Abstract
Background
The fundamental cause of overweight and obesity is consumption of calorie-dense foods. We have introduced a zero-calorie sweet sugar, d-psicose (d-allulose), a rare sugar that has been proven to have strong antihyperglycemic and antihyperlipidemic effects, and could be used as a replacement of natural sugar for the obese and diabetic subjects.
Aim
Above mentioned efficacy of d-psicose (d-allulose) has been confirmed in our previous studies on type 2 diabetes mellitus (T2DM) model Otsuka Long-Evans Tokushima Fatty (OLETF) rats with short-term treatment. In this study we investigated the long-term effect of d-psicose in preventing the commencement and progression of T2DM with the mechanism of preservation of pancreatic β-cells in OLETF rats.
Methods
Treated OLETF rats were fed 5% d-psicose dissolved in water and control rats only water. Nondiabetic control rats, Long-Evans Tokushima Otsuka (LETO), were taken as healthy control and fed water. To follow the progression of diabetes, periodic measurements of blood glucose, plasma insulin, and body weight changes were continued till sacrifice at 60 weeks. Periodic in vivo body fat mass was measured. On sacrifice, pancreas, liver, and abdominal adipose tissues were collected for various staining tests.
Results
d-Psicose prevented the commencement and progression of T2DM till 60 weeks through the maintenance of blood glucose levels, decrease in body weight gain, and the control of postprandial hyperglycemia, with decreased levels of HbA1c in comparison to nontreated control rats. This improvement in glycemic control was accompanied by the maintenance of plasma insulin levels and the preservation of pancreatic β-cells with the significant reduction in inflammatory markers. Body fat accumulation was significantly lower in the treatment group, with decreased infiltration of macrophages in the abdominal adipose tissue.
Conclusion
Our findings suggest that the rare sugar d-psicose could be beneficial for the prevention and control of obesity and hyperglycemia with the preservation of β-cells in the progression of T2DM.
Introduction
Overweight and obesity are not only the leading risk factors for global deaths but also the major contributor to other serious diseases worldwide, including heart disease, cancers, and type 2 diabetes. Mainly because of the uncontrolled intake of high-fat and high-cholesterol diet, the prevalence of overweight and rate of obesity drastically increase. Following this, type 2 diabetes mellitus (T2DM) inevitably arises, which warrants attentive consideration of the vital role of food for prevention and treatment.
The inextricably interlinked overweight, obesity, and T2DM, usually commencing from obesity, if not arrested, might be associated with life-threatening consequences of cardiac and metabolic diseases, high blood pressure, atherosclerosis, and T2DM.Citation1 Moreover, chronic intake of high calories forces the pancreatic β-cells to produce more insulin in order to cope with body demands to maintain glucose homeostasis, which, in turn, increases β-cell function and mass.Citation2 When the pancreas fails, insulin resistance becomes decompensated and hyperglycemia is detected. Among the leading mechanisms of insulin resistance, nonresolving low-grade inflammation that involves a number of inflammatory cytokines,Citation3 lipids and their metabolites, and reactive oxygen species (ROS) have been mentioned.Citation4 It is found that macrophages accumulate in inflammation tissue and discharge cytokinesCitation3 such as proinflammatory cytokines, interleukin 6 (IL-6), interleukin (IL-1β), and tumor necrosis factor α (TNF-α). Obesity itself is also closely related to systemic adipose inflammation with aggregation of macrophages, and high levels of those cytokines from them aggravate altogether insulin resistance with the progression of T2DM.Citation4 Although regular exercise and low-calorie diet could improve hyperglycemia and associated complications related to T2DM, most patients need medication. Although some antihyperglycemic products can improve the interrelated complications, they cause weight gain and, in turn, be associated with high blood pressure and dyslipidemia. Pharmacological approaches such as sulfonylureas and metformin have not been able to adequately improve the consequences of insulin resistance so far.
Therefore, attention has been focused on the study of herbal medicines that may not only provide better protection with lesser side effects but also ameliorate the associated comorbidities with the prevention of β-cell failure.Citation5 Additionally, with the intense attention of researchers and the growing interest of the general public concerning the treatment and prevention of diseases, functional foods have been explored.Citation6 It has been shown that functional foods contribute to the improvement of overall health and reduce the occurrence of diseases.Citation7 Recently, in an attempt to produce functional foods aimed at low calorie and less sugar intake, various foods with d-psicose added as a substitute for sugar have been prepared and marketed locally as FOSHU (Food for Specific Health Use).
d-Psicose (d-allulose), one of the rare sugars, has been proven to have antihyperglycemic, antihyperlipidemic, and anti-inflammatory effects. Owing to less availability, the biological functions of d-psicose have not been sufficiently explored. However, the innovation of a unique production method developed by IzumoriCitation8 enabled a number of investigations. Studies have shown that d-psicose is effective experimentally against obesity and T2DM in normalCitation9,Citation10 and in T2DM rat models,Citation11–Citation13 and clinically in healthyCitation14 and borderline diabetic humans.Citation15 Thus, these studies reflect the commercial use of d-psicose as a sugar substitute against hyperglycemia and hyperlipidemia. The possible mechanisms of controlling high glucose levels, such as potency of absorption of d-psicose over d-glucose from the intestineCitation16 and inhibition of the activities of enzymes glucoamylase and maltase, have been investigated.Citation10 Our previous study showed that d-psicose could play a role in the metabolic regulation in T2DM OLETF (Otsuka Long-Evans Tokushima Fatty) rats through maintaining blood glucose levels and preventing fat accumulation in the abdomen.Citation12 The possible mechanisms to maintain hyperglycemia may be through the translocation of glucokinase activity, which has been shown to be lower in hyperglycemic or diabetic condition,Citation17 as well as through the protection of the pancreatic β-cells from injury caused by hyperglycemia.Citation13
d-Psicose has also been shown to inhibit hepatic fatty acid synthase as the mechanism of fat control, followed by decreased body weight gain, when compared to d-glucose.Citation18 In addition to the antihyperglycemic and antihyperlipidemic effects of d-psicose, its zero-calorie credit and 70% relative sweetnessCitation9 also attracted some of the food companies to produce various foodstuffs containing d-psicose. US Food and Drug Administration (FDA) recognized d-psicose as Generally Recognized as Safe (GRAS) and has permitted its use as a food ingredient, with GRN Number 400.Citation19
In our previous studies,Citation12,Citation13 we fed OLETF rats with d-psicose for 3 months till the age of 20 weeks, around when these rats usually develop mild hyperglycemia.Citation20 However, in this study we continued d-psicose feeding till the age of 60 weeks in order to study several issues such as whether d-psicose could maintain prolonged sustained normoglycemia; protect β-cells against severe damage observed at this stage; control the cytokines related to inflammation since amelioration of oxidative stress and modulation of proinflammatory parameters have been shown to be beneficial for T2DM patients.Citation21
Materials and methods
Animals, drugs, foods, and drinks
Both OLETF and LETO (Long-Evans Tokushima Otsuka) rats were bought from Otsuka Pharmaceutical Co. (Tokushima, Japan) at 4-week age and were allowed to adapt for 2 weeks. The animals were maintained at 23°C±2°C and 55%±5% humidity with 12-hour light/dark cycle. d-Psicose (d-allulose or d-ribo-2-hexulose; molecular formula, C6H12O6; molecular weight, 180.156) is a rare ketohexose, named “rare sugar,” supplied by Kagawa University Rare Sugar Research Center (Miki, Kagawa, Japan). Rats were fed MF rat chow (Oriental Yeast Company Limited, Tokyo, Japan). d-Psicose was dissolved in normal tap water, and the rats were allowed to drink freely.
Experimental protocol
Six-week-old OLETF rats were divided into 2 groups (n=10 each): OLETF psicose (O-P) and OLETF control (O-C). Psicose group was given 5% d-psicose; O-C and LETO were given water only. All rats were supplied MF food pellets and their respective drinks. Body weight was measured every week till 60 weeks. Food and drink intakes were measured for three consecutive days each week and the average rat/day intake was calculated. Periodic fasting and postprandial blood glucose levels were measured using a freestyle glucose meter (YSI 2300-STAT) from tip of the tail. Plasma was collected from the blood by centrifugation and was stored at −80°C until analysis. At the end of 60 weeks, the rats were fasted for 12 hours and then anaesthetized deeply. Then, the abdomen was opened, blood was withdrawn, and necessary organs were removed, weighed, and preserved as per measurements and staining procedures. Abdominal fat was collected from the epididymal, retroperitoneal, and mesenteric spaces and weighed.
Tests and measurements
Rats were fasted 12 hours for oral glucose tolerance test (OGTT), 0-minute sample was collected, and then each rat was fed 2 g/kg body weight glucose by gavage and blood was collected at 30, 60, 90, and 120 minutes after gavage. The frozen plasma was used to measure insulin and lipids (total cholesterol [TC], triglycerides [TG], and high- and low-density lipoproteins [HDL and LDL]). Serum levels of glutathione (GSH), IL-6, TNF-α, leptin, and adiponectin were measured using rat-specific enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc, Minneapolis, MN, USA) following manufacturer’s recommendations. For in vivo body composition measurement, bioimpedance spectroscopy (ImpediVet, ImpediMed Ltd., Brisbane, QLD, Australia) was used to estimate total fat mass (FM), fat-free body mass, and body mass index (BMI).Citation22
To evaluate insulin resistance severity, homeostasis model assessment (HOMA) was calculated.Citation23 The details of this calculation are mentioned in our previous study.Citation13 To measure the inflammatory profile, the pancreas and adipose tissues were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin (HE). Light microscopy was used to evaluate adipocyte histology, and ImageJ (NIH, Bethesda, MD, USA) software was used to count the pancreas islet number, but it was very difficult to identify clearly because of the disorganized and broken status of the islets of Langerhans. Hence, the islets were counted after insulin immunostaining. However, the islet cell mass was calculated by multiplying β-cell percentage by total pancreatic weight and the percentage by insulin-positive staining. For immunostaining of insulin and glucagon, the pancreas tissue was embedded in optimal cutting temperature compound and stained with respective antibodies (Funakoshi, Japan).
Statistics
All values presented are the mean ± standard deviation (SD). Significant difference among the groups was determined by one-way analysis of variance (ANOVA), and differences between groups were analyzed by the post hoc test Hoechberg’s for equal and Games-Howell for unequal variances by using the SPSS software (version 17.0, SPSS, Chicago, IL, USA). A P-value of <0.05 was taken as significant.
Results
Antihyperglycemic effect of d-psicose
Changes in blood glucose and serum insulin concentration: Fasting blood glucose levels increased gradually in the OLETF groups and were significantly higher all through the experimental period as compared with those in nondiabetic LETO group (). In the O-C group, the glucose levels started to increase slowly from 25 weeks and then sharply till 60 weeks, whereas in the O-P group the glucose levels started to increase slightly from 45 weeks and remained constant till 60 weeks (). In contrast, glucose levels in LETO group remained unchanged until 60 weeks. The rise of blood glucose levels between O-C and O-P groups was significant from 35 weeks (P<0.01) till sacrifice. Periodic fasting serum insulin levels increased gradually from week 10 in the O-C group, peaked at 30 weeks, and decreased thereafter, even decreasing to almost zero level at week 60 (). These increases from weeks 20 to 40 were significantly higher than those in both O-P and LETO groups. In contrast, plasma insulin levels in both O-P and LETO groups increased slightly from week 10 and then remained almost unchanged until week 60. Glucose levels from the oral glucose tolerance test were significantly higher in the O-C group in all time points at every week than those in the O-P group. And the levels in the LETO group were significantly lower than in the O-C group at every week and the O-P group at weeks 40 and 60 (). Initially, at week 10 blood glucose levels returned close to base line 120 minutes after gavage in all groups, whereas the return values in 120 minutes were much higher in both O-C and O-P groups at week 40 and markedly higher in the O-C group at week 60. At week 40, plasma insulin levels in the O-C group were significantly higher than in O-P group in all time points, including 0 minutes, whereas at week 60 the levels became significantly lower than those in the O-P group ().
Figure 1 Periodic changes in fasting blood glucose (A) and insulin (B) concentrations with long-term treatment with or without rare sugar d-psicose in rats till age 60 weeks.
Abbreviations: HbA1c, hemoglobin A1c; HOMA, homeostasis model assessment; LETO, Long-Evans Tokushima Otsuka; OGTT, oral glucose tolerance test; OLETF, Otsuka Long-Evans Tokushima Fatty; O-C, OLETF control; O-P, OLETF psicose.
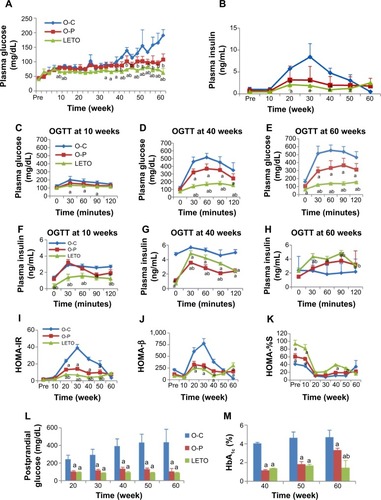
Both HOMA-IR and HOMA-β indexes were significantly higher in the O-C group than in both O-P and LETO groups, indicating the state of insulin resistance in O-C rats in which β-cells attempted to cope with increased insulin demand. In the O-P group the levels of HOMA-IR and HOMA-β were decreased and HOMA-%S (insulin sensitivity) was consistently increased ().
Postprandial blood glucose levels elevated significantly in the O-C group gradually at weeks 20 and 30 (P<0.01) and then markedly till week 60 (P<0.001) than in both O-P and LETO groups, whereas there was no difference between O-P and LETO groups (). The levels of HbA1c were significantly higher in the O-C group than in both O-P and LETO groups; however, the O-P group also showed a significantly higher value than that in LETO group at week 60, which was still significantly lower than that in O-C group ().
Antiobesity effect of d-psicose
Body weight, and food and drink consumption: Although the body weight of both the OLETF groups increased progressively with age, the body weight of all individual O-C rats was higher than that of all individual O-P rats till 33 weeks of age, and thereafter, 5 out of 9 (56%) O-C rats started to show a decline in body weight but none (0%) in the O-P group (). At 50 weeks, one more rat in the O-C group showed a decline in body weight () and the rest of the 3 rats continued to gain body weight slowly till 55 weeks and finally started to decline till 60 weeks. In contrast, with long-term d-psicose treatment, the O-P rats continued to gain body weight spontaneously with age like healthy rats till 60 weeks (). However, the average weight gain in the O-P group was significantly lower than that in the O-C group ().
Figure 2 Periodic changes in individual rat body weight of O-C (A) and O-P (B) groups with long-term treatment with or without rare sugar d-psicose in rats till age 60 weeks.
Abbreviations: LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty; O-C, OLETF control; O-P, OLETF psicose.
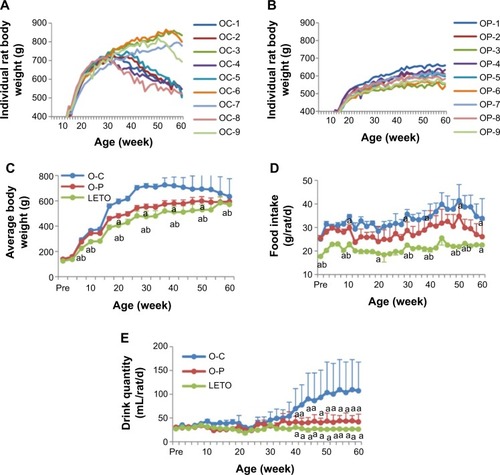
d-Psicose decreased food intake, with the average daily intake of the O-C, O-P, and LETO groups being 33.90±8.40, 26.11±7.02, and 22.63±0.34 g/rat/d, respectively (). Drink consumption was also significantly lower in the O-P group than in the O-C group from week 35 ().
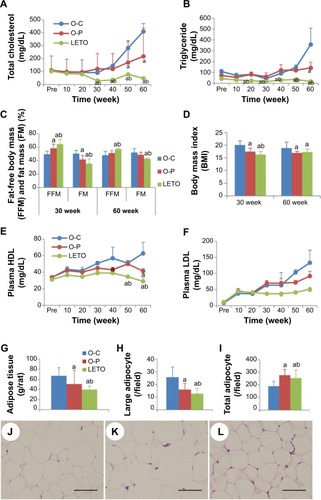
Plasma lipids, body composition, and FM: Fasting plasma concentration of TC in OLETF groups increased significantly from week 30 than that in the LETO group, and among the OLETF groups, TC level was significantly lower in the O-P group than in the O-C group at 50 and 60 weeks (). There was no significant difference in TG levels except at week 60 (). Percent FM was lower in the O-P group than in the O-C group, significantly at week 30 and nonsignificantly at week 60 (). However, FM was significantly lower with corresponding high fat-free body mass in LETO than in OLETF rats. Body mass index was significantly higher in the O-C group than in both O-P and LETO groups (). Plasma lipoproteins were significantly higher in OLETF groups than in LETO from 30 weeks onward, and significantly higher levels of HDL at weeks 40 and 60 and LDL at weeks 50 and 60 in the O-C than in the O-P () were observed. The amount of fat deposits (mesenteric + epididymal + retro-peritoneal) () and the number of large adipocytes () were significantly lower in the O-P group than in the O-C group, whereas the total number of adipocytes in the O-P group was significantly higher than that in the O-C group (). Representative H&E-stained photomicrographs of adipocytes are presented as O-C (), O-P (), and LETO ().
Figure 3 Periodic changes of blood lipid profile in rats of all groups with long-term treatment with or without rare sugar d-psicose till age 60 weeks.
Abbreviations: BMI, body mass index; FM, fat mass; FFM, fat-free mass; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty; O-C, OLETF control; O-P, OLETF psicose; HE, hematoxylin and eosin.
Anti-inflammatory and β-cell-preserving effects of d-psicose
In light microscopic findings, O-C rats showed striking hyperplastic changes in the islets of Langerhans with extensive fibrosis. Most of the islets were disorganized, fibrotic, separated into small clusters, resulting in multinodular islets which were separated by connective tissue bands. As a result, the number of islet cells was decreased (, adjacent table). Fatty degeneration was pronounced in the hypertrophied islets. These histological alterations were prominent and severe in the O-C group () in comparison to the O-P group (). In the O-C group, large or medium-size islets were hardly observed, while many were seen in both the O-P and the LETO groups. In the LETO group, these histological changes were not observed, except for some fatty degeneration (). To elucidate the islet architecture, better immunofluorescence studies using anti-insulin and antiglucagon antibodies were performed. The pattern of β-cells in the islets of LETO rats was normal, with β-cells (red color) located centrally and α-cells (green color) at the periphery (). However, the distribution was changed to a mixed pattern at week 60 in the O-C group (), where many of the insulin-positive cells lost their normal structure and were seen as a mixture of single or group of cells distributed all over the pancreas. This scattered distribution was rarely observed in the O-P group () and not at all in the LETO group. The replacement of insulin-containing granules in β-cells by fat droplets and the distorted or diminished boundary of islets were very prominent in the O-C group ().
Figure 4 Changes in light microscopic features of pancreas of rats of all groups at week 60 with long-term treatment with or without rare sugar d-psicose.
Abbreviations: GSH, glutathione; HPF, high power field; IL-6, interleukin 6; LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty; O-C, OLETF control; O-P, OLETF psicose; TNF, tumor necrotic factor; HE, hematoxylin and eosin.
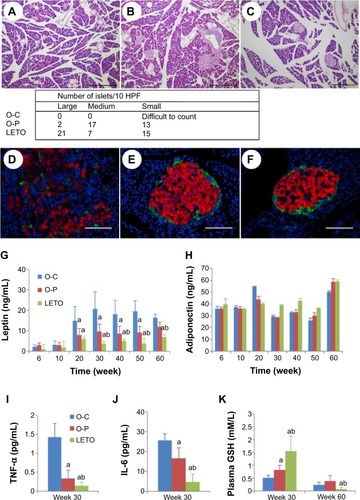
Plasma levels of leptin were elevated significantly in the O-C group than in both O-P and LETO groups from 20 weeks of age (), although there was no significant difference in plasma adiponectin levels among the groups (). As for the inflammatory cytokines, significantly high levels of both TNF-α and IL-6 were observed in the O-C group than in both O-P and LETO groups (). Plasma GSH was significantly high in the LETO group at 30 weeks of age, whereas these high levels decreased to trace amounts at 60 weeks (), although there was no significant difference among the groups at week 60.
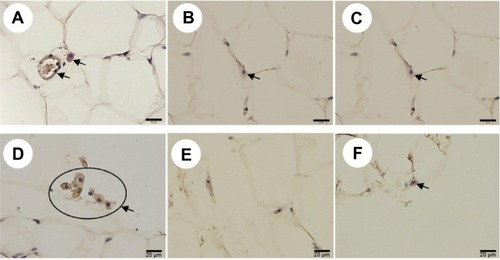
In relation to adipocyte inflammation, immunostaining of macrophage-expressed CD68 and F4/80 in adipose tissue was performed and an increased expression of both CD68 () and F4/80 () was found in the O-C group than in both O-P and LETO groups.
Figure 5 Immunohistochemical staining of CD68-expressing neutrophils (A–C) and F4/80-expressing macrophages (D–F) in adipose tissue of rats of all groups at week 60 with long-term treatment with or without rare sugar d-psicose.
Abbreviations: LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty; O-C, OLETF control; O-P, OLETF psicose.
Discussion
Our study distinctly demonstrated that long-term treatment with d-psicose until 60 weeks exerted beneficial effects against the progression of the sequences of T2DM and pancreatic β-cell damage in OLETF rats. Onset of T2DM occurs on failure of β-cells, and among the proposed mechanisms of β-cell failure, glucotoxicity, lipotoxicity, oxidative stress, ER stress, and amyloid depositionCitation24 were mentioned, and almost all these factors may induce islet inflammation.
Severe damage with marked β-cell loss in the nontreated O-C animals was observed with H&E staining of the pancreas, and the damage was consistent with the high levels of both serum IL-6 and TNF-α. The levels of cytokines usually rise in insulin-resistant states, such as obesity,Citation25 impaired glucose tolerance,Citation26 and T2DM.Citation27 The most important source of both pro- (IL-6, TNF-α) and anti-inflammatory (adiponectin) cytokines is visceral adipose tissue,Citation28 the aggregation of which was significantly reduced in the animals treated with d-psicose (). We think the inflammatory damage of β-cells was related to increased levels of proinflammatory cytokines in the control animals, which, in turn, contributed to the development of insulin resistance and T2DM. As shown in , the destructive damage of the islets was observed in OLETF control rats but not in d-psicose-treated OLETF rats at 60 weeks of age. Of course, no pathological damage was observed at 10 weeks of age in all groups, since diabetic onset usually starts around 20 weeks of age. Therefore, it is assumed that the improvement of insulin resistance and metabolic syndrome by d-psicose treatment was facilitated through the control of proinflammatory cytokines after the onset of diabetes in OLETF rats. A study has shown that obesity and obesity-related metabolic disturbances were entangled with elevated low-grade noninfectious inflammation and oxidative stress.Citation29 This obesity-associated inflammation originates mainly in visceral white adipose tissue.Citation30 Also, proinflammatory cytokine IL-6 is released from inflamed adipocytes, which in turn activate macrophages to release another cytokine, TNF-α.Citation31 Our results demonstrated that d-psicose treatment exhibited significantly less macrophage infiltration in comparison to nontreated control rats (), which in turn resulted in decreased serum levels of both IL-6 and TNF-α (). Hence, we could conclude that the anti-inflammatory effect of d-psicose may indirectly suppress the release of proinflammatory cytokines.
Additionally, d-psicose also exerted its anti-inflammatory effects through the significant decrease in serum leptin levels and thus contributed to the improvement of insulin resistance, although serum adiponectin levels were not altered. Accumulation of macrophages in adipose tissue is closely correlated with weight gain and insulin resistance in both rodents and humans,Citation32 and reduction of macrophage content correlates with weight loss.Citation3 The restoration of body weight by d-psicose () might be considered to be due to the improvement of insulin resistance through the control of glucose. d-Psicose prevented excess fat accumulation, confirmed by decreased in vivo FM () as well as abdominal fat weight (), with the significant reduction in body mass index ().
Among the potential mechanisms of the toxic effects of glucose on β-cell function is chronic oxidative stress because glucose can generate ROS,Citation33 which has adverse effects on islet function.Citation23,Citation34 Moreover, pancreas contains trace amounts of antioxidant enzymes and thus β-cells become liable to be injured by oxidative stress. Elevation in GSH level and super oxide dismutase may compensate oxidative stress. Studies have shown d-psicose as a strong antioxidant through the scavenging of ROS and expression of antioxidant enzymes.Citation35–Citation37 d-Psicose, in the present study, significantly restored the antioxidant GSH (), consistent with an earlier report,Citation38 although we were unable to detect super oxide dismutase. Control of blood glucose level remains the gold standard in the prevention and management of diabetic complications.Citation39 Treatment of OLETF rats with d-psicose was found to control blood glucose levels uniquely till the end of 60 weeks (). The oral glucose tolerance test also revealed that the glucose tolerance was improved () with the significant restoration of insulin () secretion after the glucose load. Insulin resistance initially leads to hyperinsulinemia,Citation40 followed by hypoinsulinemia when β-cell volume is reduced through ROS-induced injury. Consistently, the present data showing initial hyperinsulinemia and hypoinsulinemia afterward suggest β-cell proliferation followed by damage in the control rats. d-Psicose treatment prevented β-cell injury through its antioxidative effects and thus improved glucose tolerance, consistent with Lee et al’s findings in OLETF rats.Citation41 Moreover, glycated hemoglobin A1c is considered another gold standard in monitoring glucose control in view of the distinct relation between glucose and A1c in both fasting and postprandial periods.Citation42,Citation43 d-Psicose was able to reduce HbA1c level () through maintaining normoglycemia during both fasting () and postprandial () periods. Moreover, postprandial hyperglycemia itself is hazardous for vascular diseases and thus considered as a predictive tool for myocardial infarction and mortality.Citation44 Vascular diseases are mentioned as crucial results of elevated lipoproteins which may also affect β-cell survival and function.Citation45 Our study confirmed the antihyperlipidemic effect of d-psicose with significant decrease in serum LDL (), although the protective HDL levels remained static all through (). Studies have also demonstrated that insulin resistance is a predisposing factor for dyslipidemiaCitation46 and that diabetic dyslipidemia is typically marked by elevated TC, TG, and LDL and decreased HDL, and these constitute an important risk factor for T2DM.Citation47 d-Psicose treatment also resulted in significant decreases in plasma TC, TG, and LDL levels when compared to those in control ().
The presented data strongly suggest the potential of d-psicose in the commencement of diabetes rather than being a “remedy” after diabetes is already established. Although we have not tested this part, further investigation remains on process to clarify this issue. However, our results demonstrated that prolonged treatment with the rare sugar d-psicose maintained blood glucose levels, lipid profile with the amelioration of oxidative stress, and proinflammatory cytokines in T2DM in OLETF rats.
Finally, in addition to making functional foods, d-psicose might be used as a combination drug, not only for antihyperglycemic effect but also to counter some side effects marked by the chronic use of antidiabetic agents.
Acknowledgments
We are very grateful to Professor Ken Izumori for supplying d-psicose from the Rare Sugar Research Center, Kagawa University. We thank Arif-Ul-Hasan for technical assistance and Teruyoshi Izumi for animal care.
Disclosure
The authors report no conflicts of interest in this work.
References
- LarssonBSvardsuddKWelinLWilhelmsenLBjorntorpPTibblinGAbdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913Br Med J (Clin Res Ed)19848814011404
- RahierJGuiotYGoebbelsRMSempouxCHenquinJCPancreatic β-cell mass in European subjects with type 2 diabetesDiabetes Obes Metab200810suppl 4324218834431
- KoenenTBStienstraRvan TitsLJHyperglycemia activates caspase-1 and TXNIP-mediated IL-1b transcription in human adipose tissueDiabetes20116051752421270263
- DandonaPAljadaABandyopadhyayAInflammation: the link between insulin resistance, obesity and diabetesTrends Immunol2004254714698276
- OhkitaMKisoYMatsumuraYPharmacology in health foods: improvement of vascular endothelial function by French maritime pine bark extract (Flavangenol)J Pharmacol Sci201111546146521436602
- DuHvan derA DLBoshuizenHCDietary fiber and subsequent changes in body weight and weight circumference in European men and womenAm J Clin Nutr20099132933620016015
- BellSJGoodrickGKA functional food product for the management of weightCrit Rev Food Sci Nutr20024216317811934132
- IzumoriKIzumoring: a strategy for bioproduction of all hexosesJ Biotechnol200612471772216716430
- MatsuoTSuzukiHHashiguchiMIzumoriKd-Psicose is a rare sugar that provides no energy to growing ratsJ Nutr Sci Vitaminol200248778012026195
- MatsuoTIzumoriKd-psicose inhibits intestinal α-glucosidase and suppresses glycemic response after carbohydrate ingestion in ratsTech Bull Fac Agric Kag Univ2006582732
- BaekSHParkSJLeeHGd-psicose, a sweet monosaccharide, ameliorates hyperglycemia, and dyslipidemia in C57BL/6J db/db miceJ Food Sci201075H49H5320492234
- HossainMAKitagakiSNakanoDRare sugar d-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka long-evans Tokushima fatty (OLETF) ratsBiochem Biophys Res Commun201140571221187061
- HossainAYamaguchiFMatsunagaTRare sugar d-psicose protects pancreas β-islets and thus improves insulin resistance in OLETF ratsBiochem Biophys Res Commun201242571772322877751
- IidaTKishimotoYYoshikawaYAcute d-psicose administration decreases the glycemic responses to an oral maltodextrin tolerance test in normal adultsJ Nutr Sci Vitaminol20085451151419155592
- HayashiNIidaTYamadaTStudy on the postprandial blood glucose suppression effect of d-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjectsBiosci Biotechnol Biochem20107451051920208358
- HishiikeTOgawaMHayakawaSTransepithelial transports of rare sugar d-psicose in human intestineJ Agric Food Chem2013617381738623844903
- ToyodaYItoYTanigawaKMiwaIImpairment of glucokinase translocation in cultured hepatocytes from OLETF and GK rats, animal models of type 2 diabetesArch Histol Cytol20006324324810989935
- MatsuoTBabaYHashiguchiMTakeshitaKIzumoriKSuzukiHDietary d-psicose, a C-3 epimer of d-fructose, suppresses the activity of hepatic lipogenic enzymes in ratsAsia Pac J Clin Nutr20011023323711708315
- MuWZhangWFengYBoJZhouLRecent advances on applications and biotechnological production of d-psicoseAppl Microbiol Biotechnol2012941461146722569636
- KawanoKHirashimaTMoriSSaitohYKurosumiMNatoriTSpontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strainDiabetes199241142214281397718
- ButlerAEJansonJBonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetesDiabetes200310211012502499
- MoonJRSmithAETobkinSETotal body water changes after an exercise intervention tracked using bioimpedance spectroscopy: a deuterium oxide comparisonClin Nutr20092851652519500888
- MatthewsDRHoskerJPRudenskiASNaylorBATreacherDFTurnerRCHomeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in manDiabetologia1985284124193899825
- RobertsonRPHarmonJTranPOPoitoutVBeta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetesDiabetes200453suppl 1S119S12414749276
- Mohamed-AliVGoodrickSRaweshASubcutaneous adipose tissue releases interleukin-6 but not tumor necrosis factor-alpha, in vivoJ Clin Endocrinol Metab199782419642009398739
- MullerSMartinSKoenigWImpaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptorsDiabetologia20024580581212107724
- ZhangYProencaRMaffeiMBaroneMLeopoldLFriedmanJMPositional cloning of the mouse obese gene and its human homologueNature19943724254327984236
- MollerDEBergerJPRole of PPARs in the regulation of obesity-related insulin sensitivity and inflammationInt J Obes Relat Metab Disord200327S17S2114704738
- HummastiSHotamisligilGSEndoplasmic reticulum stress and inflammation in obesity and diabetesCirc Res201010757959120814028
- GregorMFHotamisligilGSInflammatory mechanisms in obesityAnnu Rev Immunol20112941544521219177
- BoselloOZamboniMVisceral obesity and the metabolic syndromeObes Rev20001475612119645
- CancelloRHenegarCViguerieNReduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight lossDiabetes2005542277228616046292
- OtaniMYamamotoMHaradaMOtsukiMEffect of long- and short-term treatments with pravastatin on diabetes mellitus and pancreatic fibrosis in the Otsuka-Long-Evans-Tokushima Fatty ratBr J Pharmacol201015946247320015084
- DonathMYBoni-SchnetzlerMEllingsgaardHEhsesJAIslet inflammation impairs the pancreatic beta-cell in type 2 diabetesPhysiology20092432533119996363
- MurataASekiyaKWatanabeYA novel inhibitory effect of d-allose on production of reactive oxygen species from neutrophilsJ Biosci Bioeng200396899116233490
- MuraoKYuXCaoWMd-psicose inhibits the expression of MCP-1 induced by high-glucose stimulation in HUVECsLife Sci20072659259917655880
- SunaSYamaguchiFKimuraSTokudaMJitsunariFPreventive effect of d-psicose, one of rare ketohexoses, on di-(2-ethylhexyl) phthalate (DEHP)-induced testicular injury in ratToxicol Lett200717310711717698303
- AksoyNVuralHSabuncuTAlsoySEffects of melatonin on oxidative-antioxidative status of tissues in streptozotocin-induced diabetic ratsCell Biochem Funct20032112112512736900
- BavarvaJHNarasimhacharyaAVAntihyperglycemic and hypolipidemic effects of Costus speciosus in alloxan-induced diabetic ratsPhytother Res20082262062618444247
- ReavenGMBanting lecture 1988. Role of insulin in human diseaseDiabetes198837159516073056758
- LeeERyuGRKoSHAntioxidant treatment may protect pancreatic beta cells through the attenuation of islet fibrosis in an animal model of type 2 diabetesBiochem Biophys Res Commun201141439740221971557
- WooVShestakovaMVØrskovCCerielloATargets and tactics: the relative importance of HbA, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetesInt J Clin Pract2008621935194219166440
- AvignonARadauceanuAMonnierLNonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetesDiabetes Care199720182218269405900
- Navigator Study GroupEffect of nateglinide on the incidence of diabetes and cardiovascular eventsN Engl J Med20103621463147620228402
- RoehrichMEMooserVLenainVInsulin-secreting beta-cell dysfunction induced by human lipoproteinsJ Biol Chem2003278183681837512594227
- TaskinenMRDiabetic dyslipidaemia: from basic research to clinical practiceDiabetologia20034673374912774165
- ShinASungJShinHKimJDietary intake, eating habits, and metabolic syndrome in Korean menJ Am Diet Assoc200910963364019328258
