Abstract
Orally available disease-modifying drugs for relapsing-remitting multiple sclerosis (MS) represent an unmet need for this chronic and debilitating disease. Among 5 currently investigated drugs at phase 3 clinical stage, promising efficacy data for fingolimod and oral cladribine have recently been published. However, benefits need to be weighed against the risks to define the role of these compounds within current treatment regimens. In this review, data on the efficacy of a promising compound, oral cladribine, are discussed and balanced with known and anticipated risks in a postmarketing era, and finally gives an outlook on the potential place of this drug in treatment algorithms for MS in the future.
Introduction
Multiple sclerosis (MS) is a chronic and debilitating immune-mediated disease of the central nervous system (CNS). MS is not a homogeneous disease entity and therefore, as new therapeutics emerge, will require individual therapy regimens in the future. As a chronic, so far not curable disease, therapy is required for an indefinite – if not life long – period of time. In current concepts of MS treatment, drugs that influence immunological reactions are used to alter the course of this disorder and to finally reduce the grade of disability. MS, most relevant for the development of new treatment options, is a disease of low mortality in a young population and treatment primarily seems to be effective in the early inflammatory state of disease when patients suffer only from a low grade of impairment. In addition, no definite surrogate parameters exist to predict the individual course of disease in its early stages, and the individual grade of disability in the future cannot be anticipated with certainty. Thus, ideal treatment of MS would fulfill the following general criteria:
– maximal efficacy (ideal: cure)
– minimal adverse effects (ideal: none)
– maximal compliance (ideal: 100%)
– easy dosing regimes.
Currently available first-line therapeutics are characterized by their favorable and well-defined safety profile. Since the early 1990s, these disease-modifying drugs (DMD) have been implemented as treatments for MS. Ever since, interferon beta (IFNβ) or glatiramer acetate (GA) has become the standard of care for relapsing-remitting MS (RRMS).Citation1 Different formulations of IFNβ are available, including IFNβ-1a for weekly intramuscular (IM) or 3 times weekly subcutaneous (SC) administration, or IFNβ-1b SC every other day. GA is a synthetic oligopeptide and requires daily administration. In randomized controlled phase 3 trials, all of these agents showed to be superior to placebo regarding clinical end points.Citation2–Citation15 Recently published comparative trials did not provide evidence for superiority of one or the other first-line DMD.Citation16–Citation18 In addition, data on comparative paraclinicial efficacy is controversial,Citation12,Citation15,Citation19–Citation24 and a possible tendency towards a slight advantage on magnetic resonance imaging (MRI) criteria for IFNβ compared with GA goes along with slightly unfavorable tolerability rates (mainly higher rate of flulike symptoms). Thus, individual decision for one or the other agent is currently based on the preferred route of application (SC or IM) and the individual tolerability of the agent used. The main advantage of these first-line DMD agents for RRMS is their established positive safety profile. Main drawbacks of these agents are
– limited efficacy
– limited compliance and long-term acceptance by patients.
The latter mainly relates to their SC or IM mode of application. Local adverse effects at the sites of injection impair quality of life and long-term acceptance by patients.Citation25–Citation30
Promising new targets in MS therapy have been defined within the last decades and target-specific treatment options became available. Some of these treatment options have been tested in clinical trials, and have shown very promising results regarding efficacy. But, as outlined above, to play a role as first-line therapeutics in MS, these drugs need to display a reasonable safety profile in patients on long-term therapy or even life-long therapy. This matter of risk:benefit ratio became strikingly apparent when natalizumab was introduced in the therapy for MS. Natalizumab was the first drug of rational drug design approved for MS therapy, a humanized monoclonal IgG4-antibody, specifically designed to target a critical step of leukocyte migration into areas of inflammation within the CNS.Citation31,Citation32 Phase 3 clinical trials have clearly shown its advantages: high efficacy and high rates of compliance by intravenous (IV) monthly infusion.Citation33,Citation34 However, immediately after the completion of a phase 3 trial that led to its approval, safety issues, and most notably the risk of progressive multifocal leukoencephalopathy (PML), became apparent.Citation35–Citation37 Restriction of natalizumab to patients with highly active MS or patients, not responding to first-line treatment, was not congruent with the inclusion criteria of these studies but based on risk–benefit considerations. Just recently, new cases of PML occurring in patients receiving natalizumab monotherapy have been published and the long-term safety data might further limit its use in the future.Citation38–Citation40 Interestingly, these safety issues are most likely not only restricted to natalizumab but also are relevant for other currently investigated drugs of this second generation of target-specific immunosuppressive monoclonal antibodies. One lesson to be learned from natalizumab and other compounds such as rituximab, efalizumab, or alemtuzumab in drug development is the awareness that target specificity does not guarantee disease-specific efficacy. Although the mode of action of these drugs seems to be highly specific, their administration to young and otherwise healthy patients results in a severe alteration of immunocompetence going along with an increased risk of potential life-threatening infections (eg, risk of PML in natalizumab, efalizumab,Citation41 or rituximab)Citation42 or autoimmune (eg, risk of autoimmune thrombocytopenia and thyroid disease in alemtuzumab)Citation43 complications. Thus, the risk–benefit consideration is crucial and, although low, the risk of a potential life-threatening complication in MS population demands a critical patient selection and high standards of safety surveillance plans.
This is also an issue of concern in the development of new oral drugs for MS treatment. Easy dosing regimens and a convenient mode of administration are the most relevant advantages of this group of drugs. For these reasons, approval of an oral drug would be highly appreciated by patients improving quality of life and increasing adherence to therapy.Citation44,Citation45 Among 5 oral therapies currently in phase 3 clinical trials (fingolimod, laquinimod, fumeric acetate, teriflunomide, and oral cladribine; ), fingolimod and oral cladribine have already completed phase 3 clinical trials that were just recently published.Citation46–Citation49 In this review, data on efficacy of a promising compound, oral cladribine, are contrasted with known and potential risks. As the manufacturer already applied to the US Food and Drug administration and Europe, the Middle East and Africa for approval, risks and benefits of this drug need to be discussed to define the potential role within established treatment concepts.
Table 1 Oral drugs in clinical development for multiple sclerosis
Cladribine and its mode of action
Carson et alCitation50,Citation51 discovered that the lymphopenia observed in an inherited disorder of adenosine deaminase deficiency was caused by the accumulation of deoxyadenosine nucleotides within lymphocytes. Based on this observation, this group started to synthesize therapeutic purine nucleoside analogs, including cladribine (2-chlorodeoxyadenosine), to preferentially target lymphocytes.Citation50,Citation51 Cladribine is a prodrug requiring intracellular phosphorylation to become an active purine nucleoside analog. The prodrug is resistant to degradation by adenosine desaminase and is able to enter cells via purine nucleoside transporters.Citation52 Once within the cell, cladribine undergoes initial phosphorylation by deoxycytidine kinase (DCK) to finally become the active 2-chlorodeoxyadenosinetriposphate.Citation53 To inactivate cladribine-triphosphate nucleotides and to prevent intracellular accumulation, dephosphorylation by 5′-nucleotidase (5′-NTase) is required. Compared with other cell types, resting and activated lymphocytes have high levels of DCK but low levels of 5′-NTase. Thus, cladribine becomes particularly activated to its active form within lymphocytes making these cell types preferentially vulnerable to its effects.Citation54 The accumulation of cladribine nucleotides leads to breaks in DNA strands, interferes with DNA synthesis and repair, and ultimately results in a sustained reduction of lymphocyte counts.Citation55 Therefore, the main immunosuppressive effect of cladribine is mediated via immune cell depletion, of both the proliferating and the quiescent lymphocytes.Citation50 At doses used in clinical trials for MS, cladribine differentially affects CD4+, CD8+, and CD19+ lymphocyte subpopulations, possibly related to differences in the DCK/5′-NTase-ratio.Citation56,Citation57 CD4+ T cells are preferentially reduced compared with CD8+ T cells, resulting in a lower CD4/CD8 ratio, affecting both naive and memory T cells. Although CD19+ B-cell reduction occurs rapidly, recovery from the nadir is seen earlier and more pronounced compared with T cells.Citation56–Citation60 Recent evidence indicates that cladribine may also impede the influx of T cells into the CNS, and might also influence levels of soluble adhesion molecular levels such as sICAM or sE-Selectin.Citation61,Citation62 In addition, cladribine may exert immunomodulatory effects on proinflammatory cytokine profiles: Mean values of Interleukin-2 (IL-2) and soluble interleukin-2 receptor levels measured 12 months after cladribine treatment for chronic progressive MS were found to be lowered.Citation63 IL-8-levels were decreased in cerebrospinal fluid (CSF) of cladribine-treated RRMS patients, whereas CCL-5 levels were decreased both in CSF and serum.Citation64 These and other data suggests that cladribine not only has an leukocyte depleting effect, but also may exert a direct effect on effectors T-cell function.Citation59,Citation65
Pharmacokinetics of cladribine
Cladribine is rapidly absorbed and its oral bioavailability varies between 37% and 51%.Citation53 The terminal half-life varies from 5.7 to 19.7 hours. In CSF, the concentration has been reported to be approximately 25% of that in plasma in patients without CNS disease, indicating the ability of cladribine to cross the blood–brain barrier. The renal clearance of cladribine is about 51% of total clearance and 21%–35% of an IV-administered dose is excreted un-metabolized in the urine.Citation53
Efficacy of cladribine in clinical trials
Efficacy data with parenteral cladribine
Cladribine has been primarily used for reduction of aberrant lymphocyte populations in a variety of hematological disorders, and the parenteral formulation is treatment of choice for hairy cell leukemia.Citation66–Citation68 In addition, cladribine has been tested in autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematodus-associated glomerulonephritis.Citation69,Citation70 In MS, parenteral cladribine has been evaluated for relapsing or progressive forms. The MS-Scripps-trialCitation71 was a 2-year, placebo-controlled, double-blind, crossover study started in 1992 to evaluate cladribine IV for chronic progressive MS. In the first year, patients were given cladribine 0.1 mg/kg/day IV for 7 days as 4-monthly courses (total dose, 2.8 mg/kg or placebo). During the second year, patients of the first year’s placebo group were given 0.10, 0.05, and 0.05 mg/kg/day IV for 7 consecutive days in 3 successive monthly courses (total dose, 1.4 mg/kg). In the Scripps-C-trial,Citation72 an 18-month, placebo-controlled, double-blind study in the treatment of patients with RRMS, patients received either placebo or cladribine 0.07 mg/kg/day SC for 5 consecutive days as 6-monthly courses (total cumulative dose, 2.1 mg/kg). In the MS-001-trial, safety and efficacy were evaluated in patients with progressive MS, assigned to receive placebo or cladribine 0.07 mg/kg/day SC for 5 consecutive days for every 4 weeks for either 2 or 6 cycles (total dose, 0.7 or 2.1 mg/kg), followed by placebo for a total of 8 cycles. To summarize, efficacy data of these most relevant phase 2/3 clinical studies in 262 involved patients, parenteral cladribine showed positive results in patients with both relapsing and progressive forms of MS. A total of 183 patients received cumulative doses of 0.7–2.8 mg/kg of cladribine and individual results were suggestive not only for improvement of MRI-criteria (the number and volume of T1 gadolinium-enhancing lesions, the accumulation of T2 lesion volume), but also for neurological outcome measures (relapse rate and disability progression). Based on this treatment experience, a regimen for oral cladribine was developed and recently investigated in phase 3 clinical trial settings.Citation73
Efficacy data with oral cladribine
The clarity trial
Study design
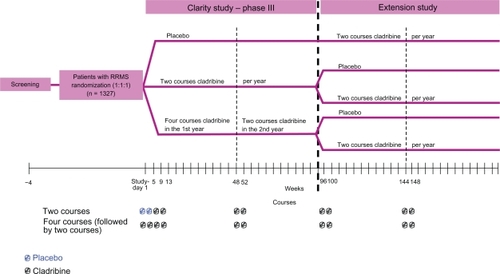
Results of the CLAdRIbine Tablets treating MS orallY (CLARITY) Trial, as one of 3 phase 3 clinical trials for oral drugs for MS to be completed, have been recently published.Citation46 CLARITY was performed in a randomized, double-blind, placebo-controlled, multicenter, 96-week setting with 3 parallel groups. Patients with RRMS,Citation74 aged 18–65, who had at least 1 relapse within 12 months before study entry, and a score of no more than 5.5 on the Kurtzke Expanded Disability Status Scale (EDSS)Citation75 were included. Patients with previous immunosuppressive treatment and patients with abnormal platelet, neutrophil, or leukocyte counts were excluded. In total, between 2005 and 2007, 1,326 patients were assigned in a 1:1:1 ratio to receive either 3.5 mg/kg or 5.25 mg/kg or matching placebo. The study drug was administered as short courses, each consisting of one or two 10-mg cladribine tablets or matching placebo given once daily for the first 4 or 5 days. In the 5.25-mg/kg group, patients received 4 courses of cladribine in the first 48-week treatment period. In the 5.25-mg/kg-group, patients received 2 courses of cladribine, followed by 2 courses of placebo. Four courses of placebo were administered to the placebo group. In all groups, courses were started at day 1, followed by courses at weeks 5, 9, and 13. In the second 48-week period, both cladribine groups received 2 courses of cladribine, and the placebo group received 2 courses of placebo, starting at weeks 48 and 52 (). After week 24, rescue therapy with IFNβ-1a SC was available for patients with more than 1 relapse or a sustained increase in the EDSS score.
The primary end point was the rate of relapse at 96 weeks. A relapse was defined as an increase of 2 points in at least 1 functional system of the EDSS or an increase of 1 point in at least 2 functional systems in the absence of fever, lasting for at least 24 hours and to have been preceded by at least 30 days of clinical stability or improvement. Secondary clinical outcome measures were the proportion of patients who were relapse-free, the time to sustained progression of disability (time to a sustained increase of at least 1 point in the EDSS score or an increase of at least 1.5 points if the baseline EDSS score was 0), the time to the first relapse, and the proportion of patients receiving rescue therapy with IFNβ-1a SC. Secondary MRI end points were the mean number of lesions per patient per scan at 96 weeks for gadolinium-enhancing T1-weighted lesions, active T2-weighted lesions, and combined unique lesions (new gadolinium-enhancing T1-weighted lesions or new nonenhancing or enlarging T2-weighted lesions).
Study results
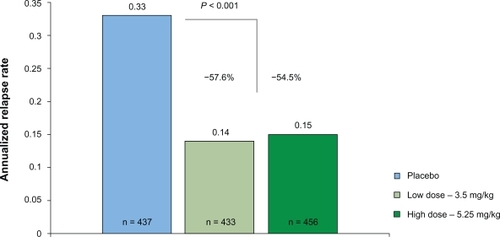
The annualized relapse rate at 96 weeks was significantly reduced in both treatment groups, as compared with placebo (0.14 in the cladribine 3.5-mg group and 0.15 in the cladribine 5.25-mg group vs 0.33 in the placebo group; ). Thus, relative reductions of the annualized relapse rate were 57.6% and 54.5%, respectively (P < 0.001). The proportion of patients who remained relapse-free at 96 weeks was significantly higher in both cladribine groups than in placebo (79.7% and 78.9% vs 60.9%; P < 0.001). There was a significant relative reduction in the risk of 3-month sustained progression of disability in both cladribine groups, as compared with placebo (33% reduction for cladribine 3.5 mg, 31% reduction for cladribine 5.25-mg group) with corresponding increase in the odds for remaining free of 3-month sustained disability progression. Additional clinical outcome measures, such as the time to the first relapse or the need for rescue therapy with IFNβ-1a SC were also in favor for both of the cladribine treatment groups.
Regarding MRI outcome measures, patients in the cladribine 3.5-mg group and cladribine 5.25-mg group had significant lower mean numbers of lesions per patient per scan than those in the placebo group for gadolinium-enhancing T1 lesions (0.12 and 0.11 vs 0.91 for placebo), active T2 lesions (0.38 and 0.33 vs 1.43 for placebo), and combined unique lesions (0.43 and 0.38 vs 1.72 for placebo).
Ongoing phase 3 clinical trials
Following completion of the CLARITY study, patients are given the opportunity to participate in the 96-week phase 3b extension study (ClinicalTrials.gov identifier: NCT00641537; ). Patients originally randomized to placebo will receive oral cladribine, whereas those originally randomized to cladribine will be rerandomized to either cladribine tablets or placebo. This study has been primarily designed to provide information on the longer term safety and tolerability of oral cladribine administered for an additional third and forth year in patients with RRMS, including clinical laboratory testing, electrocardiograms, and review of adverse events. Clinical efficacy measures are secondary end points to evaluate the sustained effects of treatment. Estimated primary completion date is September 2011.
The Oral Cladribine Added ON To Rebif New Formulation in Patients With Active Relapsing Disease (ONWARD)-trial (ClinicalTrials.gov identifier: NCT00436826) is a 96-week, randomized, double-blind, placebo-controlled, phase 2b trial in patients with active MS. This study evaluates the safety and tolerability of oral cladribine compared with placebo as an add-on therapy to IFNβ treatments in patients with active RRMS or secondary progressive MS with superimposed relapses. Clinical end points and MRI criteria are secondary outcome measures in this study. Estimated primary completion date is October 2013.
The Oral Cladribine in Early MS (ORACLE) – trial (ClinicalTrials.gov identifier: NCT00725985) is a 96-week randomized, double blind, 3-arm, placebo-controlled, multicenter, phase 3 trial to evaluate the safety and efficacy of oral cladribine vs placebo to prevent or delay conversion to definite MS (revised McDonald criteria)Citation76 in patients with a first clinical demyelinating event at high risk of converting to MS. Subjects must have a minimum of 2 clinically silent lesions on the screening MRI. Depending upon the clinical course of their MS, subjects will proceed from the initial treatment period to an open-label IFNβ-period or, if no progression to MS has been noted after the initial treatment period, to either open-label low-dose cladribine or no additional treatment. Estimated primary completion date is October 2012.
The safety and tolerability profile of cladribine
Parenteral cladribine has been in use for treatment of MS, hematological malignancies, and other indications for over 15 years, providing a comparable established safety profile for the drug.Citation73 However, safety data of oncology patients cannot directly be transferred to MS patients, as the former population is often exposed to additional cytotoxic chemotherapy, and both populations are likely to differ in immune competence. Still, from indications other than MS, myelosup-pression and infections have been noted. Escalating dose regimens have been associated with, though typically transient, toxicity to stem cells. In particular, patients with poor bone marrow reserve experienced marked thrombocytopenia with repeated dosing.Citation59,Citation63 Toxicity seems to be dose-dependant and administration of cladribine at a dosage above the recommended 0.1 mg/kg has been associated more frequently with myelosuppression, systemic infections, acute nephrotoxicity, and neuropathies. A significantly increased risk of secondary malignancies has not been noted in patients treated with cladribine for lymphoma.Citation68,Citation77,Citation78 For treatment of MS with parenteral cladribine, a combined analysis was performed using data from 268 patients enrolled in Scripps-studies. Adverse events occurring most frequently in all groups were upper respiratory tract infections (32% cladribine group vs 24% placebo), headaches (28% cladribine group vs 38% placebo), and injection-site reactions (24% cladribine group vs 25% placebo).Citation79 The incidence of serious adverse events was similar in patients receiving cladribine at doses of 0.7–2.1 mg/kg or placebo (11–15% vs 17%).Citation79 Although parenteral cladribine has shown to be teratogenic in mice and rabbits, there is no direct evidence for teratogenicity in humans.Citation59 Nevertheless, this potential side effect needs to be taken into account.
Most valid data derive from the recently published oral CLARITY trial in MS population.Citation46 As expected from parenteral trials, lymphocytopenia (mostly graded as mild or moderate) is more frequently seen among patients receiving cladribine compared with placebo. Severe neutropenia was reported in 3 patients (1 in the 3.5-mg group and 2 in the 5.25-mg group). In 1 patient of the latter group, severe thrombocytopenia and pancytopenia occurred, associated with an exacerbation of latent tuberculosis. Infections or infestations (graded mild or moderate in around 99% in all groups) were reported in 47.7% of the patients in the cladribine 3.5-mg group, 48.9% of those in the cladribine 5.25-mg group, and 42.5% of those in the placebo group. Herpes zoster infections occurred in 20 cladribine-treated patients (8 patients in the 3.5-mg group and 12 in the 5.25-mg group). All cases of herpes zoster were restricted to neighboring dermatomes, including 1 case of herpes zoster oticus. There were 3 uncomplicated cases of primary varicella, 1 in each study group. Adverse events leading to treatment discontinuation were seen in 3.5% of patients in the cladribine 3.5-mg group, 7.9% of those in the cladribine 5.25-mg group, and 2.1% of those in the placebo group. The incidence of serious adverse events was 8.4% in the cladribine 3.5-mg group, 9.0% in the cladribine 5.25-mg group, and 6.4% in the placebo group. There were 3 cases of malignancies in the cladribine 3.5-mg group (melanoma, pancreas carcinoma, and ovarian carcinoma). One case of cervical carcinoma in situ was also reported in the cladribine 5.25-mg group in a human papillomavirus type 16 positive individual. A choriocarcinoma was diagnosed in 1 patient in the cladribine 5.25-mg group approximately 9 months after completion of the study. There were 4 deaths during the study and 2 after study discontinuation, equally distributed across the 3 study groups. Causes of death were acute myocardial infarction and metastatic pancreatic carcinoma in the cladribine 3.5-mg group, drowning and cardiopulmonary arrest (considered secondary to exacerbation of latent tuberculosis) in the cladribine 5.25-mg group, and suicide and hemorrhagic stroke in the placebo group.
Perspective: potential of oral formulations in MS treatment with a focus on cladribine
The long-awaited publication of successful and well-conducted phase 3 clinical trials of oral drugs for RRMS is promising news for more than 2 million people worldwide suffering from this chronic, disabling disease, as well as for their treating physicians. Among 5 currently investigated drugs at phase 3 clinical stage, efficacy and safety data for fingolimod (FTY720 Research Evaluating Effects of Daily Oral therapy in Multiple Sclerosis [FREEDOMS] and Trial Assessing Injectable IFN vs FTY720 Oral in Relapsing Remitting Multiple Sclerosis [TRANSFORMS]) and cladribine (CLARITY) have been recently published.Citation46–Citation49 From a patient’s perspective, the approval of oral therapies would definitely be appreciated, reducing restrictions on lifestyle and hope for more efficient treatment. Compared with other oral drugs, it is the only therapy with potential of short-course dosing. From the physician’s perspective, oral medication may promise improvement of treatment adherence. However, regarding the potential of drugs like cladribine, 3 key questions still need to be answered.
First, is cladribine treatment superior to currently available drugs regarding efficacy? Only head-to-head trials can give firm conclusion on the efficacy of cladribine vs established injectable therapies. These trials still need to be undertaken. Mainly due to the differences in severity of disease, comparing data across clinical trials is extremely problematic. Currently running studies are designed to evaluate cladribine as add-on to IFNβ and for early MS, but only comparative head-to-head trials will answer this question.
Second, do benefits exceed the risks in a long-term perspective? We do not know by now, whether or not adverse effects seen in the recently published trials of cladribine and fingolimod are the only safety issues to consider. Occurrence of herpes virus infections, as seen among patients receiving cladribine or fingolimod, indicate an alteration of endogenous viral immunosurveillance by these promising orals. In addition, 3 cases of solid tissue cancers (pancreatic, ovarian, and melanoma) occurred among patients receiving cladribine. Keeping in mind the still unsolved and ongoing natalizumab-experience with occurrence of most relevant safety concerns in the postmarketing area,Citation35–Citation40 we cannot anticipate the long-term safety from the recently published phase 3 clinical trials. Particularly, with regard to rare opportunistic infections such as PML, only ongoing extension trials such as the CLARTIY-Extension trial and, in case of approval, critical patient selection and high standards of postmarketing safety surveillance programs will enable us to estimate the risk and prevent harm.
Third, what would be the potential role of cladribine within established treatment concepts of RRMS? Similar to natalizumab, not efficacy data from phase 3 clinical trials, but safety data from still running trials and of a potential post-marketing era, will finally answer this question. Cladribine, because of the known teratogenicity, should not be used in pregnancy, but also with caution in young female of potential child bearing capacity. In addition, as cladribine most likely alters viral immunosurveillance, it should not be used in combination with other immunosupressives such as natalizumab or mitoxantrone, and even pretreatment with these agents could possibly put patients at higher risks. As efficacy data are strong and oral drugs are highly appreciated by most of the patients, cladribine could potentially play a role in patients refractory to or patients not tolerating first-line treatment. In any case, individual decisions will be required and based on risk–benefit considerations in dialog with the well-informed patient, supported by high standards of postmarketing safety surveillance programs.
Disclosure
Drs Kieseier, Wiendl, and Hartung have received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Health Care, Biogen Idec, Merck Serono, Novartis, sanofi-aventis, and TEVA. Drs Stüve and Warnke have nothing to disclose.
References
- WiendlHToykaKVRieckmannPBasic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendationsJ Neurol2008255101449146319005625
- The IFNB Multiple Sclerosis Study GroupInterferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trialNeurology19934346556618469318
- PatyDWLiDKInterferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study GroupNeurology19934346626678469319
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study GroupRandomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosisLancet19983529139149815049820297
- PRISMS Study Group and the University of British Columbia MS/MRI Analysis GroupPRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MSNeurology200156121628163611425926
- LiDKPatyDWMagnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing-remitting multiple sclerosis. Prevention of relapses and disability by Interferon-beta1a subcutaneously in multiple sclerosisAnn Neurol199946219720610443885
- LiuCBlumhardtLDRandomised, double blind, placebo controlled study of interferon beta-1a in relapsing-remitting multiple sclerosis analysed by area under disability/time curvesJ Neurol Neurosurg Psychiatry199967445145610486390
- JohnsonKPBrooksBRCohenJACopolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trialNeurology1995457126812767617181
- ComiGFilipiMWolinskyJSfor the European/Canadian Glatiramer Acetate Study GroupEuropean/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging-measured disease activity and burden in patients with relapsing multiple sclerosisAnn Neurol20014929029711261502
- JacobsLDCookfairDLRudickRAIntramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG)Ann Neurol19963932852948602746
- SimonJHJacobsLDCampionMMagnetic resonance studies of intramuscular interferon beta-1a for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research GroupAnn Neurol19984379879450771
- HartungHPHigh-dose, high-frequency recombinant interferon beta-1a in the treatment of multiple sclerosisExpert Opin Pharmacother20091029130919236200
- PerumalJFilippiMFordCGlatiramer acetate therapy for multiple sclerosis: a reviewExpert Opin Drug Metab Toxicol200621019102917125414
- FrancisGBenefit-risk assessment of interferon-beta therapy for relapsing multiple sclerosisExpert Opin Drug Saf2004328930315268647
- DurelliLVerdunEBarberoPIndependent Comparison of Interferon (INCOMIN) Trial Study GroupEvery-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN)Lancet20023591453146011988242
- MikolDDBarkhofFChangPComparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trialLancet Neurol200871090391418789766
- CadavidDWolanskyLJSkurnickJEfficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME studyNeurology200972231976198319279320
- O’ConnorPFilippiMArnasonB250 mug or 500 mug interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre studyLancet Neurol200981088989719729344
- BarberoPBerguiMVersinoEINCOMIN Trial Study GroupEvery-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis (INCOMIN Trial) II: analysis of MRI responses to treatment and correlation with NabMult Scler200612727616459722
- SchwidSRThorpeJShariefMEVIDENCE (Evidence of Interferon Dose-Response: European North American Comparative Efficacy) Study Group; University of British Columbia MS/MRI Research Group. Enhanced benefit of increasing interferon beta-1a dose and frequency in relapsing multiple sclerosis: the EVIDENCE StudyArch Neurol20056278579215883267
- PanitchHGoodinDSFrancisGEVIDENCE Study GroupEVidence of Interferon Dose-response: European North American Comparative Efficacy; University of British Columbia MS/MRI Research Group. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE TrialNeurology2002591496150612451188
- PanitchHGoodinDFrancisGEVIDENCE (EVidence of Interferon Dose-response: European North American Comparative Efficacy) Study Group and the University of British Columbia MS/MRI Research Group. Benefits of high-dose, high-frequency interferon beta-1a in relapsing-remitting multiple sclerosis are sustained to 16 months: final comparative results of the EVIDENCE trialJ Neurol Sci2005239677416169561
- LublinFDWhen marketing and science intersect: do patients with MS benefit?Neurology2002591480148112451185
- KieburtzKMcDermottMNeeded in MS: Evidence, not EVIDENCENeurology2002591482148312451186
- CohenBARieckmannPEmerging oral therapies for multiple sclerosisInt J Clin Pract2007611922193017784852
- RíoJPorcelJTéllezNFactors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosisMult Scler20051130630915957512
- CoxDStoneJManaging self-injection difficulties in patients with relapsing-remitting multiple sclerosisJ Neurosci Nurs20063816717116817668
- CohenBAAdherence to disease-modifying therapy for multiple sclerosisInt J MS Care2006Suppl3237
- CramerJACuffelBJDivanVPatient satisfaction with an injection device for multiple sclerosis treatmentActa Neurol Scand200611315616216441244
- TremlettHLOgerJInterrupted therapy: stopping and switching of the beta-interferons prescribed for MSNeurology20036155155412939437
- RansohoffRMNatalizumab for multiple sclerosisN Engl J Med2007356252622262917582072
- RiceGPAHartungHCalabresiPAAnti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationaleNeurology20056481336134215851719
- PolmanCHO’ConnorPWHavrdovaEA randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosisN Engl J Med2006354989991016510744
- RudickRAStuartWHCalabresiPANatalizumab plus interferon beta-1a for relapsing multiple sclerosisN Engl J Med2006354991192316510745
- Kleinschmidt-DeMastersBKTylerKLProgressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosisN Engl J Med2005353436937415947079
- Langer-GouldAAtlasSWGreenAJBollenAWPelletierDProgressive multifocal leukoencephalopathy in a patient treated with natalizumabN Engl J Med2005353437538115947078
- Van AsscheGVan RanstMSciotRProgressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s diseaseN Engl J Med2005353436236815947080
- HartungHNew cases of progressive multifocal leukoencephalopathy after treatment with natalizumabLancet Neurol200981283119081511
- LindåHvon HeijneAMajorEOProgressive multifocal leukoencephalopathy after natalizumab monotherapyN Engl J Med20093611081108719741229
- WenningWHaghikiaALaubenbergerJTreatment of progressive multifocal leukoencephalopathy associated with natalizumabN Engl J Med20093611075108019741228
- MolloyESCalabreseLHTherapy: targeted but not trouble-free: efalizumab and PMLNat Rev Rheumatol20095841841919648939
- CarsonKREvensAMRicheyEAProgressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the research on adverse drug events and reports projectBlood20091134834484019264918
- ColesAJCompstonDASSelmajKWAlemtuzumab vs interferon beta-1a in early multiple sclerosisN Engl J Med2008359171786180118946064
- KieseierBCWiendlHOral disease-modifying treatments for multiple sclerosis: the story so farCNS Drugs200721648350217521228
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- GiovannoniGComiGCookSA placebo-controlled trial of oral cladribine for relapsing multiple sclerosisN Engl J Med2010362541642620089960
- KapposLRadueEO’ConnorPA placebo-controlled trial of oral fingolimod in relapsing multiple sclerosisN Engl J Med2010362538740120089952
- CohenJABarkhofFComiGOral fingolimod or intramuscular interferon for relapsing multiple sclerosisN Engl J Med2010362540241520089954
- CarrollWMOral therapy for multiple sclerosis – sea change or incremental step?N Engl J Med2010362545645820089958
- CarsonDAWassonDBTaetleRYuASpecific toxicity of 2 chlorodeoxyadenosine toward resting and proliferating human lymphocytesBlood1983627377436136305
- CarsonDAKayeJSeegmillerJELymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s)Proc Natl Acad Sci U S A19777456775681202960
- SipeJCCladribine tablets: a potential new short-course annual treatment for relapsing multiple sclerosisExpert Rev Neurother201010336537520187859
- LiliemarkJThe clinical pharmacokinetics of cladribineClin Pharmacokinet1997321201319068927
- KawasakiHCarreraCJPiroLDSavenAKippsTJCarsonDARelationship of deoxycytidine kinase and cytoplasmic 50-nucleotidase to the chemotherapeutic efficacy of 2-chlorodeoxyadenosineBlood1993815976018094016
- GriffigJKoobRBlakleyRLMechanisms of inhibition of DNA synthesis by 2-chlorodeoxyadenosine in human lymphoblastic cellsCancer Res198949692369282573423
- RieckmannPComiGCookSEffects of cladribine tablets on peripheral lymphocyte subtypes implicated in multiple sclerosis immunopathogenesis: surface marker analysis for a subset of patients from the 96 week, Phase III, double-blind, placebo-controlled CLARITY study. Meeting Abstract. Annual Congress of European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Duesseldorf, Germany, 2009 Sep 9–12Mult Scler200915S248S249
- SalvatCCurchodMLGuedjECellular expression profiling of genes involved in the cladribine metabolic pathway: insights into mechanism of action in multiple sclerosisProcedings and Abstracts of the Annual Congress of European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS)Dusseldorf, Germany2009 Sep 9–12 (Poster presentation P280).
- RiceGPFilippiMComiGCladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Cladribine MRI Study GroupNeurology2000541145115510720289
- HartungHAktasOKieseierBGiancarlo ComiGCDevelopment of oral cladribine for the treatment of multiple sclerosisJ Neurol2010257216317019921304
- Soelberg-SorensenPComiGCookSEffects of cladribine tablets on haematological profiles in patients with relapsing-remitting multiple sclerosis (RRMS) in the 96 week, phase III, double-blind, placebo-controlled CLARITY study. Meeting Abstract. Annual Congress of European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Dusseldorf, Germany, 2009 Sep 9–12Mult Scler200915S137
- KopadzeTDobertMLeussinkVIDehmelTKieseierBCCladribine impedes in vitro migration of mononuclear cells: a possible implication for treating multiple sclerosisEur J Neurol20091640941219175384
- Mitosek-SzewczykKStelmasiakZBartosik-PsujekHBelniakEImpact of cladribine on soluble adhesion molecules in multiple sclerosisActa Neurol Scand2010 [Epub ahead of print]
- JaniecKWajgtAKondera-AnaszZEffect of immunosuppressive cladribine treatment on serum leucocytes system in two-year clinical trial in patients with chronic progressive multiple sclerosisMed Sci Monit20017939811208501
- Bartosik-PsujekHBelniakEMitosek-SzewczykKDoboszBStelmasiakZInterleukin-8 and RANTES levels in patients with relapsing-remitting multiple sclerosis (RR-MS) treated with cladribineActa Neurol Scand200410939039215147461
- LaugelBChallierJSiegfriedCChvatchkoYWeissertRGalibertLCladribine exerts a modulatory effect of T cell activationMult Scler200814S52S53
- JuliussonGChristiansenIHansenMMOral cladribine as primary therapy for patients with B-cell chronic lymphocytic leukemiaJ Clin Oncol199614216021668683250
- OguraMMorishimaYKobayashiYDurable response but prolonged cytopenia after cladribine treatment in relapsed patients with indolent non-Hodgkin’s lymphomas: results of a Japanese phase II studyInt J Hematol20048026727715540903
- SavenAPiroLD2-Chlorodeoxyadenosine: a newer purine analog active in the treatment of indolent lymphoid malignanciesAnn Intern Med19941207847917908507
- SchirmerMMurEPfeifferKPThalerJKonwalinkaGThe safety profile of low-dose cladribine in refractory rheumatoid arthritis. A pilot trialScand J Rheumatol1997263763799385350
- DavisJCJrAustinHIIIBoumpasDA pilot study of 2-chloro-20-deoxyadenosine in the treatment of systemic lupus erythematosus-associated glomerulonephritisArthritis Rheum1998413353439485092
- BeutlerESipeJCRomineJSKoziolJAMcMillanRZyroffJThe treatment of chronic progressive multiple sclerosis with cladribineProc Natl Acad Sci U S A199693171617208643695
- RomineJSSipeJCKoziolJAZyroffJBeutlerEA double-blind, placebo-controlled, randomized trial of cladribine in relapsing-remitting multiple sclerosisProc Assoc Am Physicians199911135449893155
- LeistTPVermerschPThe potential role for cladribine in the treatment of multiple sclerosis: clinical experience and development of an oral tablet formulationCurr Med Res Opin2007232667267617880754
- McDonaldWICompstonAEdanGRecommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosisAnn Neurol20015012112711456302
- KurtzkeJFRating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS)Neurology198333144414526685237
- PolmanCHReingoldSCEdanGDiagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”Ann Neurol200558684084616283615
- ChesonBDVenaDABarrettJFreidlinBSecond malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemiasJ Clin Oncol1999172454246010561309
- KurzrockRStromSSEsteyESecond cancer risk in hairy cell leukemia: analysis of 350 patientsJ Clin Oncol199715180318109164188
- CookSA combined analysis of data from four randomized, double-blind, placebo-control led trials of parenteral cladribine and one open-label pilot study to assess the safety and tolerability profile of repeated periods of cladribine treatment in patients with progressive or relapsing multiple sclerosis (P02. 180)Neurology American Academy of Neurology MeetingChicago2008
- KapposLAntelJComiGOral fingolimod (FTY720) for relapsing multiple sclerosisN Engl J Med2006355111124114016971719
- O’ConnorPWLiDFreedmanMSA phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapsesNeurology200666689490016567708
- PolmanCBarkhofFSandberg-WollheimMTreatment with laquinimod reduces development of active MRI lesions in relapsing MSNeurology200564698799115781813
- KapposLGoldRMillerDHEfficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb studyLancet200837296481463147218970976