0.001). Rates of reduction in symptoms, assessed on the basis of the LCS scores, were similar between patients who received gefitinib and those who received carboplatin-paclitaxel (51.5% versus 48.5%; OR, 1.13, 95% CI, 0.90–1.42, P = 0.30).
An agent that might improve QoL and give relief from symptoms without bringing heavy toxicity is particularly relevant for poor PS or elderly patients. For this reason QoL and pulmonary symptom relief were important parameters in INVITE and INSTEP trials.Citation45,Citation46 In the INVITE trial overall QoL improvement and pulmonary symptom improvement (PSI) rates were 24.3% and 36.6% (for gefitinib) and 10.9% and 31.0% (for vinorelbine), respectively.Citation45 On the contrary, in the INSTEP trial no statistical difference was seen either for QoL improvement (21.1% versus 20%) and PSI (28.3% versus 28.3%) in patients treated with gefitinib or BSC.Citation46
Conclusions
Gefitinib is a well tolerated anticancer agent proven to be effective in both chemotherapy-naive and pretreated NSCLC patients. Due to its efficacy and favorable toxicity profile, it can be considered as a treatment option for those patients who cannot receive standard chemotherapy because of age, comorbidities or poor performance status.
As evidenced by data obtained in the subgroup analysis of the IPASS and First-SIGNAL trials and by the results of WJTOG3405 and NEJ002 studies, specific mutations of EGFR tyrosine kinase binding domain are related to an increased response rate and progression-free survival in patients treated with gefitinib compared to standard first-line chemotherapy treatment.
The discovery of these molecular predictors opens a new way in the management of advanced NSCLC, in which gefitinib is expected to have its larger impact. In clinical practice, given the low rate of EGFR mutations in Caucasian population (10%–15%), mutation analysis should be recommended in those patients who present at least one of the clinical or pathological features, which are related to a higher probability of mutation, such as female gender, non-smoking history, Asian ethnicity and adenocarcinoma histology. In patients harboring EGFR mutation, an up-front treatment with gefitinib should be considered.
EGFR-TKI resistance represents another major issue for research. The point mutation T790M of the EGFR gene and MET amplification are known to be involved in the majority of cases of acquired resistance to gefitinib. Open questions also remain for the potential use of gefitinib as maintenance therapy. As the trials undertaken so far were performed on unselected patients there is need to assess if gene mutated patients could derive a real benefit from a subsequent therapy with TKIs administered right after chemotherapy.
Disclosures
The authors declare no conflicts of interest.
References
- JemalASiegelRWardEHaoYXuJThunMJCancer Statistics, 2009CA Cancer J Clin200959422524919474385
- TravisWDBrambillaEMüller-HermelinkHKHarrisCCKleihuesPSobinLHPathology and genetics of tumors of the lung, pleura, thymus and heartWHO Classification of TumorsLyonIARC press2004
- Non Small Cell Lung Cancer Collaborative GroupChemotherapy in non-small cell lung cancer: a meta-analysis using update data on individual patients from 52 randomised clinical trialsBMJ199531170108999097580546
- NSCLC Meta-Analyses Collaborative GroupChemotherapy in addition to supportive care improves survival in advanced non-small-cell-lung cancer: a systematic review and meta-analysis of individual patients data from 16 randomized controlled trialsJ Clin Oncol200826284617462518678835
- WatersJSO’BrienMERThe case for the introduction of new chemotherapy agents in the treatment of advanced non-small cell lung cancer in the wake of the findings of The National Institute of Clinical Excellence (NICE)Br J Cancer200287548149012189541
- ScagliottiGVDe MarinisFRinaldiMPhase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancerJ Clin Oncol200220214285429112409326
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced Non-Small-Cell Lung CancerN Engl J Med20023462929811784875
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- ReckMVon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for non-squamous Non-Small-Cell Lung Cancer: AVAiLJ Clin Oncol200927141227123419188680
- ScagliottiGVParikhPvon PawelJPhase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemo-therapy- naive patients with advanced-stage non-small-cell lung cancerJ Clin Oncol200826213543355118506025
- GridelliCArdizzoniACiardielloFSecond-line treatment of advanced non-small cell lung cancerJ Thorac Oncol20083443044018379366
- AzzoliCGBakerSJrTeminSAmerican Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancerJ Clin Oncol200927366251626619917871
- BunnPAJrFranklinWEpidermal Grow Factor receptor expression, signal pathways, and inhibitors in non-small cell lung cancerSemin Oncol200229Suppl 14S38S44
- AstraZeneca, Macclesfield, UK. Data on file 2009.
- AlbanellJRRojoFAverbuchSPharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopatologic and molecular consequences of receptor inhibitionJ Clin Oncol200220111012411773160
- NakagawaKTamuraTNegoroSPhase I pharmacokinetics trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (‘Iressa’, ZD1839) in Japanese patients with solid malignant tumorsAnn Oncol200314692293012796031
- HauraEBSommersEBackerAPilot phase II study of preoperative gefitinib in early stage non small cell lung cancer with assessment of intra-tumor gefitinib levels and tumor target modulation [abstract]J Clin Oncol200725SupplS18
- McKillopDMcCormickADMillarACytochrome P450-dependent metabolism of gefitinibXenobiotica2005351395015788367
- WakelingAEGuySPWoodburnJRZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapyCancer Res200662205749575412384534
- Di GennaroEBarbarinoMBruzzeseFCritical role of both p27KIP1 and p21CIP1/WAF1 in the anti-proliferative effect of ZD1839 (Iressa), an epidermal growth factor receptor tyrosine kinase inhibitor, in head and neck squamous carcinoma cellJ Cell Physiol2003195113915012599217
- CappuzzoFMagriniECeresoliGLAkt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancerJ Natl Cancer Inst200496151133114115292385
- CiardielloFKurodaJPuthalakathHInhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitorClin Cancer Res2001751459146511350918
- BaselgaJRischinDRansonMPhase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor typesJ Clin Oncol200220214292430212409327
- RansonMHammondLAFerryDZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trialJ Clin Oncol20022092240225011980995
- HerbstRSMaddoxAMRothenbergMLSelective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trialJ Clin Oncol200220183815382512228201
- FukuokaMYanoSGiacconeGMulti-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancerJ Clin Oncol200321122237224612748244
- KrisMGNataleRBHerbstRSEfficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small-cell lung cancer. A randomized trialJAMA2003290162149215814570950
- ThatcherNChangAParikhPGefitinib plus best supportive care in previously treated patients with refractory advanced non-small- cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer)Lancet20053661527153716257339
- ChangAParikhPThongprasertSGefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: subset analysis from the ISEL studyJ Thorac Oncol20061884785517409969
- ShepherdFARodrigues PereiraJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- HirschFRVarella-GarciaMBunnPAJrMolecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancerJ Clin Oncol200624315034504217075123
- TsaoMSSakuradaACutzJCErlotinib in lung cancer – molecular and clinical predictors of outcomeN Engl J Med2005353213314416014883
- ZhuC-Qda Cunha SantosGDingKRole of KRAS and EGFR as biomarkers for response to erlotinib in National Cancer Institute of Canada Clinical Trials Group study BR-21J Clin Oncol200826264268427518626007
- CuferTVrdoljakEGaafarRPhase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIB or IV) non-small-cell lung cancerAnticancer Drugs200617440140916549997
- KimESHirschVMokTGefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trialLancet20083721809181819027483
- MaruyamaRNishiwakiYTamuraTPhase III study, V-15–32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancerJ Clin Oncol200826264244425218779611
- LeeDHParkKKimJHRandomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapyClin Cancer Res20101641307131420145166
- DouillardJYShepherdFAHirshVMolecular predictors of out-come with gefitinib and docetaxel in previously treated non-small- cell lung cancer: data from the randomized phase III INTEREST TrialJ Clin Oncol Epub 2009 Dec 28
- ShepherdFADouillardJFukuokaMComparison of gefitinib and docetaxel in patients with pretreated advanced non-small cell lung cancer (NSCLC): Meta-analysis from four clinical trials [abstract]J Clin Oncol200927SupplS15
- GiacconeGHerbstRSManegoldCGefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial – INTACT 1J Clin Oncol200422577778414990632
- HerbstRSGiacconeGSchillerJHGefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial – INTACT 2J Clin Oncol200422578579414990633
- CiardielloFCaputoRBiancoRAntitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitorClin Cancer Res2000652053206310815932
- HerbstRSPragerDHermannRTRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancerJ Clin Oncol20052325892899
- GatzemeierUPluzanskaASzczesnaAPhase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation TrialJ Clin Oncol200725121545155217442998
- CrinòLCappuzzoFZatloukalPGefitinib versus vinorelbine in chemotherapy-naïve elderly patients with advanced non-small-cell lung cancer (INVITE) a randomized, phase II studyJ Clin Oncol200826264253426018779612
- GossGFerryDLaurieSRandomized phase II study of gefitinib compared with placebo in chemotherapy-naïve patients with advanced non-small-cell lung cancer and poor performance statusJ Clin Oncol200927132253226019289623
- ArgirisAMittalNGefitinib as first-line, compassionate use therapy in patients with advanced non-small cell lung cancerLung Cancer200443331732215165090
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- PaezJGJannePALeeJCEGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapyScience20043041497150015118125
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A200410136133061331115329413
- HanSWKimTYHwangPGPredictive and prognostic impact of epidermal growth factor receptor mutation in non – small-cell lung cancer patients treated with gefitinibJ Clin Oncol200523112493250115710947
- MitsudomiTKosakaTEndohHMutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with post-operative recurrenceJ Clin Oncol200523112513252015738541
- TakanoTOheYSakamotoHEpidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancerJ Clin Oncol200523286829683715998907
- Cortes-FunesHGomezCRosellREpidermal growth factor receptor activating mutations in Spanish gefitinib-treated non-small-cell lung cancer patientsAnn Oncol20051671081108615851406
- TaronMIchinoseYRosellRActivating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemo-refractory lung adenocarcinomasClin Cancer Res200511165878588516115929
- AsahinaHYamazakiKKinoshitaIA phase II trial of gefitinib as first-line therapy for advanced non-small-cell lung cancer with epidermal growth factor receptor mutationsBr J Cancer2006958998100417047648
- InoueASuzukiTFukuharaTProspective phase II study of gefitinib for chemotherapy naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutationsJ Clin Oncol200624213340334616785471
- YangCYuCShihJSpecific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapyJ Clin Oncol200826162745275318509184
- SequistLVMartinsRGSpigelDFirst-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutationsJ Clin Oncol200826152442244918458038
- InoueAKobayashiKUsuiKFirst-line Gefitinib for patients with advanced non-small-cell lung cancer harbouring epidermal growth factor receptor mutations without indication for chemotherapyJ Clin Oncol20092791394140019224850
- SutaniANagaiYUdagawaKGefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clampBr J Cancer200695111483148917106442
- YoshidaKYatabeYParkJYProspective validation of prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancerJ Thorac Oncol200721222817410005
- SunagaNTomizawaYYanagitaniNPhase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapyLung Cancer200756338338917368623
- TamuraKOkamotoIKashiiTMulticentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403)Br J Cancer200898590791418283321
- SugioKUramotoHOnitsukaTProspective phase II study of gefitinib in non-small cell lung cancer with epidermal growth factor receptor mutationsLung Cancer200964331431818992959
- CappuzzoFLigorioCJännePAProspective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non small-cell lung cancer: The ONCOBELL trialJ Clin Oncol200725162248225517538169
- WestHLFranklinWAMcCoyJGefitinib therapy in advanced bronchioloalveolar carcinoma: Southwest Oncology Group Study S0126J Clin Oncol200624121807181316622257
- LeeDHHanJYLeeHGGefitinib as a first-line therapy of advanced or metastatic adenocarcinoma of the lung in never-smokersClin Cancer Res20051183032303715837758
- MokTWuYLThongprasertSGefitinib or carboplatin/paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- LeeJSParkKKimSWA randomized phase III study of gefitinib (IRESSATM) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung [abstract]J Thor Oncol20094SupplPRS.4
- MitsudomiTMoritaSYatabeYGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harboring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trialLancet Oncol Epub 2009 Dec 21
- KobayashiKInoueAMaemondoMFirst-line gefitinib versus first-line chemotherapy by carboplatin (CBDCA) plus paclitaxel (TXL) in non-small cell lung cancer (NSCLC) patients (pts) with EGFR mutations: A phase III study (002) by North East Japan Gefitinib Study Group [abstract]J Clin Oncol200927SupplS15
- TakedaKHidaTSatoTRandomized phase III trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: results of a West Japan Thoracic Oncology Group Trial (WJTOG0203)J Clin Oncol Epub 2009 Dec 28
- KellyKChanskyKGasparLEPhase III trial of maintenance Gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023J Clin Oncol200826152450245618378568
- OchsJSRationale and clinical basis for combining gefitinib (IRESSA, ZD1839) with radiation therapy for solid tumorsInt J Radiat Oncol Biol Phys200458394194914967454
- TakanoTOheYKusumotoMRisk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinibLung Cancer20044519310415196739
- AndoMOkamotoIYamamotoNPredictive factors for interstitial lung Disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinibJ Clin Oncol200624162549255616735708
- CellaDHerbstRSLynchTJClinically meaningful improvement in symptoms and quality of life for patients with non-small-cell lung cancer receiving gefitinib in a randomized controlled trialJ Clin Oncol200523132946295415699477
- SekineIIchinoseYNishiwakiYQuality of life and disease-related symptoms in previously treated Japanese patients with non-small-cell lung cancer: results of a randomized phase III study (V-15–32) of gefitinib versus docetaxelAnn Oncol20092091483148819282468