Abstract
Background
At this time, several antibiotics have been investigated as possibilities for aerosol administration, but local therapy has been found to be more efficient in several diseases.
Materials and methods
The drugs linezolid (Zyvox), vancomycin (Voncon), and daptomycin (Cubicin) were tested with three jet nebulizers with seven different residual cups and different loadings. Moreover, three ultrasound nebulizers were again tested with these drugs, with different loadings and mouthpiece attachments.
Results
When drugs are combined with particular cup designs, they significantly lower the droplet size to 1.60 and 1.80 μm, which represents the best combination of Zyvox and cup G and Cubicin and cup D, respectively. Cup design D is suggested as the most effective cup for lowering the droplet size (2.30 μm) when considering a higher loading level (8 mL).
Conclusion
Modification of current drugs from dry powder to solution is possible, and the residual cup design plays the most important role in droplet size production when the nebulization systems have the same properties.
Background
The respiratory system has been a target for local therapies for many years. Several drugs for systemic diseases are currently being investigated for aerosol administration.Citation1–Citation7 The respiratory system has several defense mechanisms that the aerosol droplets or dry powder have to bypass in order to deposit in the alveoli.Citation8 The beating cilia, mucus, and macrophages are the most important obstacles. The function of beating cilia, macrophages, and mucus production is modified according to underlying respiratory diseases. Chronic obstructive pulmonary disease, cystic fibrosis, and asthma disable the efficiency of the defense mechanisms. Moreover, the thick mucus disables the absorption of deposited drugs.Citation9
The lung surface is more than 100 mCitation2, and therefore the absorption of the aerosol drugs through the vessels of the alveoli is fast.Citation10 There are several factors that influence aerosol droplet production, and the most important ones can be summarized as follows: jet nebulizer flow rate,Citation11 design of the residual cup,Citation12 residual cup filling on initiation of nebulization,Citation13 residual cup loading,Citation14 charge of the drug molecules,Citation15 tapping of the residual cup during nebulization,Citation13,Citation16 chemical formula,Citation17,Citation18 viscosity,Citation11 surface tension, and concentration of drug solution.
Other factors affecting the droplet size after production can be summarized as the following: humidity within the airway environment, airway turbulence, architecture of aerosol droplet, and temperature within the airways.Citation17,Citation19–Citation21 The salts within the chemical structure of the drug formulation are responsible for the absorption of water from the environment and expansion of the molecule. The dry powder as a form of drug administration absorbs water from the environment according to the porosity of the particles, and further hydration may cause either “expansion” or “contraction” of the molecule. The shape of the dry powder particle plays an important role in the induction of cough. If one axis of a dry powder particle is more extensive, the mucosa of the respiratory system will be irritated and cough will be induced. However, the inhaled droplets/particles should not exceed 5 μm in size to reach the alveoli.Citation22
The major differences between aerosol administration and dry powder administration include the time of aerosol production. Inhaled insulin was one of the first drugs administered percutaneously and transformed so it could be administered as an aerosol.Citation23 There has been extensive investigation regarding the safety of aerosol administration of systemic therapies.Citation24,Citation25 The knowledge gained from inhaled insulin indicates that an underlying respiratory disease/exacerbation of this disease or respiratory tract infection changes the systemic absorption of an aerosol-administered drug.Citation23 In recent years, extensive investigation with inhaled antibiotics has been performed, and there are already several products on the market.Citation6,Citation7 Moreover; experimentation has been made to enhance aerosol drug delivery.Citation26 In our current research, we investigated whether daptomycin, vancomycin, and linezolid could be administered as an aerosol with jet nebulizers or ultrasound nebulizers, and which could be the optimal combination of residual cup design and residual cup loading.
Materials and methods
Drugs
The following drugs were used: vancomycin hydrochloride 500 mg per vial (Voncon; Hospira Inc., Lake Forest, IL, USA), daptomycin 500 mg per vial (Cubucin; Novartis International AG, Basel, Switzerland), and linezolid (Zyvox; Pfizer, Inc., New York, NY, USA), supplied as a ready-to-use sterile isotonic solution for intravenous infusion. Each milliliter contains 2 mg linezolid. Inactive ingredients are sodium citrate, citric acid, and dextrose in an aqueous vehicle for intravenous administration. The sodium (Na+) content is 0.38 mg/mL (5 mEq/300 mL bag, 3.3 mEq/200 mL bag, and 1.7 mEq/100 mL bag).
Aerosol production systems
Jet nebulizers and residual cups
Three jet nebulizers were chosen from our department for the experiment: MaxiNeb® (Flexicare Medical Ltd, Mountain Ash, UK; 6 L/minute and 35 psi), Sunmist® (DeVilbiss Health Care, Inc., Somerset, PA, USA; 5–7 L/minute and 35 psi), and Invacare® (DeVilbiss Health Care, Inc.; 4–8 L/minute and 36 psi; ).
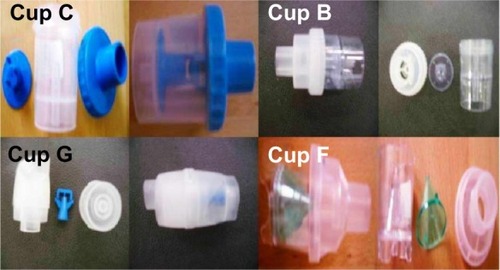
In total, seven residual cups were chosen for evaluation, four with a capacity of no more than 6 mL, and three with a capacity of no more than 10 mL. The designs for the large residual cups will be mentioned as A, D, and E (). The small residual cups will be mentioned as B, C, F, and G () The large residual cups were not used with a capacity of more than 8 mL, as explained in the Discussion. The residual cup loadings were 2, 4, 6, and 8 mL (8 mL only for large cups).
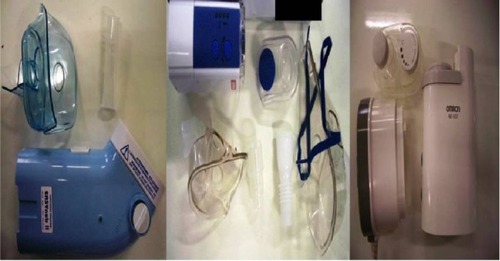
Ultrasound nebulizers
Three ultrasound nebulizers were chosen from the market (). The first was NE-U07 (Omron, Kyoto, Japan). Compact and weighing less than 350 g, it includes a 10 mL medication cup and generates uniform micro-sized vapor particles. The second ultrasound nebulizer was a portable EasyNeb® II (Flaem Nuova, Martino, Italy), with the following operating specifications; drug maximum capacity, 8 mL; frequency, 2.4 MHz; nebulization capacity (adjustable), 0–0.7 mL/minute approximately (tests performed with saline 0.9%); particle size, 2.13 μm mass median aerodynamic diameter (MMAD); sound level at 10 cm, 50 db; operating temperature, minimum of 10°C, maximum of 40°C; and air humidity, minimum of 10%, maximum of 95% relative humidity. The third ultrasound nebulizer was a portable GIMA, Gessate, Italy (Choice Smart Health Care Company Limited, Wan Chai, Hong Kong, No G2061259328002) with the following operating specifications: particle size, 3–5 μm; frequency, 2.5 MHz; medication cup capacity, 1–6 mL; sound level at 10 cm, <50 db; operating temperature, minimum of 10°C, maximum of 40°C; and air humidity, minimum of 10%, maximum of 95% relative humidity. The loadings were 2 and 4 mL, as this was the amount of the residual cup for each of the three ultrasound nebulizers.
Droplet measurement
The size distribution of the droplets and their mean diameter (d 32) were calculated using a Malvern Mastersizer 2000 apparatus (Malvern Instruments Ltd, Malvern, Worcestershire, UK; ), equipped with a Scirocco module (Malvern Instruments Ltd). The device has been modified to be able to spray the generated droplets directly perpendicular to the laser beam.Citation6,Citation26–Citation29 A refractive index of 1.33 has been used for the sprayed droplets. The measurements were made under ambient temperature.
Milling
The daptomycin and vancomycin powders were milled in a planetary ball mill (Pulverisette-5; Fritsch, Idar-Oberstein, Germany) equipped with Agate bowls (500 mL) and 6 balls (20 mm, 20 g) with a rotational speed of approximately 200 rpm, which results in an acceleration of about 7.5 ×g. We initiated our milling at 120 minutes and acquired a MMAD of 5 μm or less (measured with the Mastersizer 2000). After milling, we collected powder of the same weight from each drug and diluted it with 2 mL 0.9% NaCl in an effort to simulate a future method/compound of administration.
Statistical analysis
Data (MMAD) were treated using analysis of variance (ANOVA) three times, depending on the fixed factors and their particular levels employed each time. A four-way ANOVA included three Gram-positive antibiotics (Zyvox, Voncon, Cubicin), three nebulizers (Invacare, Sunmist, MaxiNeb), and seven residual cups (A–G) at three loading levels (2, 4, and 6 mL). A three-way ANOVA included the same drugs and nebulizers and those cups (A, D, E) that could receive a higher load of 8 mL. A four-way ANOVA was finally resumed concerning the same drugs but three unique ultrasound devices (EasyNeb® II, Gima, NE-U07; ) adapted with two different mouthpieces (inlets 1 and 2) and at two loading levels (2 and 4 mL).
Statistically significant factors were checked for particular differences between level and interaction means by comparing the 95% confidence intervals of means. Means whose intervals do not overlap differ significantly. The probability of 0.05 was chosen as the reference level of statistical significance.
Normality and homogeneity of the ANOVA residuals were tested in parallel.
Thus, the basic differences among the three ANOVA schemes are the inclusion of residual cups, the 8 mL loading, and the mouthpiece effect on ultrasound nebulizers.
Results
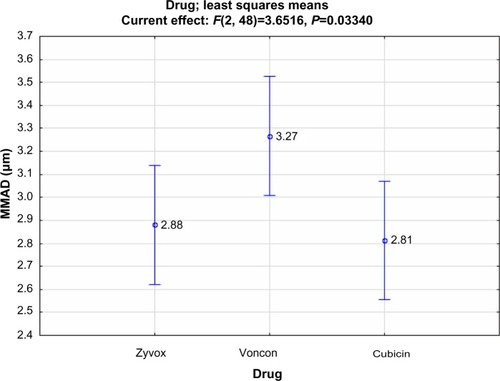
Regarding the inclusion of residual cups, ANOVA results revealed statistically significant effects for drugs (F=3.65; P=0.033), residual cups (F=5.442; P=0.0002), and the interaction term drug × residual cup (F=4.045; P=0.0002). Judging from the 95% confidence intervals of the drug means, it appears that both antibiotics, Zyvox (2.88 μm) and Cubicin (2.82 μm), provide a smaller droplet size than that of Voncon (3.27 μm).
Cup designs D (2.67 μm), A (2.40 μm), and G (2.63 μm) reduce the droplet size more than the other cups (), although the confidence intervals do not differentiate their effects clearly. These effects, however, are better clarified when the interactive results are shown (). In fact, cup designs D (1.80 μm) and G (1.60 μm) perform best in droplet reduction when combined with Cubicin and Zyvox administration, respectively.
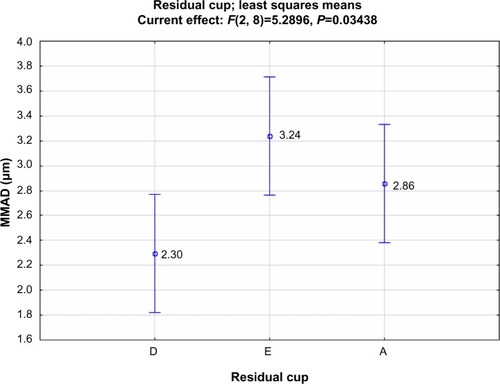
At higher load (8 mL), ANOVA’s statistically significant differences are found only among residual cups (F=5.290; P=0.034), with cup D appearing the most effective (2.30 μm; ).
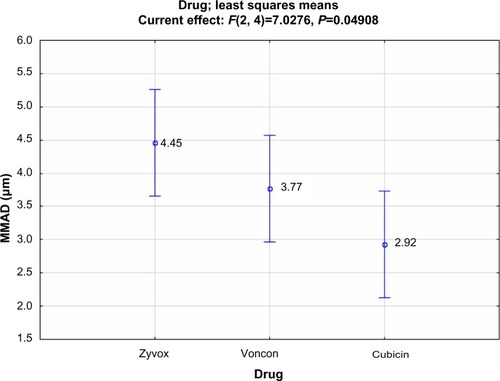
The mouthpiece does not influence the performance of the device, neither the loads nor the ultrasound nebulizers. Only the antibiotics perform slightly differently (F=7.028; P=0.049) because of the smaller droplet size (2.92 μm) produced by Cubicin (), which gives a result similar to that of .
To summarize, Gram-positive antibiotics do not lower the droplet size as effectively as other drugs do, even if they are of different natures in terms of synthesis.Citation6,Citation26 Nevertheless, when drugs are combined with particular cup designs, they significantly lower the droplet size down to 1.60 and 1.80 μm, which represent the best combination between Zyvox and cup G, and Cubicin and cup D, respectively.
Cup design D is suggested as the most effective cup for lowering the droplet size (2.30 μm) when considering a higher loading level (8 mL).
Discussion
The major findings of our study indicate that cup designs D (2.67 μm), A (2.40 μm), and G (2.63 μm) reduce the droplets more than the other cups. In fact, cup designs D (1.80 μm) and G (1.60 μm) perform best in droplet reduction when combined with Cubicin and Zyvox administration, respectively.
At higher load (8 mL), ANOVA’s statistically significant differences are found only among residual cups (F=5.290; P=0.034), with cup D appearing to be the most effective (2.30 μm).
The mouthpiece does not influence the performance of the device, neither the loads nor the ultrasound nebulizers. Only the antibiotics perform slightly differently (F=7.028; P=0.049) because of the smaller droplet size (2.92 μm) produced by Cubicin (), which gives a similar result to that of . In conclusion, the produced droplet size depends again on particular cup designs (D and G, which are large cups). These larger cups significantly lower the droplet size down to 1.60 and 1.80 μm, which represent the best combination between Zyvox and cup G, and Cubicin and cup D, respectively. Moreover, the composition of the formulation plays a crucial role. Therefore, several more experiments with different 0.9% NaCl residual cup fillings will probably reduce droplet size even more.
Major limitations of our study include, first, the fact that we did not extensively investigate the dilution of different drugs with powder, and we did not measure the time until the residual cup was empty.
Until now, several antibiotics have been investigated for aerosol administration (eg, meropenemCitation6), and several others are on the market with aerosol administration (eg, tobramycinCitation7). The optimal combination of nebulization system, residual cup, and loading have also been investigated.Citation11 It has also been investigated whether mouthpiece design influences droplet size after nebulization of the solution.Citation26
In our current research, we investigated daptomycin, linezolid, and vancomycin. There are no previous studies regarding linezolid or daptomycin; however, there has been experimentation with the aerosol administration of vancomycin.Citation30 In the study by Maiz et al,Citation30 aerosolized vancomycin was investigated for the first time as a treatment option for patients with cystic fibrosis. It was observed that this method of treatment was safe and well-tolerated and did not induce bacterial resistance. In a study by Park et al,Citation31 multifunctional controlled-release nanoparticles of vancomycin were produced as a dry powder.
Future usage of vancomycin in in vivo models will allow us to investigate the superiority of this formulation when compared with normal aerosol.Citation31 In the study by Nettey et al,Citation32 microspheres of albumin loaded with vancomycin were produced and demonstrated superiority when compared with the solution form. There are several successful examples in which systemic administration of a drug was converted to aerosol; this is the case with insulin and tobramycin.Citation23,Citation33 The lesson that was learned was that in the case of insulin, when exacerbation of an underlying disease occurred or when respiratory infection occurred, the dosage administration had to change and glucose measurements had to be more frequent. In fact, the pharmacokinetics of the drug changed because of the alteration of the absorption from the alveoli to the systemic circulation. In contrast, tobramycin was approved for children older than 6 years who had cystic fibrosis. Tobramycin was not approved for acute cystic fibrosis exacerbation or for non-cystic fibrosis patients.Citation34
The three antibiotics are effective against Gram-positive bacteria. Linezolid is a member of the oxazolidinone class of drugs. Linezolid has been observed to be active against most Gram-positive bacteria that cause disease, including streptococci, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci.Citation35 The main indications of linezolid until now have been infections of the skin and soft tissues and pneumonia (particularly hospital-acquired pneumonia); however, off-label uses for a variety of other infections are already becoming popular. Linezolid is a completely synthetic drug that does not occur in nature and was not developed by building on a naturally occurring skeleton. After several efforts, there is now a cost-effective method of production.Citation36
Vancomycin is a naturally occurring antibiotic made by the soil bacterium Actinobacteria species Amycolatopsis orientalis. The original indication for vancomycin was for the treatment of penicillin-resistant S. aureus, a use kept alive for many years by the fact that the compound had to be given intravenously. Vancomycin has been observed to be nephrotoxic and ototoxic; moreover, vancomycin-resistant organisms are becoming common. Therefore, vancomycin is being replaced in the clinical practice by newer antibiotics such as linezolid (Zyvox), daptomycin (Cubicin), and quinupristin/dalfopristin (Synercid).Citation37
Daptomycin is a lipopeptide antibiotic used in the treatment of systemic and life-threatening infections caused by Gram-positive organisms. It is useful in treating infections caused by multiresistant bacteria. Daptomycin is approved for use in adults in the US for skin and skin structure infections caused by Gram-positive infections, right-sided S. aureus endocarditis, and S. aureus bacteriemia. A major drawback that has to be considered if it is used as aerosol treatment is that it binds avidly to pulmonary surfactant, and therefore cannot be used in the treatment of pneumonia as it is in aerosol form.Citation38 However, there seems to be a difference in working daptomycin on hematogenous pneumonia.Citation39
If one day these products reach the production arena, time of administration is an essential factor for cost–time effectiveness. Time-consuming aerosol administration can cause adverse effects, such as cough.Citation8 Finally, cup design D is suggested as the most effective cup for lowering the droplet size (2.30 μm) when considering a higher loading level (8 mL). In our future experiments, we will investigate the effect of different mouthpiece designs with the best combination of residual cup design and residual cup filling for each drug. In addition, we will record the time of administration and droplet size production with the different mouthpiece designs.
Disclosure
The authors report no conflicts of interest in this work.
References
- Zarogoulidis P Chatzaki E Porpodis K Inhaled chemotherapy in lung cancer: future concept of nanomedicine Int J Nanomedicine 2012 7 1551 1572 22619512
- Zarogoulidis P Hohenforst-Schmidt W Darwiche K 2-diethylaminoethyl-dextran methyl methacrylate copolymer nonviral vector: still a long way toward the safety of aerosol gene therapy Gene Ther 2013 20 10 1022 1028 23719068
- Zarogoulidis P Darwiche K Hohenforst-Schmidt W Inhaled gene therapy in lung cancer: proof-of-concept for nano-oncology and nanobiotechnology in the management of lung cancer Future Oncol 2013 9 8 1171 1194 23902248
- Zarogoulidis P Eleftheriadou E Sapardanis I Feasibility and effectiveness of inhaled carboplatin in NSCLC patients Invest New Drugs 2012 30 4 1628 1640 21739158
- Patel RB Smaldone GC Cuccia AD Strachan P In vitro delivery of aerosolized treprostinil via modern mechanical ventilation J Aerosol Med Pulm Drug Deliv 2013 26 4 200 207 23668545
- Zarogoulidis P Kioumis I Ritzoulis C New insights in the production of aerosol antibiotics. Evaluation of the optimal aerosol production system for ampicillin-sulbactam, meropenem, ceftazidime, cefepime and piperacillin-tazobactam Int J Pharm 2013 455 1–2 182 188 23891745
- Zarogoulidis P Kioumis I Porpodis K Clinical experimentation with aerosol antibiotics: current and future methods of administration Drug Des Devel Ther 2013 7 1115 1134
- Zarogoulidis P Darwiche K Yarmus L Defense mechanisms of the respiratory system and aerosol production systems Med Chem 2014 10 2 123 136 24007475
- Miller JK Neubig R Clemons CB Nanoparticle deposition onto biofilms Ann Biomed Eng 2013 41 1 53 67 22878680
- Ma B Darquenne C Aerosol deposition characteristics in distal acinar airways under cyclic breathing conditions J Appl Physiol (1985) 2011 110 5 1271 1282 21330617
- Clay MM Pavia D Newman SP Clarke SW Factors influencing the size distribution of aerosols from jet nebulisers Thorax 1983 38 10 755 759 6648854
- Newman SP Pellow PG Clay MM Clarke SW Evaluation of jet nebulisers for use with gentamicin solution Thorax 1985 40 9 671 676 4060108
- Kendrick AH Smith EC Wilson RS Selecting and using nebuliser equipment Thorax 1997 52 Suppl 2 S92 S101 9155861
- Kendrick AH Smith EC Denyer J Nebulizers – fill volume, residual volume and matching of nebulizer to compressor Respir Med 1995 89 3 157 159 7746906
- Kwok PC Trietsch SJ Kumon M Chan HK Electrostatic charge characteristics of jet nebulized aerosols J Aerosol Med Pulm Drug Deliv 2010 23 3 149 159 20500092
- Hoffmann N Duft D Kiselev A Leisner T Contact freezing efficiency of mineral dust aerosols studied in an electrodynamic balance: quantitative size and temperature dependence for illite particles Faraday Discuss 2013 165 383 390 24601013
- Davis JM Davies IA Some physico-chemical characteristics of the antigens of Ehrlich ascites-tumour cells Biochem Soc Trans 1980 8 4 436 437 7450169
- Buttini F Miozzi M Balducci AG Differences in physical chemistry and dissolution rate of solid particle aerosols from solution pressurised inhalers Int J Pharm 2014 465 1–2 42 51 24491530
- Chen C Zhao B Cui W Dong L An N Ouyang X The effectiveness of an air cleaner in controlling droplet/aerosol particle dispersion emitted from a patient’s mouth in the indoor environment of dental clinics J R Soc Interface 2010 7 48 1105 1118 20031985
- Lelong N Vecellio L Sommer de Gelicourt Y Tanguy C Diot P Junqua-Moullet A Comparison of numerical simulations to experiments for atomization in a jet nebulizer PLoS ONE 2013 8 11 e78659 24244334
- Wu C Lee D Zachariah MR Aerosol-based self-assembly of nanoparticles into solid or hollow mesospheres Langmuir 2010 26 6 4327 4330 20041678
- Labiris NR Dolovich MB Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications Br J Clin Pharmacol 2003 56 6 588 599 14616418
- Zarogoulidis P Papanas N Kouliatsis G Spyratos D Zarogoulidis K Maltezos E Inhaled insulin: too soon to be forgotten? J Aerosol Med Pulm Drug Deliv 2011 24 5 213 223 21689020
- Darwiche K Zarogoulidis P Karamanos NK Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: a future dilemma for micro-oncology Future Oncol 2013 9 4 505 525 23560374
- Zarogoulidis P Giraleli C Karamanos NK Inhaled chemotherapy in lung cancer: safety concerns of nanocomplexes delivered Ther Deliv 2012 3 9 1021 1023 23035587
- Zarogoulidis P Petridis D Ritzoulis C Internal mouthpiece designs as a future perspective for enhanced aerosol deposition. Comparative results for aerosol chemotherapy and aerosol antibiotics Int J Pharm 2013 456 2 325 331 24035789
- Zarogoulidis P Darwiche K Huang H Time recall; future concept of chronomodulating chemotherapy for cancer Curr Pharm Biotechnol 2013 14 6 632 642 24180308
- Zaric B Stojsic V Tepavac A Adjuvant chemotherapy and radiotherapy in the treatment of non-small cell lung cancer (NSCLC) J Thorac Dis 2013 5 Suppl 4 S371 S377 24102009
- Boukovinas I Tsakiridis K Zarogoulidis P Neo-adjuvant chemotherapy in early stage non-small cell lung cancer J Thorac Dis 2013 5 Suppl 4 S446 S448 24102019
- Maiz L Canton R Mir N Baquero F Escobar H Aerosolized vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infection in cystic fibrosis Pediatr Pulmonol 1998 26 4 287 289 9811080
- Park CW Li X Vogt FG Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols Int J Pharm 2013 455 1–2 374 392 23820131
- Nettey H Haswani D Oettinger CW D’Souza MJ Formulation and testing of vancomycin loaded albumin microspheres prepared by spray-drying J Microencapsul 2006 23 6 632 642 17118879
- Zarogoulidis P Petridis D Ritzoulis C Further experimentation of inhaled; LANTUS, ACTRAPID and HUMULIN with todays’ production systems Int J Pharm 2013 458 1 39 47 24140545
- Prober CG Walson PD Jones J Committee on Infectious Diseases and Committee on Drugs Technical report: precautions regarding the use of aerosolized antibiotics Pediatrics 2000 106 6 E89 11099632
- Swaney SM Aoki H Ganoza MC Shinabarger DL The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria Antimicrob Agents Chemother 1998 42 12 3251 3255 9835522
- Brickner SJ Hutchinson DK Barbachyn MR Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections J Med Chem 1996 39 3 673 679 8576909
- Moellering RCJr Vancomycin: a 50-year reassessment Clin Infect Dis 2006 42 Suppl 1 S3 S4 16323117
- Baltz RH Daptomycin: mechanisms of action and resistance, and biosynthetic engineering Curr Opin Chem Biol 2009 13 2 144 151 19303806
- Henken S Bohling J Martens-Lobenhoffer J Efficacy profiles of daptomycin for treatment of invasive and noninvasive pulmonary infections with Streptococcus pneumoniae Antimicrob Agents Chemother 2010 54 2 707 717 19917756